Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
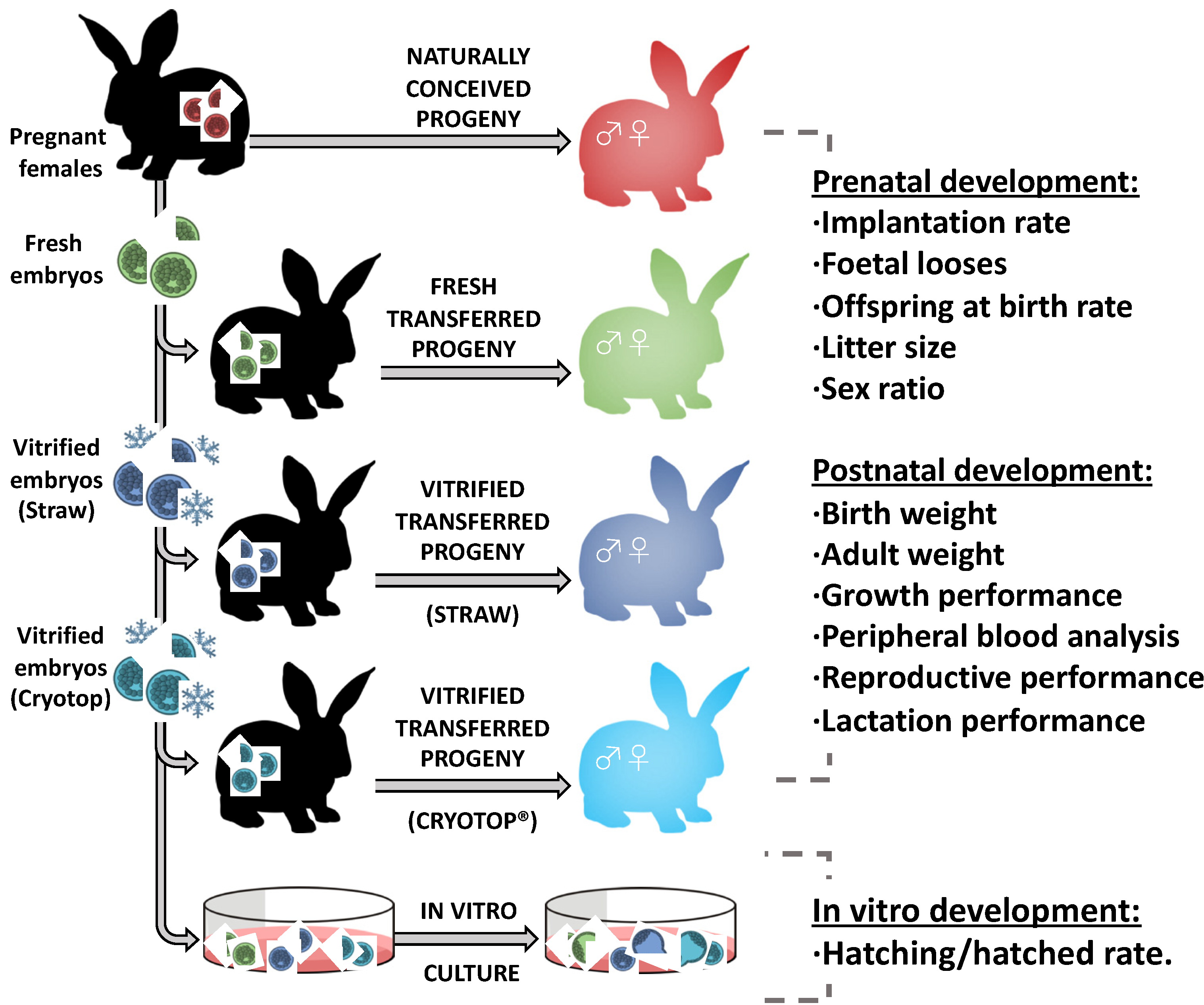
2.1. Experimental Design
2.2. Embryo Vitrification
2.3. In Vitro Culture
2.4. Embryo Transfer
2.5. Prenatal Development
2.6. Postnatal Growth Performance and Body Weight Study
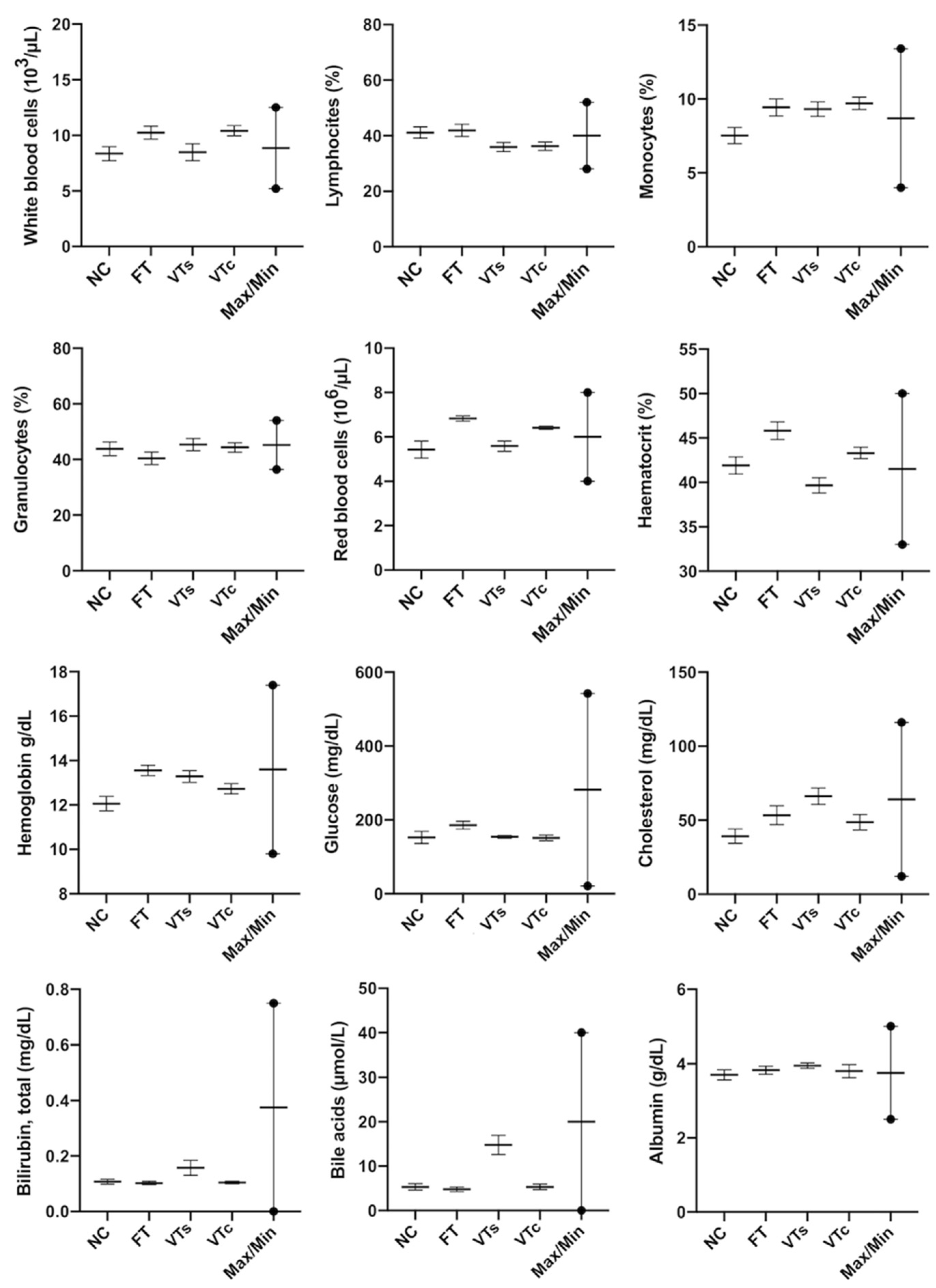
2.7. Determination of Haematological and Biochemical Parameters of Peripheral Blood
2.8. Male Reproductive Performance: Seminal Traits, Fertility Rate, and Induced Litter Size
2.9. Female Reproductive Performance: Pregnancy Rate, Litter Size, and Number of Liveborns
2.10. Lactation Performance: Milk Yield, Milk Composition, and Nutritional Potential
2.11. Statistical Analysis
3. Results
3.1. Effect of Embryo Vitrification on the Embryonic In Vitro Development
3.2. Effect of In Vitro Embryo Manipulation during Vitrification on Implantation, Foetal Losses, Offspring Rate, and Sex Ratio
3.3. Postnatal Growth Performance and Body Weight
3.4. Healthy Status: Peripheral Blood Parameters
3.5. Reproductive Performances
3.6. Lactation Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABN | Percentage of abnormal forms |
| ALH | Amplitude of lateral head displacement |
| ART | Assisted reproductive technologies |
| BCF | Beat cross-frequency |
| CON | Spermatic concentration |
| DMSO | Dimethyl sulfoxide |
| EDTA | Ethylenediaminetetraacetic acid |
| EG | Ethylene glycol |
| ESHRE | European Society of Human Reproduction and Embryology |
| FT | Fresh-transferred |
| GLM | General linear model |
| LIN | Linearity coefficient |
| MOT | Percentage of sperm motility |
| NAR | Percentage of normal apical ridge |
| NC | Naturally conceived |
| PRO | Percentage of progressive motility |
| SCC | Somatic cell count |
| Spz | Spermatozoa |
| STR | Straightness coefficient |
| TSE | Total sperm per ejaculate |
| VAP | Average path velocity |
| VCL | Curvilinear velocity |
| VIA | Percentage of viable sperm |
| VSL | Straight-line velocity |
| VT | Vitrified-transferred |
| VTc | Vitrified-transferred using Cryotop |
| VTs | Vitrified-transferred using ministraws |
| WOB | Wobble coefficient |
References
- Ng, K.Y.B.; Mingels, R.; Morgan, H.; Macklon, N.; Cheong, Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review. Hum. Reprod. Update 2018, 24, 15–34. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, S.; Hurtado, M.A.S.; Gutiérrez, H.; Margallo, F.M.S.; Romar, R.; Latorre, R.; Coy, P.; Albors, O.L. Mimicking physiological O2 tension in the female reproductive tract improves assisted reproduction outcomes in pig. Mol. Hum. Reprod. 2018, 24, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J. Developmental plasticity and its relevance to assisted human reproduction. Hum. Reprod. 2018, 33, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Vrooman, L.A.; Bartolomei, M.S. Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod. Toxicol. 2017, 68, 72–84. [Google Scholar] [CrossRef]
- Servick, K. Unsettled questions trail IVF’s success. Science 2014, 345, 744–746. [Google Scholar] [CrossRef]
- Skelly, A.; Dettori, J.; Brodt, E. Assessing bias: The importance of considering confounding. Evid. Based. Spine. Care. J. 2012, 3, 9–12. [Google Scholar] [CrossRef]
- Chen, M.; Heilbronn, L.K. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). J. Dev. Orig. Health Dis. 2017, 8, 388–402. [Google Scholar] [CrossRef]
- Halliday, J.; Lewis, S.; Kennedy, J.; Burgner, D.P.; Juonala, M.; Hammarberg, K.; Amor, D.J.; Doyle, L.W.; Saffery, R.; Ranganathan, S.; et al. Health of adults aged 22 to 35 years conceived by assisted reproductive technology. Fertil. Steril. 2019, 112, 130–139. [Google Scholar] [CrossRef]
- Juonala, M.; Lewis, S.; McLachlan, R.; Hammarberg, K.; Kennedy, J.; Saffery, R.; McBain, J.; Welsh, L.; Cheung, M.; Doyle, L.W.; et al. American Heart Association ideal cardiovascular health score and subclinical atherosclerosis in 22–35-year-old adults conceived with and without assisted reproductive technologies. Hum. Reprod. 2020, 35, 232–239. [Google Scholar] [CrossRef]
- Duranthon, V.; Chavatte-Palmer, P. Long term effects of ART: What do animals tell us? Mol. Reprod. Dev. 2018, 85, 348–368. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Heras, S.; Gómez-Redondo, I.; Planells, B.; Fernández-González, R.; Pericuesta, E.; Laguna-Barraza, R.; Pérez-Cerezales, S.; Gutiérrez-Adán, A. Embryo responses to stress induced by assisted reproductive technologies. Mol. Reprod. Dev. 2019, 86, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Feuer, S.K.; Rinaudo, P.F. Physiological, metabolic and transcriptional postnatal phenotypes of in vitro fertilization (IVF) in the mouse. J. Dev. Orig. Health Dis. 2017, 8, 403–410. [Google Scholar] [CrossRef]
- De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V.; et al. ART in Europe, 2015: Results generated from European registries by ESHRE. Hum. Reprod. Open 2020, 2020, hoz038. [Google Scholar] [CrossRef]
- Sparks, A.E.T. Human embryo cryopreservation-methods, timing, and other considerations for optimizing an embryo cryopreservation program. Semin. Reprod. Med. 2015, 33, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Hargreave, M.; Jensen, A.; Hansen, M.K.; Dehlendorff, C.; Winther, J.F.; Schmiegelow, K.; Kjær, S.K. Association between Fertility Treatment and Cancer Risk in Children. JAMA J. Am. Med. Assoc. 2019, 322, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Norrman, E.; Petzold, M.; Clausen, T.D.; Henningsen, A.K.; Opdahl, S.; Pinborg, A.; Rosengren, A.; Bergh, C.; Wennerholm, U.B. Type 1 diabetes in children born after assisted reproductive technology: A register-based national cohort study. Hum. Reprod. 2020, 35, 221–231. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef]
- Arav, A. Cryopreservation of oocytes and embryos. Theriogenology 2014, 81, 96–102. [Google Scholar] [CrossRef]
- Saragusty, J.; Arav, A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction 2011, 141, 1–19. [Google Scholar] [CrossRef]
- Vicente, J.S.; García-Ximénez, F. Osmotic and cryoprotective effects of a mixture of DMSO and ethylene glycol on rabbit morulae. Theriogenology 1994, 42, 1205–1215. [Google Scholar] [CrossRef]
- Vicente, J.S.; Viudes-De-Castro, M.P.; García, M.L. In vivo survival rate of rabbit morulae after vitrification in a medium without serum protein. Reprod. Nutr. Dev. 1999, 39, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Dominguez, X.; Marco-Jimenez, F.; Viudes-de-Castro, M.P.; Vicente, J.S. Minimally invasive embryo transfer and embryo vitrification at the optimal embryo stage in rabbit model. J. Vis. Exp. 2019, 147. [Google Scholar] [CrossRef] [PubMed]
- Besenfelder, U.; Strouhal, C.; Brem, G. A Method for Endoscopie Embryo Collection and Transfer in the Rabbit. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 1998, 45, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Blasco, A.; Gómez, E. A note on growth curves of rabbit lines selected on growth rate or litter size. Anim. Prod. 1993, 57, 332–334. [Google Scholar] [CrossRef]
- Maertens, L.; Lebas, F.; Szendrö, Z. Rabbit milk: A review of quantity, quality and non-dietary affecting factors. World Rabbit Sci. 2006, 14, 204–230. [Google Scholar] [CrossRef]
- Novakovic, B.; Lewis, S.; Halliday, J.; Kennedy, J.; Burgner, D.P.; Czajko, A.; Kim, B.; Sexton-Oates, A.; Juonala, M.; Hammarberg, K.; et al. Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nat. Commun. 2019, 10, e3922. [Google Scholar] [CrossRef]
- Seki, S.; Mazur, P. The dominance of warming rate over cooling rate in the survival of mouse oocytes subjected to a vitrification procedure. Cryobiology 2009, 59, 75–82. [Google Scholar] [CrossRef]
- Mazur, P.; Seki, S. Survival of mouse oocytes after being cooled in a vitrification solution to −196 °C at 95° to 70,000 °C/min and warmed at 610° to 118,000 °C/min: A new paradigm for cryopreservation by vitrification. Cryobiology 2011, 62, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.; Catalano, P.N.; Gurkan, U.A.; Khimji, I.; Demirci, U. Emerging technologies in medical applications of minimum volume vitrification. Nanomedicine 2011, 6, 1115–1129. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Lavara, R.; Jiménez-Trigos, E.; Vicente, J.S. In vivo development of vitrified rabbit embryos: Effects of vitrification device, recipient genotype, and asynchrony. Theriogenology 2013, 79, 1124–1129. [Google Scholar] [CrossRef]
- Saenz-De-Juano, M.D.; Marco-Jimenez, F.; Schmaltz-Panneau, B.; Jimenez-Trigos, E.; Viudes-De-Castro, M.P.; Penaranda, D.S.; Jouneau, L.; Lecardonnel, J.; Lavara, R.; Naturil-Alfonso, C.; et al. Vitrification alters rabbit foetal placenta at transcriptomic and proteomic level. Reproduction 2014, 147, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Riesche, L.; Bartolomei, M.S. Assisted Reproductive Technologies and the Placenta: Clinical, Morphological, and Molecular Outcomes. Semin. Reprod. Med. 2018, 36, 240–248. [Google Scholar] [CrossRef]
- Tan, K.; Wang, Z.; Zhang, Z.; An, L.; Tian, J. IVF affects embryonic development in a sex-biased manner in mice. Reproduction 2016, 151, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; An, L.; Miao, K.; Ren, L.; Hou, Z.; Tao, L.; Zhang, Z.; Wang, X.; Xia, W.; Liu, J.; et al. Impaired imprinted X chromosome inactivation is responsible for the skewed sex ratio following in vitro fertilization. Proc. Natl. Acad. Sci. USA 2016, 113, 3197–3202. [Google Scholar] [CrossRef]
- Maalouf, W.E.; Mincheva, M.N.; Campbell, B.K.; Hardy, I.C.W. Effects of assisted reproductive technologies on human sex ratio at birth. Fertil. Steril. 2014, 101, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, P.R.; Mittal, M.; Ohuma, E.O.; Lim, L.N.; McVeigh, E.; Granne, I.; Becker, C.M. Secondary sex ratio in assisted reproduction: An analysis of 1 376 454 treatment cycles performed in the UK. Hum. Reprod. Open 2019, 2019, hoz020. [Google Scholar] [CrossRef]
- Lin, P.Y.; Huang, F.J.; Kung, F.T.; Wang, L.J.; Chang, S.Y.; Lan, K.C. Comparison of the offspring sex ratio between fresh and vitrification-thawed blastocyst transfer. Fertil. Steril. 2009, 92, 1764–1766. [Google Scholar] [CrossRef]
- Chen, M.; Du, J.; Zhao, J.; Lv, H.; Wang, Y.; Chen, X.; Zhang, J.; Hu, L.; Jin, G.; Shen, H.; et al. The sex ratio of singleton and twin delivery offspring in assisted reproductive technology in China. Sci. Rep. 2017, 7, e7754. [Google Scholar] [CrossRef]
- Leme, L.O.; Carvalho, J.O.; Franco, M.M.; Dode, M.A.N. Effect of sex on cryotolerance of bovine embryos produced in vitro. Theriogenology 2020, 141, 219–227. [Google Scholar] [CrossRef]
- Spijkers, S.; Lens, J.W.; Schats, R.; Lambalk, C.B. Fresh and Frozen-Thawed Embryo Transfer Compared to Natural Conception: Differences in Perinatal Outcome. Gynecol. Obstet. Investig. 2017, 82, 538–546. [Google Scholar] [CrossRef]
- Chen, L.; Ni, X.; Xu, Z.; Fang, J.; Zhang, N.; Li, D. Effect of frozen and fresh embryo transfers on the birthweight of live-born twins. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 246, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Uk, A.; Collardeau-Frachon, S.; Scanvion, Q.; Michon, L.; Amar, E. Assisted Reproductive Technologies and imprinting disorders: Results of a study from a French congenital malformations registry. Eur. J. Med. Genet. 2018, 61, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Donnelly, C.G.; Rivera, R.M. Overgrowth Syndrome. Vet. Clin. North Am. Food Anim. Pract. 2019, 35, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hagen, D.E.; Elsik, C.G.; Ji, T.; Morris, C.J.; Moon, L.E.; Rivera, R.M. Characterization of global loss of imprinting in fetal overgrowth syndrome induced by assisted reproduction. Proc. Natl. Acad. Sci. USA 2015, 112, 4618–4623. [Google Scholar] [CrossRef]
- Mussa, A.; Molinatto, C.; Cerrato, F.; Palumbo, O.; Carella, M.; Baldassarre, G.; Carli, D.; Peris, C.; Riccio, A.; Ferrero, G.B. Assisted reproductive techniques and risk of Beckwith-Wiedemann syndrome. Pediatrics 2017, 140, e20164311. [Google Scholar] [CrossRef]
- Van Heertum, K.; Weinerman, R. Neonatal outcomes following fresh as compared to frozen/thawed embryo transfer in in vitro fertilization. Birth Defects Res. 2018, 110, 625–629. [Google Scholar] [CrossRef]
- Feuer, S.K.; Liu, X.; Donjacour, A.; Lin, W.; Simbulan, R.K.; Giritharan, G.; Piane, L.D.; Kolahi, K.; Ameri, K.; Maltepe, E.; et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology 2014, 155, 1956–1969. [Google Scholar] [CrossRef]
- Marshall, K.L.; Rivera, R.M. The effects of superovulation and reproductive aging on the epigenome of the oocyte and embryo. Mol. Reprod. Dev. 2018, 85, 90–105. [Google Scholar] [CrossRef]
- Baker, H.W.G. Reproductive effects of nontesticular illness. Endocrinol. Metab. Clin. North Am. 1998, 27, 831–850. [Google Scholar] [CrossRef]
- Calle, A.; Miranda, A.; Fernandez-Gonzalez, R.; Pericuesta, E.; Laguna, R.; Gutierrez-Adan, A. Male Mice Produced by In Vitro Culture Have Reduced Fertility and Transmit Organomegaly and Glucose Intolerance to Their Male Offspring. Biol. Reprod. 2012, 87, e34. [Google Scholar] [CrossRef]
- Belva, F.; Bonduelle, M.; Roelants, M.; Michielsen, D.; Van Steirteghem, A.; Verheyen, G.; Tournaye, H. Semen quality of young adult ICSI offspring: The first results. Hum. Reprod. 2016, 31, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Vellvé, K.; González-Comadran, M.; Robles, A.; Prat, M.; Torné, M.; Carreras, R.; Checa, M.A. Perinatal outcomes in children born after fresh or frozen embryo transfer: A Catalan cohort study based on 14,262 newborns. Fertil. Steril. 2017, 107, 940–947. [Google Scholar] [CrossRef]
- Sallem, A.; Santulli, P.; Barraud-Lange, V.; Le Foll, N.; Ferreux, L.; Maignien, C.; Bourdon, M.; Chapron, C.; de Ziegler, D.; Wolf, J.P.; et al. Extended culture of poor-quality supernumerary embryos improves ART outcomes. J. Assist. Reprod. Genet. 2018, 35, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Marsico, T.V.; de Camargo, J.; Valente, R.S.; Sudano, M.J. Embryo competence and cryosurvival: Molecular and cellular features. Anim. Reprod. 2019, 16, 423–439. [Google Scholar] [CrossRef]
- Mehdid, A.; Martí-De Olives, A.; Fernández, N.; Rodríguez, M.; Peris, C. Effect of stress on somatic cell count and milk yield and composition in goats. Res. Vet. Sci. 2019, 125, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, K.D.; Rutherford, K.M.D.; Wallace, J.M.; Brameld, J.M.; Stöger, R.; Alberio, R.; Sweetman, D.; Gardner, D.S.; Perry, V.E.A.; Adam, C.L.; et al. Epigenetics and developmental programming of welfare and production traits in farm animals. Reprod. Fertil. Dev. 2016, 28, 1443–1478. [Google Scholar] [CrossRef]
- Siqueira, L.G.B.; Dikmen, S.; Ortega, M.S.; Hansen, P.J. Postnatal phenotype of dairy cows is altered by in vitro embryo production using reverse X-sorted semen. J. Dairy Sci. 2017, 100, 5899–5908. [Google Scholar] [CrossRef]
- Mahsoudi, B.; Li, A.; O’Neill, C. Assessment of the Long-Term and Transgenerational Consequences of Perturbing Preimplantation Embryo Development in Mice. Biol. Reprod. 2007, 77, 889–896. [Google Scholar] [CrossRef]
- del Ciampo, L.A.; del Ciampo, I.R.L. Breastfeeding and the benefits of lactation for women’s health. Rev. Bras. Ginecol. Obstet. 2018, 40, 354–359. [Google Scholar] [CrossRef]
- Calle, A.; Fernandez-Gonzalez, R.; Ramos-Ibeas, P.; Laguna-Barraza, R.; Perez-Cerezales, S.; Bermejo-Alvarez, P.; Ramirez, M.A.; Gutierrez-Adan, A. Long-term and transgenerational effects of in vitro culture on mouse embryos. Theriogenology 2012, 77, 785–793. [Google Scholar] [CrossRef]
- Auroux, M. Long-term effects in progeny of paternal environment and of gamete/embryo cryopreservation. Hum. Reprod. Update 2000, 6, 550–563. [Google Scholar] [CrossRef] [PubMed]


| Traits | Naturally Conceived | Fresh-Transferred | Vitrified-Transferred | |
|---|---|---|---|---|
| Ministraw | Cryotop | |||
| Embryos (n) | 85 + | 96 | 87 | 101 |
| Foster mothers (n) | 6 | 6 | 6 | 7 |
| Implantation rate | 0.95 ± 0.021 a | 0.88 ± 0.034 b | 0.67 ± 0.051 c | 0.78 ± 0.041 bc |
| Foetal loss rate | 0.10 ± 0.033 b | 0.15 ± 0.039 b | 0.31 ± 0.061 a | 0.17 ± 0.042 b |
| Offspring rate | 0.86 ± 0.038 a | 0.74 ± 0.045 b | 0.52 ± 0.054 c | 0.65 ± 0.048 bc |
| Litter size | 12.2 ± 0.83 a | 11.8 ± 0.83 a | 7.5 ± 0.83 b | 9.3 ± 0.77 b |
| Sex ratio | 0.75:1 b | 1.08:1 ab | 1.33:1 a | 1.5:1 a |
| Total born (n) | 73 | 71 | 45 | 65 |
| Traits | Naturally Conceived | Fresh-Transferred | Vitrified-Transferred | |
|---|---|---|---|---|
| Ministraw | Cryotop | |||
| Semen parameters | ||||
| Pools (n) | 13 | 15 | 12 | 10 |
| CON (106 spz/ml) | 253.8 ± 31.71 ab | 317.8 ± 28.58 a | 217.3 ± 34.47 b | 248.5 ± 34.47 ab |
| MOT (%) | 88.6 ± 2.46 | 87.4 ± 2.37 | 90.3 ± 2.56 | 83.8 ± 2.95 |
| PRO (%) | 50.4 ± 2.87 ab | 43.1 ± 2.77 b | 53.1 ± 2.98 a | 42.1 ± 3.45 b |
| VIA (%) | 90.5 ± 1.60 a | 87.4 ± 1.55 ab | 84.8 ± 1.87 b | 89.6 ± 1.87 ab |
| NAR (%) | 95.1 ± 0.86 | 94.7 ± 0.80 | 93.1 ± 0.94 | 95.3 ± 1.04 |
| ABN (%) | 19.6 ± 2.01 | 19.1 ± 1.88 | 17.9 ± 2.19 | 17.1 ± 2.01 |
| Motion parameters | ||||
| VCL (μm s−1) | 98.5 ± 3.38 | 103.9 ± 3.11 | 100.3 ± 3.23 | 106.9 ± 3.96 |
| VSL (μm s−1) | 48.8 ± 2.18 a | 42.5 ± 2.09 b | 49.1 ± 2.18 a | 43.4 ± 2.67 ab |
| VAP (μm s−1) | 69.9 ± 2.25 | 66.1 ± 2.17 | 70.2 ± 2.25 | 67.9 ± 2.76 |
| LIN (%) | 48.5 ± 2.19 a | 41.2 ± 2.11 b | 49.1 ± 2.19 a | 40.6 ± 2.68 b |
| STR (%) | 69.1 ± 2.22 | 63.8 ± 2.04 | 68.1 ± 2.12 | 64.9 ± 2.59 |
| WOB (%) | 68.8 ± 1.67 ab | 64.1 ± 1.54 c | 69.8 ± 1.60 a | 64.4 ± 1.96 cb |
| ALH (μm) | 2.3 ± 0.12 ab | 2.3 ± 0.12 ab | 2.0 ± 0.12 b | 2.5 ± 0.15 a |
| BCF (Hz) | 9.8 ± 0.49 | 9.8 ± 0.47 | 9.9 ± 0.49 | 9.7 ± 0.69 |
| Fertility rate | 0.97 ± 0.019 | 0.94 ± 0.028 | 0.93 ± 0.030 | 0.92 ± 0.031 |
| Litter size | 12.1 ± 0.38 | 11.7 ± 0.40 | 11.9 ± 0.43 | 12.3 ± 0.41 |
| Traits | Naturally Conceived | Fresh-Transferred | Vitrified-Transferred | |
|---|---|---|---|---|
| Ministraw | Cryotop | |||
| Inseminated females | 16 | 12 | 20 | 18 |
| Reproductive performance | ||||
| Pregnant females | 16 | 11 | 20 | 17 |
| Litter size | 10.5 ± 0.65 | 9.1 ± 0.69 | 10.2 ± 0.62 | 9.1 ± 0.65 |
| Liveborn | 8.5 ± 0.68 | 8.9 ± 0.85 | 8.6 ± 0.60 | 8.5 ± 0.66 |
| Lactation performance | ||||
| Milk yield (g/day) | 261.9 ± 12.21a | 206.5 ± 13.44 b | 255.2 ± 10.98 a | 219.6 ± 11.26 b |
| Dry matter (%) | 36.3 ± 0.56 a | 33.5 ± 0.59 b | 36.0 ± 0.54 a | 33.94 ± 0.56 b |
| Fat (%) | 21.6 ± 0.51a | 18.5 ± 0.53 b | 20.5 ± 0.48 a | 18.3 ± 0.51b |
| Protein (%) | 10.9 ± 0.17 b | 11.0 ± 0.18 ab | 11.5 ± 0.17 a | 11.2 ± 0.18 ab |
| Lactose (%) | 2.5 ± 0.08 b | 2.4 ± 0.08 b | 2.5 ± 0.08 b | 2.8 ± 0.08 a |
| Somatic cells (103/mL) | 371.9 ± 101.09 b | 557.3 ± 113.92 ab | 408.1 ± 98.77 b | 725.3 ± 101.09 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Dominguez, X.; Vicente, J.S.; Marco-Jiménez, F. Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits. Animals 2020, 10, 804. https://doi.org/10.3390/ani10050804
Garcia-Dominguez X, Vicente JS, Marco-Jiménez F. Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits. Animals. 2020; 10(5):804. https://doi.org/10.3390/ani10050804
Chicago/Turabian StyleGarcia-Dominguez, Ximo, José Salvador Vicente, and Francisco Marco-Jiménez. 2020. "Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits" Animals 10, no. 5: 804. https://doi.org/10.3390/ani10050804
APA StyleGarcia-Dominguez, X., Vicente, J. S., & Marco-Jiménez, F. (2020). Developmental Plasticity in Response to Embryo Cryopreservation: The Importance of the Vitrification Device in Rabbits. Animals, 10(5), 804. https://doi.org/10.3390/ani10050804





