The three dietary treatments fed to the dairy cows in the present study during the dry period are commonly used in commercial farms based on the feed that can be produced on these farms. The diets covered the requirements of the cows without excess. The dietary treatments were characterized by different nutritional compositions, which could influence the milk yield, milk composition and metabolism of the dairy cows. The main differences were linked to starch content, DVE and OEB values. The lactation diet was similar for the three treatments.
4.1. Effect of the Diet during the Dry Period on Milk Production and Composition
The results of this study showed that the composition of diets during the dry period had no effect on the milk yield and a limited effect on the milk FA profile. Few studies had observed the effect of the dry-period diet composition on milk production and composition. According to the results of Kokkonen et al. [
31], the concentrate level in the diet given during the dry period could not affect milk yield during early and mid-lactation. Holcomb et al. [
32] did not show an effect of the forage percentage in the diet during the dry period on the milk yield. The nature of forage [
33] and concentrate [
34] used in the diet during the dry period had no effect on the milk yield. However, in the first week of lactation, Litherland et al. [
33] observed that the dairy cows produced more milk when they received orchardgrass rather than wheat straw. Many studies did not demonstrate an effect of the dietary energy level during the dry period on the milk yield [
12,
35], whereas, Gruber et al. [
17] observed an increase in the milk yield when the energy and nutrient supply of the diet during the dry period was above 75%. Jolicoeur et al. [
36] demonstrated that different management during the dry period had no effect on the milk yield. In our study, we expect that the diet during lactation can mitigate the effect of the dry-period diet on the milk traits. This observation is in agreement with the results of Dann et al. [
35].
The C18:2n-6 concentration was significantly higher in the milk collected at week +3 relative to parturition for the cows receiving the MIXED treatment than those receiving the CORN treatment. For the same period, the C18:3n-3 concentration was significantly higher when the cows received the MIXED treatment compared to the CONC treatment. In accordance with our results, Morel et al. [
34] observed an impact of the diet given during the dry period on the C18:2n-6 concentration, but only during the first week of lactation. During the dry period, this FA was stored in the adipose tissue and appeared to be mobilized directly during lactation. During early lactation, the milk fat was affected not only by the diet during lactation but also by the composition of the adipose tissue mobilized [
37]. As observed by Conner et al. [
38] in rabbits, the FA were mobilized selectively and proportionally to the degree of unsaturation and the chain length. That could explain the significant interaction observed in the present study between the dietary treatment and the week of lactation for the C18:2n-6. Furthermore, the C18:2 in the milk can be from C18:1, thanks to the action of desaturase. In the colostrum, Mann et al. [
39] showed that the dry-period diet energy level had an effect on the FA profile. The FA de novo concentration increased when the dairy cows received a higher energy diet. However, in this study, the milk FA profile was weakly affected by the diet intake during the dry period. This result needs further confirmation. The results of the present study show that farmers can use different diets during the dry period without negatively affecting milk production and composition during early lactation.
4.2. Effect of the Diet during the Dry Period on the BCS and Serum Metabolites
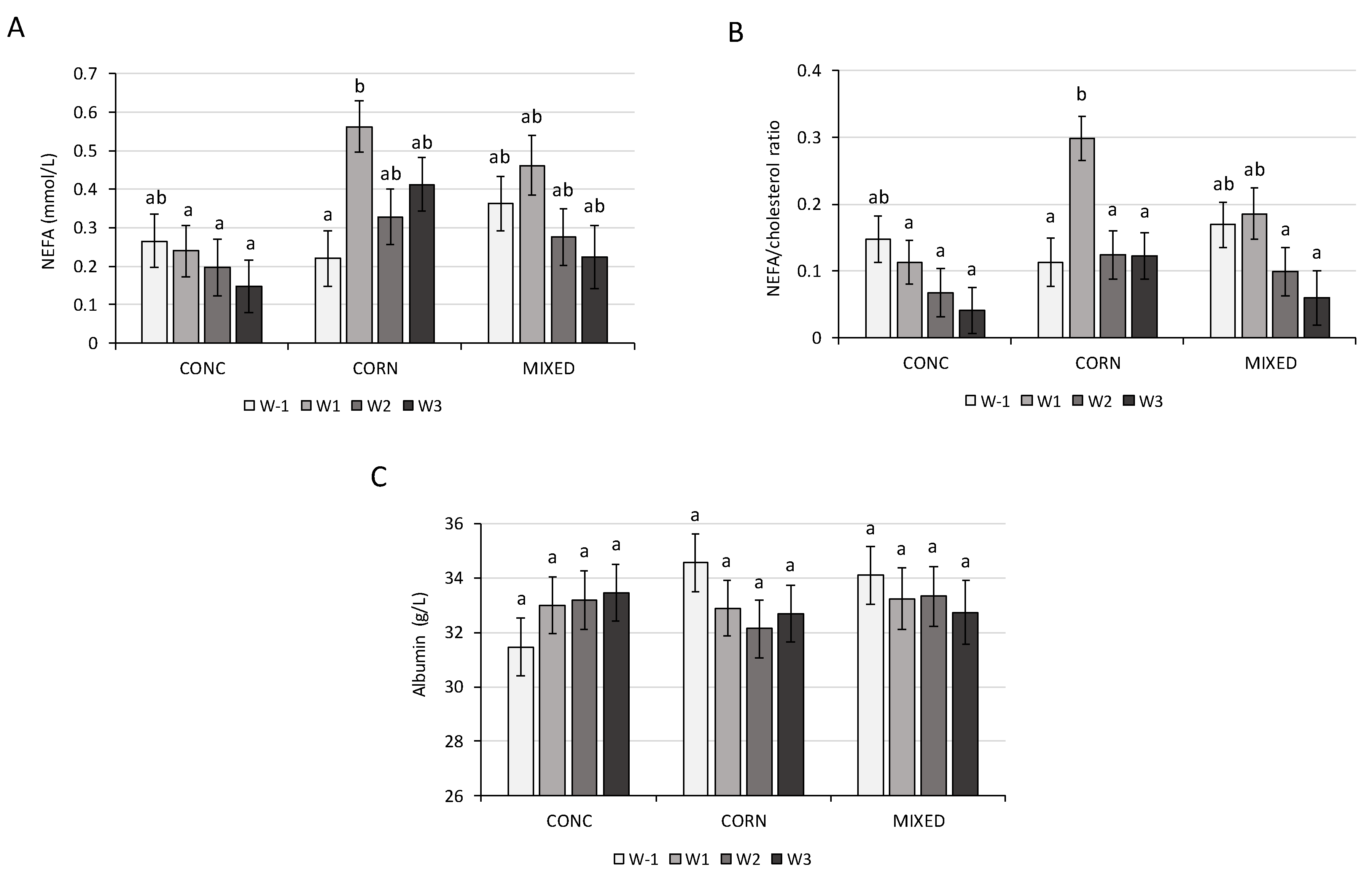
The decrease in the BCS and the concomitant increase in the serum NEFA and BHB after calving observed in our trial showed that the dairy cows had an NEB in early lactation. This situation is described by other authors [
8]. In accordance with our results, many studies did not observe a significant effect of the diet during the dry period on the BCS in lactation [
33,
40]. However, the diet could have an effect on the BCS during the dry period, according to the energy level of the diet [
35]. In our study, the dietary treatment had an impact on the serum NEFA concentration. It is indicated that the NEFA concentration in the blood should not be higher than 0.4 mmol/L for dry cows and the BHB concentration in the blood should not be higher than 1.2 mmol/L for cows in early lactation [
41]. In the present study, the BHB mean concentrations were higher than the recommendations, at weeks +2 and +3 in the CONC treatment (
Table A1). The BHB concentration in this latter group is known to be a sign of clinical ketosis. However, in our trial, it induced no clinical symptoms and had no impact on milk yield According to the present results, NEFA concentrations increased significantly after calving only in the CORN treatment. Many studies observed a significant effect of the dry-period diet on the NEFA concentration [
32,
42]. In our study, the higher NEFA concentration at week +1 relative to parturition in the CORN treatment could be explained by a higher mobilization of fat because the dairy cows had a high BCS during this period. The high NEFA concentrations observed in the CORN treatment at week +1 could be an indicator of hepatic lipidosis (
Table A1) [
43] Furthermore, dairy cows have a lipidosis risk if the NEFA/cholesterol ratio is above 0.2 [
43]. The cows in the CORN treatment receiving a higher amount of starch were the only ones to have this risk, especially at week +1. According to Drackley et al. [
44], a high-starch diet during the dry period could increase the proportion of internal fat, which is more conductive to mobilization and can increase the risk of hepatic lipidosis. The starch from maize, which is more lipogenic, could lead more frequently to fat cow syndrome. In our trial, higher fat mobilization had no effect on the concentrations of BHB and glucose or on the milk yield. The lack of dry treatment effect observed on the BHB is in agreement with the results of different studies [
12,
45]. However, Kokkonen et al. [
31] showed an increase in the BHB concentration in early lactation when the concentrate level of the diet had been increased during the dry period. Dann et al. [
35] also showed that an energy restriction in the far-off dry period treatment could decrease the BHB concentration. In our study, the dairy cows can have a BHB concentration above 1.2 mmol/L, considered as subclinical ketosis [
46]. However, it should be noticed that NEFA concentrations cannot be used to explain the BHB concentration. In this study, the correlation between the NEFA and BHB metabolites was very poor (r = −0.14), considering the dry and lactation periods. Contrary to the results of different studies, the increase in NEFA and BHB did not seem to affect the milk production [
46,
47] and the health [
5] of the dairy cows. Our results showed that the serum BHB concentration, especially in the dry cows, was not a good indicator of metabolic stress.
After calving, the serum glucose decreased because of the consumption by the mammary gland (85% of the total glucose in the first three weeks without insulin dependence) and, because of the modification in the liver, induced an increase in the NEFA and BHB in the blood [
48,
49]. The variations in the glucose and cholesterol observed over time in our study were in accordance with the results of previous studies [
45,
49]. Cholesterol is the principal lipid molecule exported by the liver in lipoproteins in ruminants and is linked to the capacity of the liver to export lipids [
42]. Cholesterol concentration with albumin can give information on the activity of the liver and the pro- or anti-inflammatory state of the cows [
50]. In accordance with our results, the serum glucose [
32,
51] and cholesterol [
35] were not affected by the diet during the dry period. The recommended cholesterol thresholds are 75 and 100 mg/dL for transition and early lactation, respectively. This last level was not reached in the three treatments at week +1 relative to parturition. Our results demonstrated that the increase in serum cholesterol was nevertheless dependent on the dietary treatment after calving. To our knowledge, the effect of diet on the serum cholesterol variation, during the dry period, was not studied. According to Weber et al. [
49], the interaction between the dry period duration and the time had a significant effect on the serum cholesterol concentration.
Protein metabolism can be evaluated by the determination of albumin, total serum proteins, and urea in the blood. Total serum proteins and albumin concentrations can give an estimation of the globulin concentration. A high total serum protein concentration with a low albumin concentration can indicate a change in protein synthesis during an inflammatory process. In our study, albumin and total serum proteins were not influenced by diet. Bjerre-Harpøth et al. [
6] observed that the dietary energy level during the dry period affected the serum albumin concentration in early lactation. The variation of the serum protein in their study agreed with our results. Contrary to Bjerre-Harpøth et al. [
6], we did not observe an increase in serum albumin during the lactation of the dairy cows. According to Moorby et al. [
8], the levels of two factors affected the plasma urea N concentration: feed intake and dietary protein content. Urea could also be linked to the synchronization and ratio of energy and proteins in the rumen. In our study, we observed that the serum urea could be affected by the dietary treatment given during the dry period. Moorby et al. [
8] also observed an effect of the diet during the dry period on the serum urea concentration. In the present study, in contrast to the results of Moorby et al. [
8], the CP level does not seem to explain the variation of the serum urea, but is consistent with the DVE level. In early lactation, we observed an increase in the serum urea concentration, probably due to a higher content of the diet in CP and DVE. We observed that the serum urea was significantly lower in the MIXED treatment than in the CONC treatment. According to the results of Mcnamara et al. [
52], a low serum urea concentration could be explained by a greater capture of N in the rumen. It seemed that the capture of N was more efficient in the MIXED treatments than in the CONC treatment. The higher urea concentration observed suggests a waste of nitrogen.
The serum vitamin B
12 is synthesized in the rumen and could provide information on rumen function. In our study, the serum vitamin B
12 concentration of the dairy cows varied according to their diet during the dry period. It would seem that the impact of the dry-period diet on the serum vitamin B
12 had never been studied before. Higher concentrations were observed in the MIXED treatment compared to the CONC treatment. It is also well documented that the milk vitamin B
12 concentration could be affected by the diet during lactation [
53,
54]. These results show that the diet during the dry period could affect the serum vitamin B
12. The composition of the diet and the physiological stage of the cow could affect the bacteria population of the rumen and the capacity to synthesize or use the vitamin B
12. In the present study, the serum vitamin B
12 decrease observed after parturition is in accordance with the results of Girard and Matte [
55], which indicated that the serum vitamin B
12 concentration varied over time. In early lactation, the vitamin B
12 concentration was lower because of a high demand for this vitamin in the production of colostrum and milk [
56] and in propionate utilization by the liver using a vitamin B
12 dependent pathway [
57]. In early lactation, as observed in our study, the serum vitamin B
12 concentration stayed low and constant [
58]. In the CONC and CORN treatments, the decrease was more severe. The MIXED diet provided starch from corn, cellulose, hemicellulose, pectin and soluble proteins from grass silage, offering a pattern of fermentation favorable for the synthesis of different elements including vitamin B. By contrast, the CONC treatment was the richest in starch. By contrast, the CONC diet contained cellulose from straw and other nutrients came from concentrate, inducing a faster fermentation profile and an increase in volatile fatty acids probably linked to concentrate intake. The CORN diet was the richest in starch and presented a negative OEB value, indicating that there is a lack of available N in the rumen. This situation can be detrimental to some categories of microbes and could lead to sub optimal fermentation. In the two latter diets (i.e., CORN and CONC), the nitrogen source was mainly storage proteins, which are less degradable in the rumen than the intracellular proteins forms included in MIXED treatment. The CORN and CONC treatments seemed to be less efficient than MIXED diet for rumen fermentation. Furthermore, the transition to a lactation diet partially composed of these silages is easier, as shown by the higher vitamin B
12 content in the serum. According to our results, the more appropriate dry-period diet seems to be MIXED treatment.
Our study shows that, in early lactation in dairy cows, the milk production and composition is less affected by the dietary treatment than the metabolism of the cows.