The Effect of Age and Sampling Site on the Outcome of Staphylococcus aureus Infection in a Rabbit (Oryctolagus cuniculus) Farm in Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Breeding
2.2. Sampling
2.3. Isolation and Identification of Staphylococcus Aureus
2.4. Genotypic Characterization
2.5. Statistical Analysis
3. Results
3.1. Sample Collection and Bacterial Isolation
3.2. Genotypic Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef]
- Cucarella, C.; Tormo, M.A.; Ubeda, C.; Trotonda, M.P.; Monzon, M.; Peris, C.; Amorena, B.; Lasa, I.; Penades, J.R. Role of biofilm-associated protein bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 2004, 72, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.M.L.; Lloyd, D.H.; Lindsay, J.A. Staphylococcus aureus host specificity: Comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology 2008, 154, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Sakwinska, O.; Giddey, M.; Moreillon, M.; Morisset, D.; Waldvogel, A.; Moreillon, P. Staphylococcus aureus Host Range and Human-Bovine Host Shift. Appl. Environ. Microbiol. 2011, 77, 5908–5915. [Google Scholar] [CrossRef] [PubMed]
- Penadés, M.; Viana, D.; García-Quirós, A.; Muñoz-Silvestre, A.; Moreno-Grua, E.; Pérez-Fuentes, S.; Pascual, J.J.; Corpa, J.M.; Selva, L. Differences in virulence between the two more prevalent Staphylococcus aureus clonal complexes in rabbitries (CC121 and CC96) using an experimental model of mammary gland infection. Vet. Res. 2020, 51, 11. [Google Scholar] [CrossRef]
- Smyth, D.S.; Feil, E.J.; Meaney, W.J.; Hartigan, P.J.; Tollersrud, T.; Fitzgerald, J.R.; Enright, M.C.; Smyth, C.J. Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus. J. Med. Microbiol. 2009, 58, 1343–1353. [Google Scholar] [CrossRef]
- Weinert, L.A.; Welch, J.J.; Suchard, M.A.; Lemey, P.; Rambaut, A.; Fitzgerald, J.R. Molecular dating of human-to-bovid host jumps by Staphylococcus aureus reveals an association with the spread of domestication. Biol. Lett. 2012, 8, 829–832. [Google Scholar] [CrossRef]
- Viana, D.; Comos, M.; McAdam, P.R.; Ward, M.J.; Selva, L.; Guinane, C.M.; González-Muñoz, B.M.; Tristan, A.; Foster, S.J.; Ross Fitzgerald, J.; et al. A single natural nucleotide mutation alters bacterial pathogen host-tropism. Nat. Genet. 2015, 47, 361–366. [Google Scholar] [CrossRef]
- Mørk, T.; Tollersrud, T.; Kvitle, B.; Jørgensen, H.J.; Waage, S. Comparison of Staphylococcus aureus Genotypes Recovered from Cases of Bovine, Ovine, and Caprine Mastitis. J. Clin. Microbiol. 2005, 43, 3979–3984. [Google Scholar] [CrossRef]
- Hermans, K.; De Herdt, P.; Devriese, L.A.; Hendrickx, W.; Godard, C.; Haesebrouck, F. Colonization of rabbits with Staphylococcus aureus in flocks with and without chronic staphylococcosis. Vet. Microbiol. 1999, 67, 37–46. [Google Scholar] [CrossRef]
- Agnoletti, F.; Cocchi, M.; Bano, L.; Guolo, A.; Bacchin, C.; Drigo, I.; Mazzolini, E. Validation of a sampling method to detect healthy rabbit Staphylococcus aureus carriers. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008; pp. 899–903. [Google Scholar]
- Okerman, L.; Devriese, L.A.; Maertens, L.; Okerman, F.; Godard, C. Cutaneous staphylococcosis in rabbits. Vet. Rec. 1984, 114, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Viana, D.; Selva, L.; Segura, P.; Penadés, J.R.; Corpa, J.M. Genotypic characterization of Staphylococcus aureus strains isolated from rabbit lesions. Vet. Microbiol. 2007, 121, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Hermans, K.; Devriese, L.A.; Haesebrouck, F. Rabbit staphylococcosis: Difficult solutions for serious problems. Vet. Microbiol. 2003, 91, 57–64. [Google Scholar] [CrossRef]
- Corpa, J.M.; Hermans, K.; Haesebrouck, F. Main pathologies associated with Staphylococcus aureus infections in rabbits: A review. World Rabbit Sci. 2009, 17, 115–125. [Google Scholar] [CrossRef]
- Viana, D.; Selva, L.; Callanan, J.J.; Guerrero, I.; Ferrian, S.; Corpa, J.M. Strains of Staphylococcus aureus and pathology associated with chronic suppurative mastitis in rabbits. Vet. J. 2011, 190, 403–407. [Google Scholar] [CrossRef]
- Vancraeynest, D.; Hermans, K.; Martel, A.; Vaneechoutte, M.; Devriese, L.A.; Haesebrouck, F. Antimicrobial resistance and resistance genes in Staphylococcus aureus strains from rabbits. Vet. Microbiol. 2004, 101, 245–251. [Google Scholar] [CrossRef]
- Ortega, C.; Simón, M.C.; Alonso, J.L.; Mateo, A. Caracterización y riesgos para la salud pública de la antibiorresistencia de Staphylococcus aureus en la cunicultura intensiva. Rev. Sci. Tech. Off. Int. Epiz. 2009, 28, 1119–1128. [Google Scholar] [CrossRef]
- Agnoletti, F.; Mazzolini, E.; Bacchin, C.; Bano, L.; Berto, G.; Rigoli, R.; Muffato, G.; Coato, P.; Tonon, E.; Drigo, I. First reporting of methicillin-resistant Staphylococcus aureus (MRSA) ST398 in an industrial rabbit holding and in farm-related people. Vet. Microbiol. 2014, 170, 172–177. [Google Scholar] [CrossRef]
- Loncaric, I.; Künzel, F. Sequence type 398 meticillin-resistant Staphylococcus aureus infection in a pet rabbit. Vet. Dermatol. 2013, 24, 370-e84. [Google Scholar] [CrossRef]
- Loncaric, I.; Künzel, F.; Licka, T.; Simhofer, H.; Spergser, J.; Rosengarten, R. Identification and characterization of methicillin-resistant Staphylococcus aureus (MRSA) from Austrian companion animals and horses. Vet. Microbiol. 2014, 168, 381–387. [Google Scholar] [CrossRef]
- Holmes, M.A.; Harrison, E.M.; Fisher, E.A.; Graham, E.M.; Parkhill, J.; Foster, G.; Paterson, G.K. Genomic Analysis of Companion Rabbit Staphylococcus aureus. PLoS ONE 2016, 11, e0151458. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, L.; Hermans, K.; Duchateau, L.; Haesebrouck, F. High and low virulence Staphylococcus aureus strains in a rabbit skin infection model. Vet. Microbiol. 2007, 125, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.B.; Skov, R.; Pallesen, L.V. Detection of methicillin resistance in coagulase-negative staphylococci and in staphylococci directly from simulated blood cultures using the EVIGENE MRSA Detection Kit. J. Antimicrob. Chemother. 2003, 51, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Stegger, M.; Andersen, P.S.; Kearns, A.; Pichon, B.; Holmes, M.A.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef]
- Devriese, L.A.; Hendrickx, W.; Godard, C.; Okerman, L.; Haesebrouck, F. A new pathogenic Staphylococcus aureus type in commercial rabbits. J. Vet. Med. B 1996, 43, 313–315. [Google Scholar] [CrossRef]
- Vancraeynest, D.; Haesebrouck, F.; Hermans, K. Multiplex PCR assay for the detection of high virulence rabbit Staphylococcus aureus strains. Vet. Microbiol. 2007, 121, 368–372. [Google Scholar] [CrossRef]
- Ridom SpaServer. Available online: https://www.spaserver.ridom.de (accessed on 18 September 2019).
- Fortinbras SpaTyper. Available online: http://spatyper.fortinbras.us (accessed on 14 November 2019).
- The R Project for Statistical Computing. Available online: https://www.r-project.org (accessed on 20 January 2020).
- Moreno-Grúa, E.; Pérez-Fuentes, S.; Muñoz-Silvestre, A.; Viana, D.; Fernández-Ros, A.B.; Sanz-Tejero, C.; Corpa, J.M.; Selva, L. Characterization of Livestock-Associated Methicillin-Resistant Staphylococcus aureus Isolates Obtained From Commercial Rabbitries Located in the Iberian Peninsula. Front. Microbiol. 2018, 9, 1812. [Google Scholar] [CrossRef]
- Fall, C.; Richard, V.; Dufougeray, A.; Biron, A.; Seck, A.; Laurent, F.; Breurec, S. Staphylococcus aureus nasal and pharyngeal carriage in Senegal. Clin. Microbiol. Infect. 2014, 20, O239–O241. [Google Scholar] [CrossRef]
- Holzknecht, B.J.; Hardardottir, H.; Haraldsson, G.; Westh, H.; Valsdottir, F.; Boye, K.; Karlsson, S.; Kristinsson, K.G.; Gudlaugsson, O. Changing Epidemiology of Methicillin-Resistant Staphylococcus aureus in Iceland from 2000 to 2008: A Challenge to Current Guidelines. J. Clin. Microbiol. 2010, 48, 4221–4227. [Google Scholar] [CrossRef]
- Von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. Study Group. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef]
- Neradova, K.; Jakubu, V.; Pomorska, K.; Zemlickova, H. Methicillin-resistant Staphylococcus aureus in veterinary professionals in 2017 in the Czech Republic. BMC Vet. Res. 2020, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Zambrano-Mila, M.; Rodrigueza, A.S.; Rivera-Olivero, I.A.; Salas-Ruedac, M.; Caceres-Orellanad, M.V.; de Waarda, J.H.; Garcia-Bereguiai, M.A. Methicillin resistant Staphylococcus aureus carriage among guinea pigs raised as livestock in Ecuador. One Health 2020, 9, 100118. [Google Scholar] [CrossRef] [PubMed]
- Segura, P.; Martinez, J.; Peris, B.; Selva, L.; Viana, D.; Penades, J.R.; Corpa, J.M. Staphylococcal infections in rabbit does on two industrial farms. Vet. Rec. 2007, 160, 869–872. [Google Scholar] [CrossRef]
- Boydell, P.; Cooke, S.W.; Deeb, B.; Flecknell, P.A.; Dermod Malley, A.; Meredith, A.; Morris, T.H.; Redrobe, S.; Scarff, D.H.; Walshaw, S.O. Manual of Rabbit Medicine and Surgery; Flecknell, P.A., Ed.; British Small Animal Veterinary Association: Gloucester, UK, 2000; pp. 33–38, 47–55, 63–68, 67–79. [Google Scholar]
- O’Hara, F.P.; Suaya, J.A.; Ray, G.T.; Baxter, R.; Brown, M.L.; Mera, R.M.; Close, N.M.; Thomas, E.; Amrine-Madsen, H. spa Typing and Multilocus Sequence Typing Show Comparable Performance in a Macroepidemiologic Study of Staphylococcus aureus in the United States. Microb. Drug Resist. 2016, 22, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K.; Piechowicz, L.; Podkowik, M.; Mroczkowska, A.; Empel, J.; Bania, J. Emergence and spread of worldwide Staphylococcus aureus clones among cystic fibrosis patients. Infect. Drug Resist. 2018, 11, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Greenwood-Quaintance, K.E.; Uhl, J.R.; Cunningham, S.A.; Chia, N.; Jeraldo, P.R.; Sampathkumar, P.; Nelson, H.; Patel, R. Molecular epidemiology of Staphylococcus aureus bacteremia in a single large Minnesota medical center in 2015 as assessed using MLST, core genome MLST and spa typing. PLoS ONE 2017, 12, e0179003. [Google Scholar] [CrossRef] [PubMed]
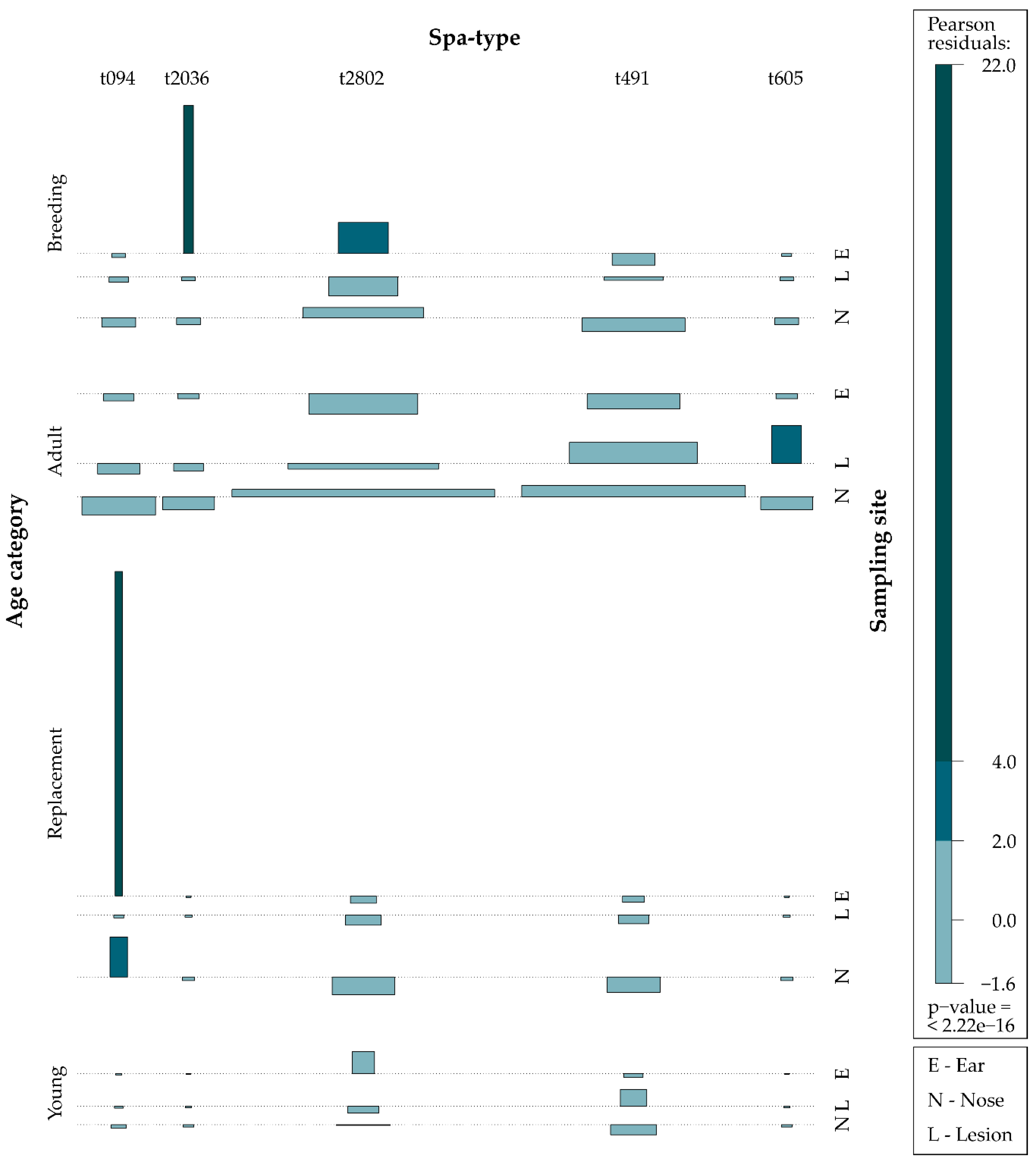
| Rabbit Age Category | No. of Animals (%) | S. aureus Frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy Anatomic Sites | Lesions | |||||||
| Nose No. (%) | Ear No (%) | Axilla No. (%) | Groin No. (%) | Perineum No. (%) | Total Isolates (%) | Isolates per Total of Lesions No. (%) | ||
| Young | 26 (6) | 4 (15.4) | 9 (34.6) | 3 (11.5) | 4 (15.4) | 6 (23.0) | 26 (4.7) | 1/5 (20) |
| Adult | 312 (78) | 139 (31.2) | 135 (30.3) | 50 (11.3) | 62 (13.9) | 59 (13.3) | 445 (80.6) | 33/39 (84.6) |
| Breeding | 27 (7) | 15 (25.9) | 18 (31.0) | 8 (13.8) | 8 (13.8) | 9 (15.5) | 58 (10.8) | 6/20 (30.0) |
| Replacement | 35 (9) | 3 (13.0) | 12 (52.3) | 2 (8.7) | 3 (13.0) | 3 (13.0) | 23 (3.9) | 0/2 (0.0) |
| Total | 400 | 161 (29.2) | 174 (31.5) | 63 (11.4) | 77 (13.9) | 77 (13.9) | 552 (93.2) | 40/66 (60.6) |
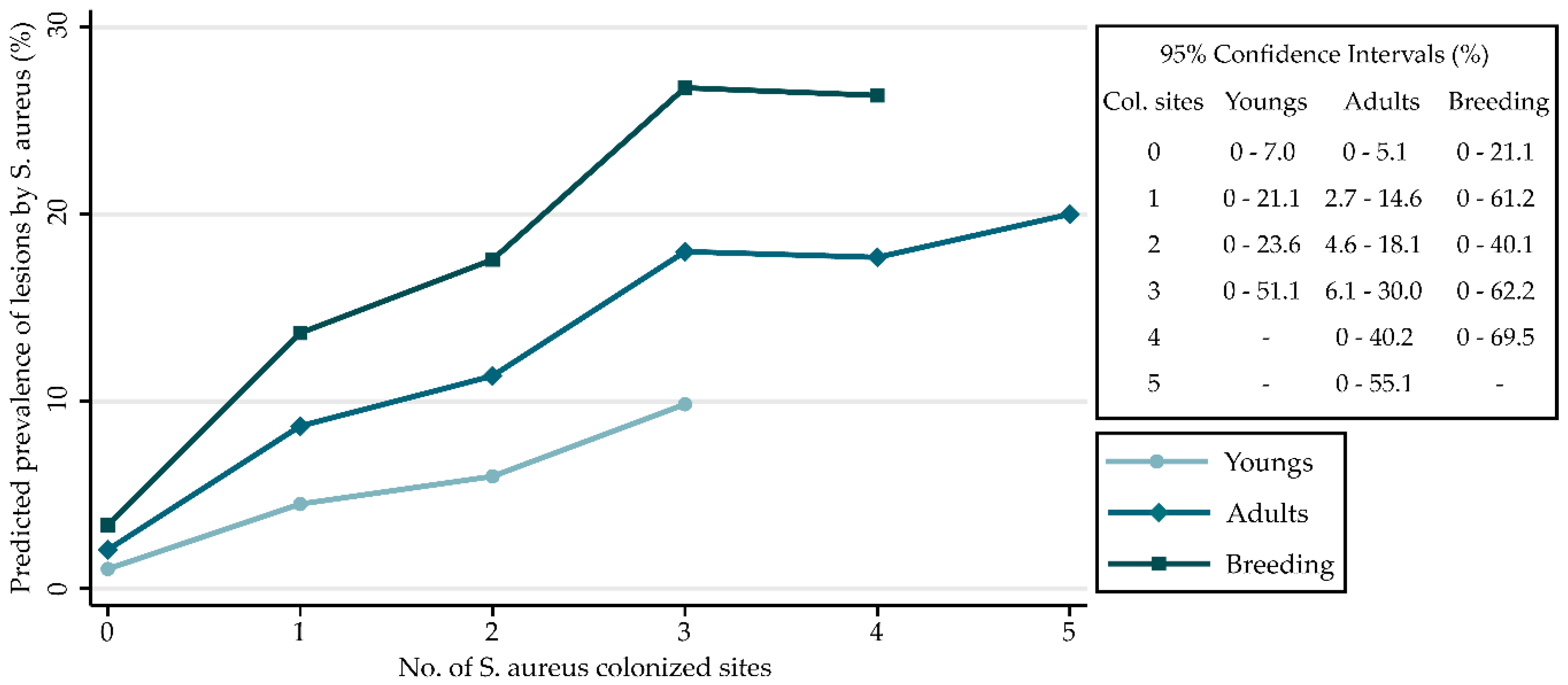
| No. of Sites Colonized by S. aureus | Rabbits with Lesions by S. aureus (%) | Crude Analysis | Controlling for Age Category | ||
|---|---|---|---|---|---|
| OR | 95% CI | OR * | 95% CI * | ||
| 0 | 2/116 (1.7) | 1 | 1 | ||
| 1 | 8/110 (7.3) | 4.5 | 0.9–21.5 | 4.5 | 0.9–21.9 |
| 2 | 12/105 (11.4) | 7.4 | 1.6–33.7 | 6.1 | 1.3–28.1 |
| 3 | 9/49 (18.4) | 12.8 | 2.6–61.9 | 10.4 | 2.1–50.8 |
| 4 | 3/15 (20.0) | 14.3 | 2.2–93.9 | 10.2 | 1.5–69.4 |
| 5 | 1/5 (20.0) | 14.3 | 1.1–191.7 | 11.9 | 0.9–160.8 |
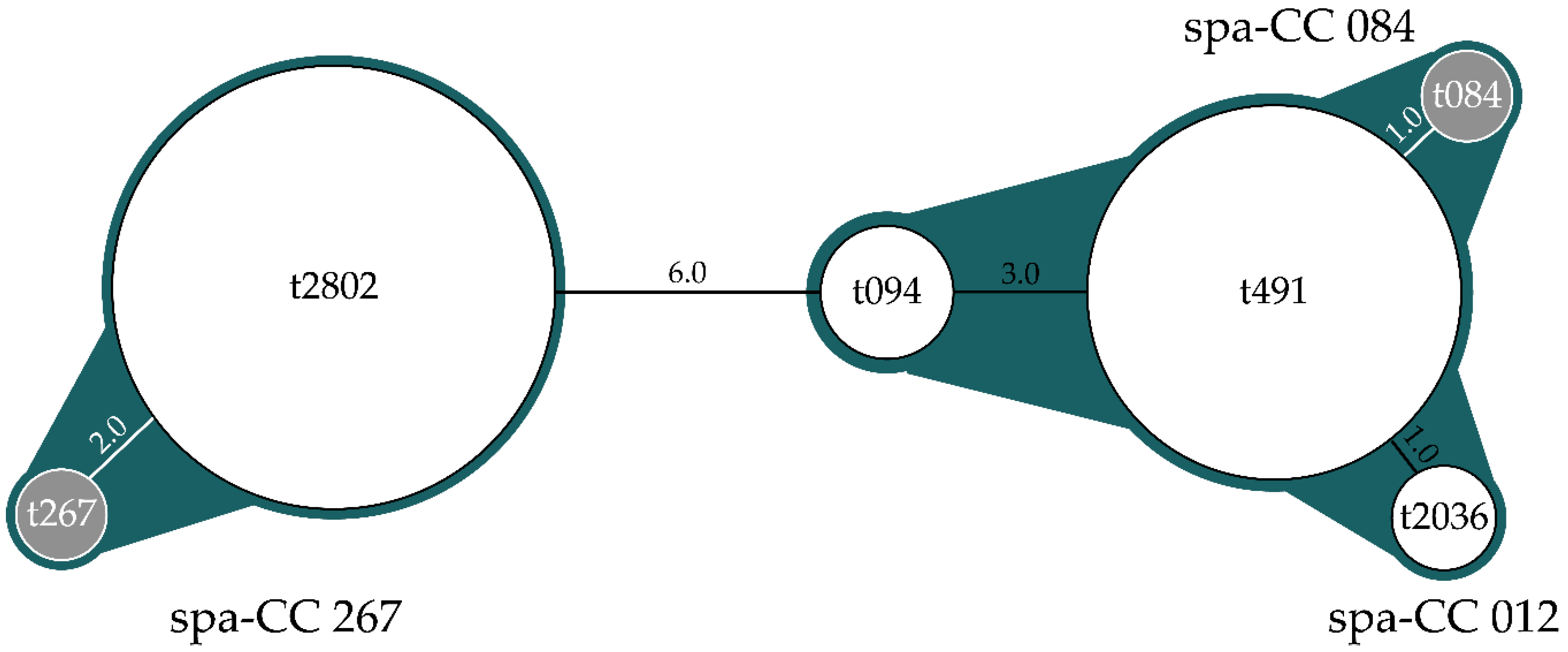
| Spa-Type | Repetitions | Spa-CC | Frequency (No) | Prevalence (%) | 95% CI |
|---|---|---|---|---|---|
| t094 | r07-r23-r12-r34-r34-r12-r12-r23 | 084 | 4 | 4.2 | 0.2–8.2 |
| t491 | r26-r23-r12-r34-r34-r12-r12-r23-r02-r12-r23 | 084 | 37 | 38.5 | 28.8–48.3 |
| t605 | r07-r23 | - | 2 | 2.1 | 0–4.9 |
| t2036 | r26-r23-r12-r34-r34-r12-r23-r02-r12-r23 | 012 | 2 | 2.1 | 0–4.9 |
| t2802 | r07-r23-r21-r17-r34-r34-r34-r33-r34 | 267 | 51 | 53.1 | 43.1–63.1 |
| Total | 96 | 100 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attili, A.-R.; Nebbia, P.; Bellato, A.; Galosi, L.; Papeschi, C.; Rossi, G.; Linardi, M.; Fileni, E.; Cuteri, V.; Chiesa, F.; et al. The Effect of Age and Sampling Site on the Outcome of Staphylococcus aureus Infection in a Rabbit (Oryctolagus cuniculus) Farm in Italy. Animals 2020, 10, 774. https://doi.org/10.3390/ani10050774
Attili A-R, Nebbia P, Bellato A, Galosi L, Papeschi C, Rossi G, Linardi M, Fileni E, Cuteri V, Chiesa F, et al. The Effect of Age and Sampling Site on the Outcome of Staphylococcus aureus Infection in a Rabbit (Oryctolagus cuniculus) Farm in Italy. Animals. 2020; 10(5):774. https://doi.org/10.3390/ani10050774
Chicago/Turabian StyleAttili, Anna-Rita, Patrizia Nebbia, Alessandro Bellato, Livio Galosi, Cristiano Papeschi, Giacomo Rossi, Martina Linardi, Eleonora Fileni, Vincenzo Cuteri, Francesco Chiesa, and et al. 2020. "The Effect of Age and Sampling Site on the Outcome of Staphylococcus aureus Infection in a Rabbit (Oryctolagus cuniculus) Farm in Italy" Animals 10, no. 5: 774. https://doi.org/10.3390/ani10050774
APA StyleAttili, A.-R., Nebbia, P., Bellato, A., Galosi, L., Papeschi, C., Rossi, G., Linardi, M., Fileni, E., Cuteri, V., Chiesa, F., & Robino, P. (2020). The Effect of Age and Sampling Site on the Outcome of Staphylococcus aureus Infection in a Rabbit (Oryctolagus cuniculus) Farm in Italy. Animals, 10(5), 774. https://doi.org/10.3390/ani10050774








