Effects of Short-Term Inhibition of Rho Kinase on Dromedary Camel Oocyte In Vitro Maturation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Media and Reagent
2.2. Ovary Collection and Oocyte Retrieval
2.3. Experiment Design and Procedure
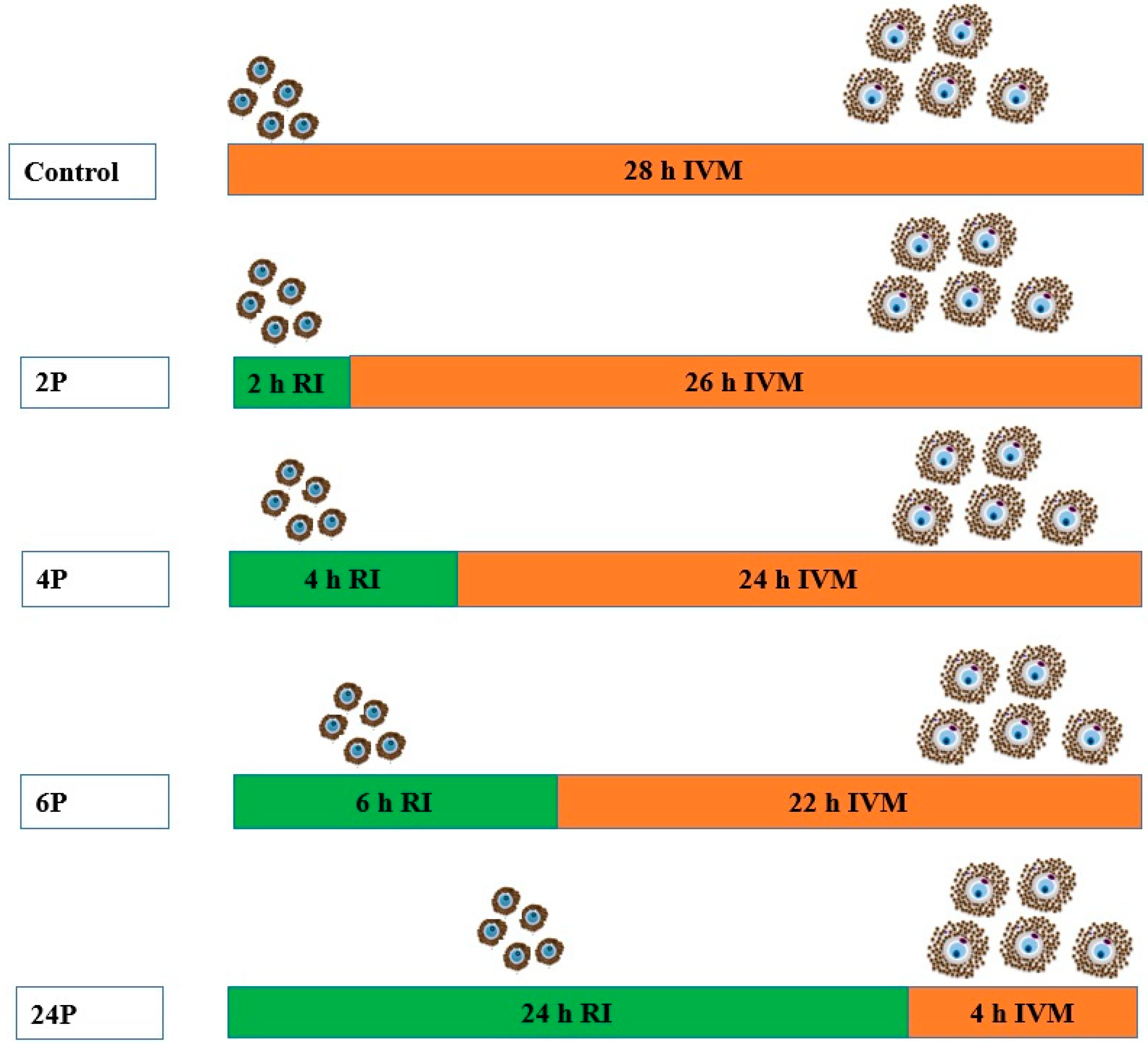
2.3.1. Experiment 1: Biphasic IVM with ROCK Inhibitor
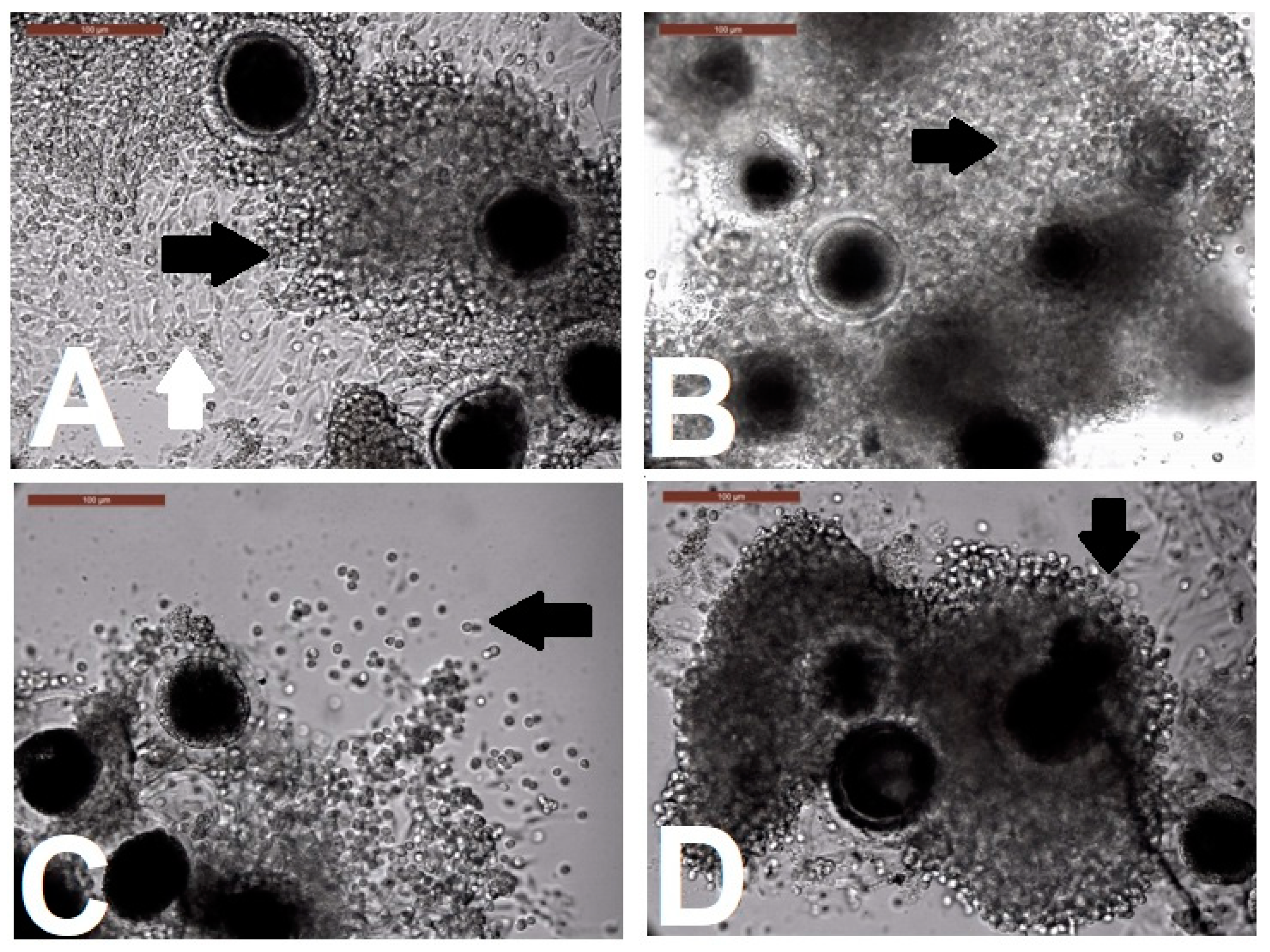
Evaluation of Cumulus Cell Expansion after Biphasic IVM
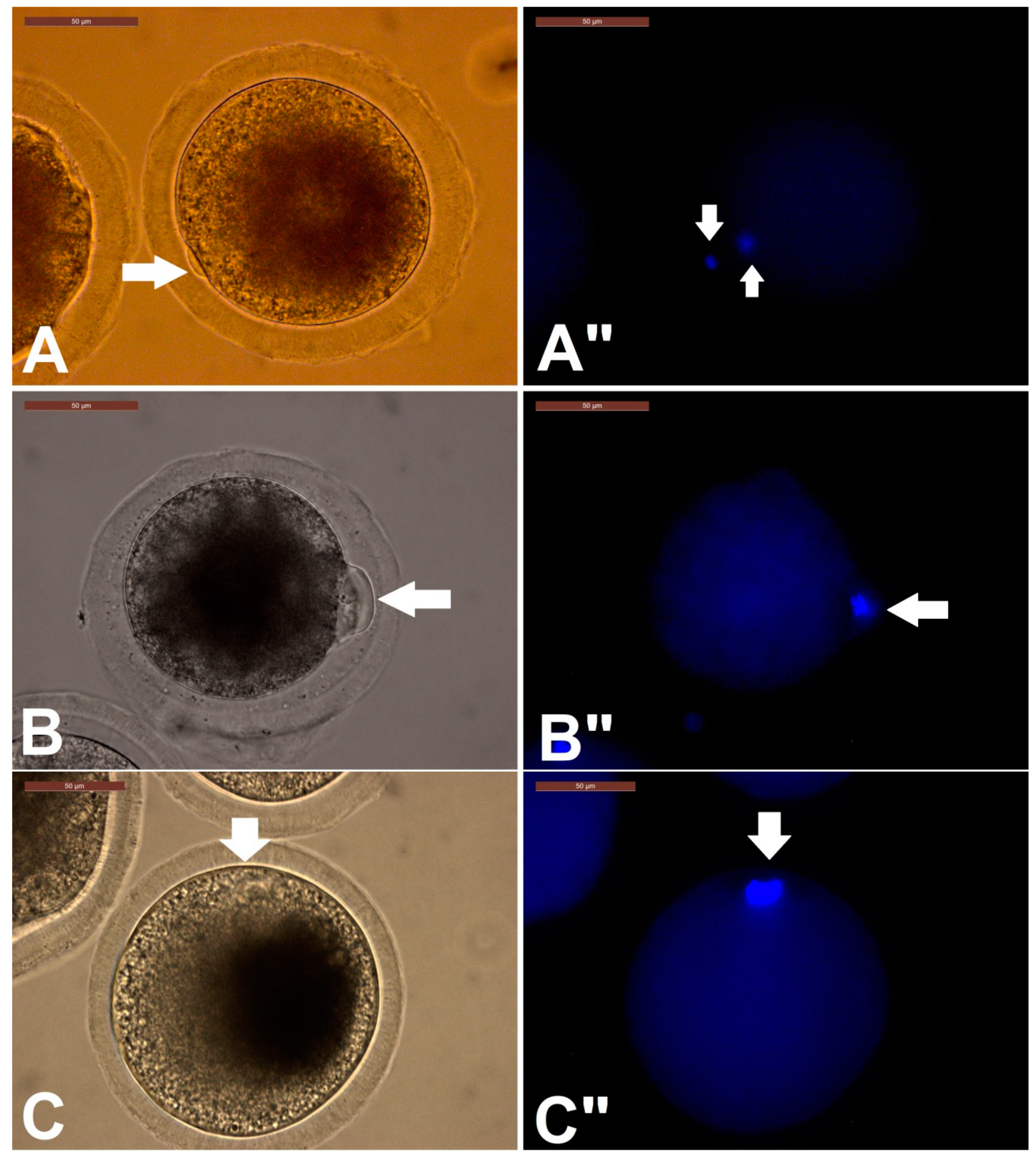
Assessment of Oocytes for Completion of Meiosis I
Oocyte Morphometric Evaluation
2.3.2. Experiment 2
Collection of COCs for RNA Isolation
Total RNA Isolation, Complimentary DNA Synthesis, and Real-Time PCR
2.3.3. Statistical Analysis
3. Results
3.1. Effect of RI on Camel IVM, Oocyte Morphometry, and Cumulus Expansion
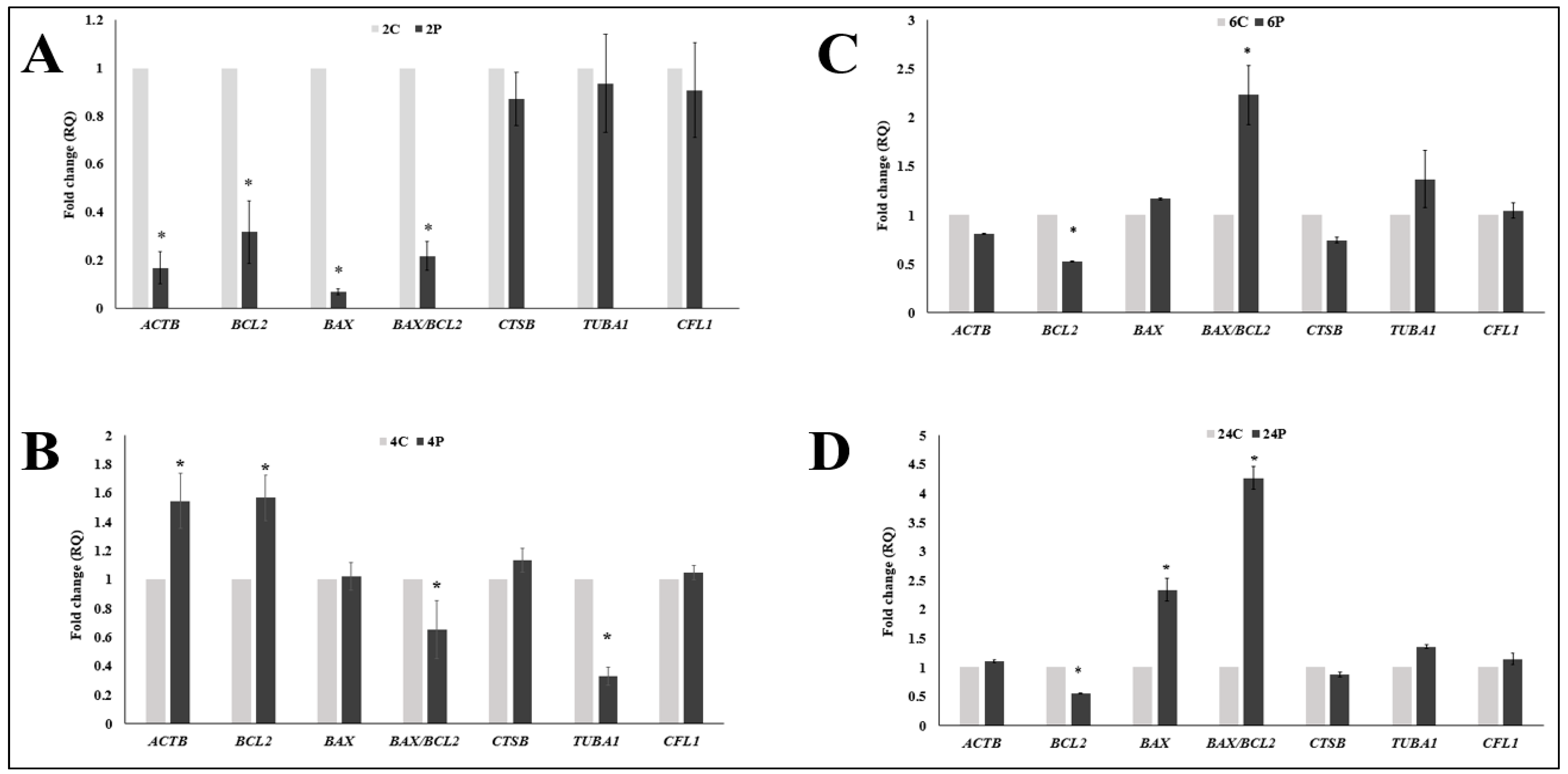
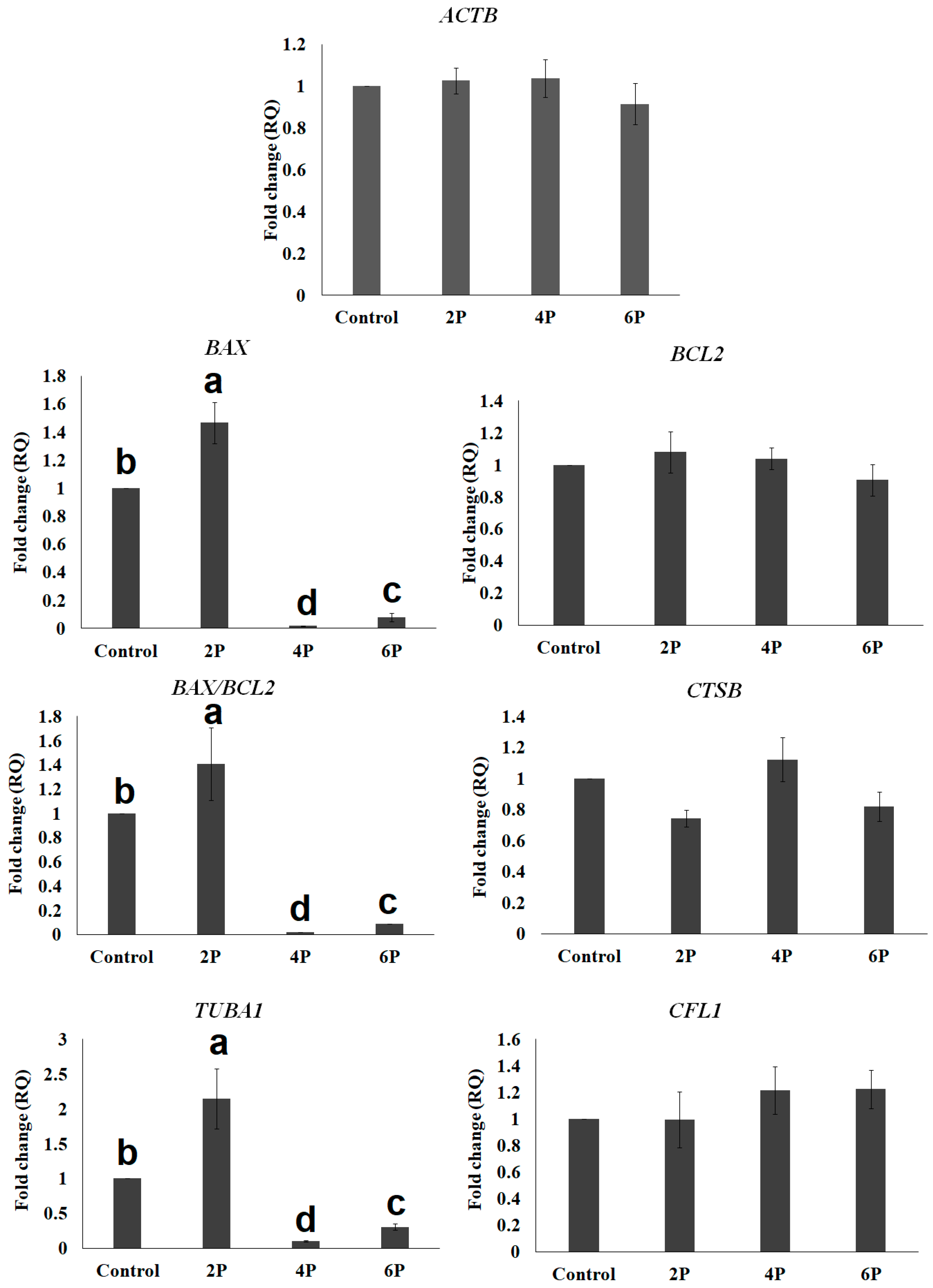
3.2. Effect of Biphasic IVM with RI on the Expression of Cytokinesis- and Apoptosis-Related mRNA Transcripts
3.3. Correlation Analysis between Oocyte Maturation, Oocyte Morphometry, and Different mRNA Transcripts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comizzoli, P.; Paulson, E.E.; McGinnis, L.K. The mutual benefits of research in wild animal species and human-assisted reproduction. J. Assist. Reprod. Genet. 2018, 35, 551–560. [Google Scholar] [CrossRef]
- Herrick, J.R. Assisted reproductive technologies for endangered species conservation: Developing sophisticated protocols with limited access to animals with unique reproductive mechanisms. Biol. Reprod. 2019, 100, 1158–1170. [Google Scholar] [CrossRef]
- Skidmore, J.A. The use of some assisted reproductive technologies in old world camelids. Anim. Reprod. Sci. 2019, 207, 138–145. [Google Scholar] [CrossRef]
- Abri, M.A.A.; Faye, B. Genetic Improvement in Dromedary Camels: Challenges and Opportunities. Front. Genet. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Monaco, D.; Rubessa, M.; El-Bahrawy, K.A.; El-Sayed, A.; Martino, N.A.; Beneult, B.; Ciannarella, F.; Dell’Aquila, M.E.; Lacalandra, G.M.; et al. Confocal fluorescence assessment of bioenergy/redox status of dromedary camel (Camelus dromedarius) oocytes before and after in vitro maturation. Reprod. Biol. Endocrinol. 2014, 12, 16. [Google Scholar] [CrossRef]
- Singh, B.; Mal, G.; Gautam, S.K.; Mukesh, M. Reproduction Biotechnology in Camelids. In Advances in Animal Biotechnology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 145–153. [Google Scholar] [CrossRef]
- Abdelkhalek, A.E.; Gabr, S.A.; Khalil, W.A.; Shamiah, S.M.; Pan, L.; Qin, G.; Farouk, M.H. In vitro production of Sudanese camel (Camelus dromedarius) embryos from epididymal spermatozoa and follicular oocytes of slaughtered animals. Pol. J. Vet. Sci. 2017, 20, 95–101. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Mahmood, A.; Yaqoob, S.H.; Alfayez, M.; Alowaimer, A.N. Effects of all-trans retinoic acid on the in vitro maturation of camel (Camelus dromedarius) cumulus-oocyte complexes. J. Reprod. Dev. 2019, 65, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, S.H.; Saadeldin, I.M.; Swelum, A.A.-A.; Alowaimer, A.N. Optimizing camel (Camelus dromedarius) oocytes in vitro maturation and early embryo culture after parthenogenetic activation. Small Rum. Res. 2017, 153, 81–86. [Google Scholar] [CrossRef]
- Fathi, M.; Moawad, A.R.; Badr, M.R. Production of blastocysts following in vitro maturation and fertilization of dromedary camel oocytes vitrified at the germinal vesicle stage. PLoS ONE 2018, 13, e0194602. [Google Scholar] [CrossRef]
- Albuz, F.K.; Sasseville, M.; Lane, M.; Armstrong, D.T.; Thompson, J.G.; Gilchrist, R.B. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 2010, 25, 2999–3011. [Google Scholar] [CrossRef]
- Edwards, R.G. Maturation in vitro of Mouse, Sheep, Cow, Pig, Rhesus Monkey and Human Ovarian Oocytes. Nature 1965, 208, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Wert, S.E.; Larsen, W.J. Meiotic resumption and gap junction modulation in the cultured rat cumulus-oocyte complex. Gamete Res. 1989, 22, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.L.; Albertini, D.F. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J. Assist. Reprod. Genet. 2009, 27, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B. Recent insights into oocyte—Follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 23–31. [Google Scholar] [CrossRef]
- Lonergan, P.; Dinnyes, A.; Fair, T.; Yang, X.; Boland, M. Bovine oocyte and embryo development following meiotic inhibition with butyrolactone I. Mol. Reprod. Dev. 2000, 57, 204–209. [Google Scholar] [CrossRef]
- Caballero, J.; Blondin, P.; Vigneault, C.; Sirard, M.-A.; Richard, F.J. The use of adenosine to inhibit oocyte meiotic resumption in Bos taurus during pre-IVM and its potential to improve oocyte competence. Theriogenology 2020, 142, 207–215. [Google Scholar] [CrossRef]
- Sanchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; Van Ranst, H.; De Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; Vos, M.D.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef]
- Li, H.J.; Sutton-McDowall, M.L.; Wang, X.; Sugimura, S.; Thompson, J.G.; Gilchrist, R.B. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Menendez-Blanco, I.; Catala, M.G.; Izquierdo, D.; Thompson, J.G.; Paramio, M.T. Biphasic in vitro maturation with C-type natriuretic peptide enhances the developmental competence of juvenile-goat oocytes. PLoS ONE 2019, 14, e0221663. [Google Scholar] [CrossRef]
- Zhu, S.; Jia, Y.J.; Pan, L.Z.; Gong, S.; Sun, M.J.; Wang, G.L.; Luo, M.J.; Tan, J.H. Meiotic block with roscovitine improves competence of porcine oocytes by fine-tuning activities of different cyclin-dependent kinases. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Maziero, R.R.D.; Guaitolini, C.R.F.; Paschoal, D.M.; Crespilho, A.M.; Monteiro, B.A.; Lima, J.S.; Sestari, D.A.O.; Landim-Alvarenga, F.D.C. Treatment with roscovitine and butyrolactone I prior to in vitro maturation alters blastocyst production. Zygote 2020, 28, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Xiong, B.; Cui, X.S.; Kim, N.H.; Rui, R.; Sun, S.C. ROCK inhibitor Y-27632 prevents porcine oocyte maturation. Theriogenology 2014, 82, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, C.-X.; Pan, L.-Z.; Gong, S.; Cui, W.; Yuan, H.-J.; Zhang, W.-L.; Tan, J.-H. Meiotic arrest with roscovitine and follicular fluid improves cytoplasmic maturation of porcine oocytes by promoting chromatin de-condensation and gene transcription. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Harb, N.; Archer, T.K.; Sato, N. The Rho-Rock-Myosin Signaling Axis Determines Cell-Cell Integrity of Self-Renewing Pluripotent Stem Cells. PLoS ONE 2008, 3, e3001. [Google Scholar] [CrossRef]
- Claassen, D.A.; Desler, M.M.; Rizzino, A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol. Reprod. Dev. 2009, 76, 722–732. [Google Scholar] [CrossRef]
- Huang, S.; Ding, C.; Mai, Q.; Xu, Y.; Zhou, C. Inhibition of Rhoassociated protein kinase increases the ratio of formation of blastocysts from single human blastomeres. Mol. Med. Rep. 2016, 13, 2046–2052. [Google Scholar] [CrossRef]
- Arayatham, S.; Tiptanavattana, N.; Tharasanit, T. Effects of vitrification and a Rho-associated coiled-coil containing protein kinase 1 inhibitor on the meiotic and developmental competence of feline oocytes. J. Reprod. Dev. 2017, 63, 511–517. [Google Scholar] [CrossRef]
- An, L.; Liu, J.; Du, Y.; Liu, Z.; Zhang, F.; Liu, Y.; Zhu, X.; Ling, P.; Chang, S.; Hu, Y.; et al. Synergistic effect of cysteamine, leukemia inhibitory factor, and Y27632 on goat oocyte maturation and embryo development in vitro. Theriogenology 2018, 108, 56–62. [Google Scholar] [CrossRef]
- Duan, X.; Liu, J.; Dai, X.-X.; Liu, H.-L.; Cui, X.-S.; Kim, N.-H.; Wang, Z.-B.; Wang, Q.; Sun, S.-C. Rho-GTPase effector ROCK phosphorylates cofilin in actin-meditated cytokinesis during mouse oocyte meiosis. Biol. Reprod. 2014, 90, 37. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Yaqoob, S.H.; Alowaimer, A.N. Morphometric assessment of in vitro matured dromedary camel oocytes determines the developmental competence after parthenogenetic activation. Theriogenology 2017, 95, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Swelum, A.A.-A.; Alowaimer, A.N. In Vitro Culture of Camelid Embryos. In Comparative Embryo Culture, Part of the Methods in Molecular Biology Book Series (MIMB, Volume 2006); Humana: New York, NY, USA, 2019; pp. 209–218. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.-A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Petrie, A.; Watson, P. Statistics for Veterinary and Animal Science; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Bahrami, M.; Morris, M.B.; Day, M.L. Amino acid supplementation of a simple inorganic salt solution supports efficient in vitro maturation (IVM) of bovine oocytes. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Vuong, L.N.; Le, A.H.; Ho, V.N.A.; Pham, T.D.; Sanchez, F.; Romero, S.; De Vos, M.; Ho, T.M.; Gilchrist, R.B.; Smitz, J. Live births after oocyte in vitro maturation with a prematuration step in women with polycystic ovary syndrome. J. Assist. Reprod. Genet. 2020, 37, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.-T.; Thompson, J.G. Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte communication and oocyte developmental competence in cattle. Anim. Reprod. Sci. 2019, 202, 49–57. [Google Scholar] [CrossRef]
- Suzuki, H.; Koyama, K.-I.; Kabashima, K.; Fang, J.; Matsuzaki, M. Temporary Inhibition of Germinal Vesicle Breakdown by Rho Kinase Inhibitor Y-27632 is Detrimental to Oocyte Maturation. J. Mammal. Ova Res. 2011, 28, 126–130. [Google Scholar] [CrossRef]
- Lee, S.R.; Xu, Y.N.; Jo, Y.J.; Namgoong, S.; Kim, N.H. The Rho-GTPase effector ROCK regulates meiotic maturation of the bovine oocyte via myosin light chain phosphorylation and cofilin phosphorylation. Mol. Reprod. Dev. 2015, 82, 849–858. [Google Scholar] [CrossRef]
- Wu, Y.-G.; Zhou, P.; Lan, G.-C.; Gao, D.; Li, Q.; Wei, D.-L.; Wang, H.-L.; Tan, J.-H. MPF Governs the Assembly and Contraction of Actomyosin Rings by Activating RhoA and MAPK during Chemical-Induced Cytokinesis of Goat Oocytes. PLoS ONE 2010, 5, e12706. [Google Scholar] [CrossRef]
- Pan, M.-H.; Wang, F.; Lu, Y.; Tang, F.; Duan, X.; Zhang, Y.; Xiong, B.; Sun, S.-C. FHOD1 regulates cytoplasmic actin-based spindle migration for mouse oocyte asymmetric cell division. J. Cell. Physiol. 2018, 233, 2270–2278. [Google Scholar] [CrossRef]
- Schofield, A.V.; Steel, R.; Bernard, O. Rho-associated coiled-coil kinase (ROCK) protein controls microtubule dynamics in a novel signaling pathway that regulates cell migration. J. Biol. Chem. 2012, 287, 43620–43629. [Google Scholar] [CrossRef] [PubMed]
- Buccione, R.; Schroeder, A.C.; Eppig, J.J. Interactions between Somatic Cells and Germ Cells throughout Mammalian Oogenesis1. Biol. Reprod. 1990, 43, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Arias-Álvarez, M.; García-García, R.M.; López-Tello, J.; Rebollar, P.G.; Gutiérrez-Adán, A.; Lorenzo, P.L. In vivo and in vitro maturation of rabbit oocytes differently affects the gene expression profile, mitochondrial distribution, apoptosis and early embryo development. Reprod. Fertil. Dev. 2017, 29, 1667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.X.; Ma, Y.Z.; Liu, Y.L.; Chen, Y.; Zhou, C.J.; Wu, S.N.; Shen, J.P.; Liang, C.G. Assessment of mouse germinal vesicle stage oocyte quality by evaluating the cumulus layer, zona pellucida, and perivitelline space. PLoS ONE 2014, 9, e105812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, Y.; Shen, C.; Zhang, L.; Wang, X. MicroRNA-375 regulates oocyte in vitro maturation by targeting ADAMTS1 and PGR in bovine cumulus cells. Biomed. Pharmacol. 2019, 118, 109350. [Google Scholar] [CrossRef]
- Zanetti, B.F.; Braga, D.P.A.F.; Setti, A.S.; Figueira, R.C.S.; Iaconelli, A., Jr.; Borges, E., Jr. Is perivitelline space morphology of the oocyte associated with pregnancy outcome in intracytoplasmic sperm injection cycles? Eur. J. Obst. Gynecol. Reprod. Biol. 2018, 231, 225–229. [Google Scholar] [CrossRef]
- Schofield, A.V.; Gamell, C.; Suryadinata, R.; Sarcevic, B.; Bernard, O. Tubulin Polymerization Promoting Protein 1 (Tppp1) Phosphorylation by Rho-associated Coiled-coil Kinase (Rock) and Cyclin-dependent Kinase 1 (Cdk1) Inhibits Microtubule Dynamics to Increase Cell Proliferation. J. Biol. Chem. 2013, 288, 7907–7917. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef]
- Duan, X.; Liu, J.; Zhu, C.-C.; Wang, Q.-C.; Cui, X.-S.; Kim, N.-H.; Xiong, B.; Sun, S.-C. RhoA-mediated MLC2 regulates actin dynamics for cytokinesis in meiosis. Cell Cycle 2016, 15, 471–477. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Duan, X.; Zhang, G.-L.; Wang, Z.-B.; Wang, Q.; Xiong, B.; Sun, S.-C. RhoA-mediated FMNL1 regulates GM130 for actin assembly and phosphorylates MAPK for spindle formation in mouse oocyte meiosis. Cell Cycle 2015, 14, 2835–2843. [Google Scholar] [CrossRef]
- Wang, H.; Guo, J.; Lin, Z.; Namgoong, S.; Oh, J.S.; Kim, N.H. Filamin A is required for spindle migration and asymmetric division in mouse oocytes. FASEB J. 2017, 31, 3677–3688. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Q.-C.; Duan, X.; Cui, X.-S.; Kim, N.-H.; Zhang, Y.; Sun, S.-C. Profilin 1 plays feedback role in actin-mediated polar body extrusion in mouse oocytes. Reprod. Fertil. Dev. 2018, 30, 752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Cao, R.; Liu, H.-L.; Cui, X.-S.; Kim, N.-H.; Rui, R.; Sun, S.-C. Small GTPase RhoA regulates cytoskeleton dynamics during porcine oocyte maturation and early embryo development. Cell Cycle 2014, 13, 3390–3403. [Google Scholar] [CrossRef] [PubMed]
- Correia, H.H.V.; Vieira, L.A.; Maside, C.; Paes, V.M.; Silva, R.F.; Alves, B.G.; Santos, F.W.; Apgar, G.A.; Rodrigues, A.P.R.; Figueiredo, J.R. Ovarian transport temperature (4 vs. 33 °C) impacts differently the in vitro development of isolated goat preantral and antral follicles. Small Rum. Res. 2017, 155, 16–23. [Google Scholar] [CrossRef]
- Namgoong, S.; Kim, N.H. Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle 2016, 15, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Uraji, J.; Scheffler, K.; Schuh, M. Functions of actin in mouse oocytes at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Roeles, J.; Tsiavaliaris, G. Actin-microtubule interplay coordinates spindle assembly in human oocytes. Nat. Commun. 2019, 10, 4651. [Google Scholar] [CrossRef]
- Wang, W.-H.; Abeydeera, L.R.; Prather, R.S.; Day, B.N. Polymerization of Nonfilamentous Actin into Microfilaments Is an Important Process for Porcine Oocyte Maturation and Early Embryo Development1. Biol. Reprod. 2000, 62, 1177–1183. [Google Scholar] [CrossRef]
- Lee, S.H.; Dominguez, R. Regulation of actin cytoskeleton dynamics in cells. Mol. Cells 2010, 29, 311–325. [Google Scholar] [CrossRef]
- Sit, S.T.; Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 2011, 124, 679–683. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Yi, K.; Guo, F.; Wang, H.; Wu, P.H.; Yang, J.; Mair, D.B.; Morales, E.A.; Kalab, P.; et al. Dynamic organelle distribution initiates actin-based spindle migration in mouse oocytes. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wei, L. Rho kinase in the regulation of cell death and survival. Arch. Immunol. Ther. Exp. 2007, 55, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, L.; Shan, C.; Cheng, Q.; Budhraja, A.; Zhou, T.; Cui, H.; Gao, N. RhoA/ROCK/PTEN signaling is involved in AT-101-mediated apoptosis in human leukemia cells in vitro and in vivo. Cell Death Dis. 2014, 5, e998. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-S.; Hara, H.; Chung, H.-J.; Hirabayashi, M.; Hochi, S. Rescue of Vitrified-Warmed Bovine Oocytes with Rho-Associated Coiled-Coil Kinase Inhibitor1. Biol. Reprod. 2013, 89. [Google Scholar] [CrossRef] [PubMed]
- Felici, M.D.; Carlo, A.D.; Pesce, M.; Iona, S.; Farrace, M.G.; Piacentini, M. Bcl-2 and Bax regulation of apoptosis in germ cells during prenatal oogenesis in the mouse embryo. Cell Death Differ. 1999, 6, 908–915. [Google Scholar] [CrossRef]
- Craig, R.W. The BCL-2gene family. Semin. Cancer Biol. 1995, 6, 35–43. [Google Scholar] [CrossRef]
- Hockenbery, D.; Nuñez, G.; Milliman, C.; Schreiber, R.D.; Korsmeyer, S.J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 1990, 348, 334–336. [Google Scholar] [CrossRef]
| Gene | Sequence (5′−3′) | Accession No. | Fragment Size (bp) |
|---|---|---|---|
| BLC2 | F: TGGATCCAGGATAACGGAGG | XM_010979993.1 | 92 |
| R: TTCAGAGACAGCCAGGAGAAA | |||
| BAX | F:CACCAAGGTGCCTGAACTGA | XM_010996357.1 | 130 |
| R: CGTGGGTGTCCCAAAGTAGG | |||
| CTSB | F: CAGATGATTGGCAGATGGGC | XM_010995147.1 | 90 |
| R: CTTCGCTGATCCTCGGTCTC | |||
| ACTB | F: ATCTGGCACCACACCTTCT | XM_010997926.1 | 137 |
| R: GGGGTGTTGAAGGTCTCGAA | |||
| TUBA1A | F: GGAGACCTGGCCAAAGTACA | XM_010997479.1 | 95 |
| R: CAGGCTTTTCCAGTGTGACG | |||
| CFL1 | F: ACGCCACCTATGAGACCAAG | XM_010995981.1 | 111 |
| R: CATCCTTGGAGCTGGCATAG | |||
| GAPDH | F: TGCTGAGTACGTTGTGGAGT | XM_010990867 | 134 |
| R: TCACGCCCATCACAAACATG |
| Parameter | Control | 2P | 4P | 6P | 24P |
|---|---|---|---|---|---|
| Number of IVM oocyte | 75 | 75 | 75 | 75 | 75 |
| Degenerated oocyte | 36.0 ± 2.2 ab | 38.6 ±1.6 ab | 17.3 ±2.2 b | 34.7 ± 1.2 ab | 44 ± 0.8 a |
| (%) | (27) | (29) | (13) | (26) | (33) |
| No polar body | 37.33 ± 0.9 a | 35.66 ± 1.6 a | 28.0 ± 2.4 a | 40 ± 0.2 a | 52 ± 0.5 a |
| (%) | (28) | (38) | (21) | (30) | (39) |
| First polar body | 26.6 ± 2.8 b | 25.57± 1.6 b | 54.67± 4.6 a | 25.3 ± 2.0 b | 4 ± 0 b |
| (%) | (20) | (8) | (41) | (19) | (3) |
| Cumulus expansion degree | 1 ± 0 b | 1 ± 0 b | 2 ± 0 a | 1 ± 0 b | 0 ± 0 c |
| No | Oocyte Diameter (µm) | Zona Pellucida Thickness (µm) | Perivitelline Space Length (µm) | Ooplasm Diameter (µm) | |
|---|---|---|---|---|---|
| 2P | 25 | 156.28 ± 2.75 a | 11.40 ± 0.35 b | 6.01 ± 0.30 a | 116.43 ± 1.9 a |
| 4P | 25 | 151.55 ± 1.76 a | 10.37 ± 0.34 bc | 3.19 ± 0.30 b | 116.67 ± 1.2 a |
| 6P | 25 | 138.82 ± 1.11 b | 9.14 ± 0.25 c | 6.55 ± 0.7 a | 103.93 ± 0.7 b |
| Control | 25 | 153.09 ± 1.83 a | 12.97 ± 0.5 a | 2.56 ± 0.41 b | 119.23 ± 2.1 a |
| Parameter | Maturation | Degeneration | Cumulus | Oocyte | Ooplasm | ACTB | BAX | BCL2 | BAX/BCL2 | CFLN Exp. | TUBA Exp. | CTSB Exp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | Expansion | Diameter | Diameter | Exp. | Exp. | Exp. | |||||
| Maturation % | 1.00 | |||||||||||
| Degeneration % | −0.83 | 1.00 | ||||||||||
| Cumulus Expansion | 0.85 | −0.99 | 1.00 | |||||||||
| Oocyte diameter | −0.08 | 0.03 | 0.13 | 1.00 | ||||||||
| Ooplasm diameter | −0.14 | −0.10 | 0.23 | 0.94 | 1.00 | |||||||
| ACTB expression | 0.65 | −0.66 | 0.77 | 0.68 | 0.65 | 1.00 | ||||||
| BAX expression | −0.56 | 0.70 | −0.58 | 0.72 | 0.55 | 0.06 | 1.00 | |||||
| BCL2 expression | 0.01 | 0.03 | 0.13 | 0.98 | 0.87 | 0.72 | 0.73 | 1.00 | ||||
| BAX/BCL2 | −0.59 | 0.71 | −0.58 | 0.72 | 0.56 | 0.05 | 1.00 | 0.73 | 1.00 | |||
| CFLN expression | 0.84 | −0.59 | 0.52 | −0.59 | −0.66 | 0.13 | −0.74 | −0.48 | −0.77 | 1.00 | ||
| TUB expression | −0.47 | 0.77 | −0.65 | 0.56 | 0.31 | −0.03 | 0.96 | 0.61 | 0.95 | −0.55 | 1.00 | |
| CTSB expression | 0.05 | −0.57 | 0.58 | 0.36 | 0.65 | 0.45 | −0.22 | 0.23 | −0.19 | −0.30 | −0.49 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukur, H.A.; Aljumaah, R.S.; Swelum, A.A.-A.; Alowaimer, A.N.; Abdelrahman, M.; Saadeldin, I.M. Effects of Short-Term Inhibition of Rho Kinase on Dromedary Camel Oocyte In Vitro Maturation. Animals 2020, 10, 750. https://doi.org/10.3390/ani10050750
Tukur HA, Aljumaah RS, Swelum AA-A, Alowaimer AN, Abdelrahman M, Saadeldin IM. Effects of Short-Term Inhibition of Rho Kinase on Dromedary Camel Oocyte In Vitro Maturation. Animals. 2020; 10(5):750. https://doi.org/10.3390/ani10050750
Chicago/Turabian StyleTukur, Hammed A., Riyadh S. Aljumaah, Ayman Abdel-Aziz Swelum, Abdullah N. Alowaimer, Mutassim Abdelrahman, and Islam M. Saadeldin. 2020. "Effects of Short-Term Inhibition of Rho Kinase on Dromedary Camel Oocyte In Vitro Maturation" Animals 10, no. 5: 750. https://doi.org/10.3390/ani10050750
APA StyleTukur, H. A., Aljumaah, R. S., Swelum, A. A.-A., Alowaimer, A. N., Abdelrahman, M., & Saadeldin, I. M. (2020). Effects of Short-Term Inhibition of Rho Kinase on Dromedary Camel Oocyte In Vitro Maturation. Animals, 10(5), 750. https://doi.org/10.3390/ani10050750







