Experimental Staphylococcus aureus Mastitis Infection Model by Teat Dipping in Bacterial Culture Suspension in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Bacterial Growth Condition
2.3. Experimental Challenge (Infection)
2.3.1. Clinical Examination of Challenged Cows
2.3.2. Sample Collection
Milk or Mammary Secretion and Blood Sample Collection
Milk or Mammary Secretion Somatic Cell Count (SCC) and Bacterial Count
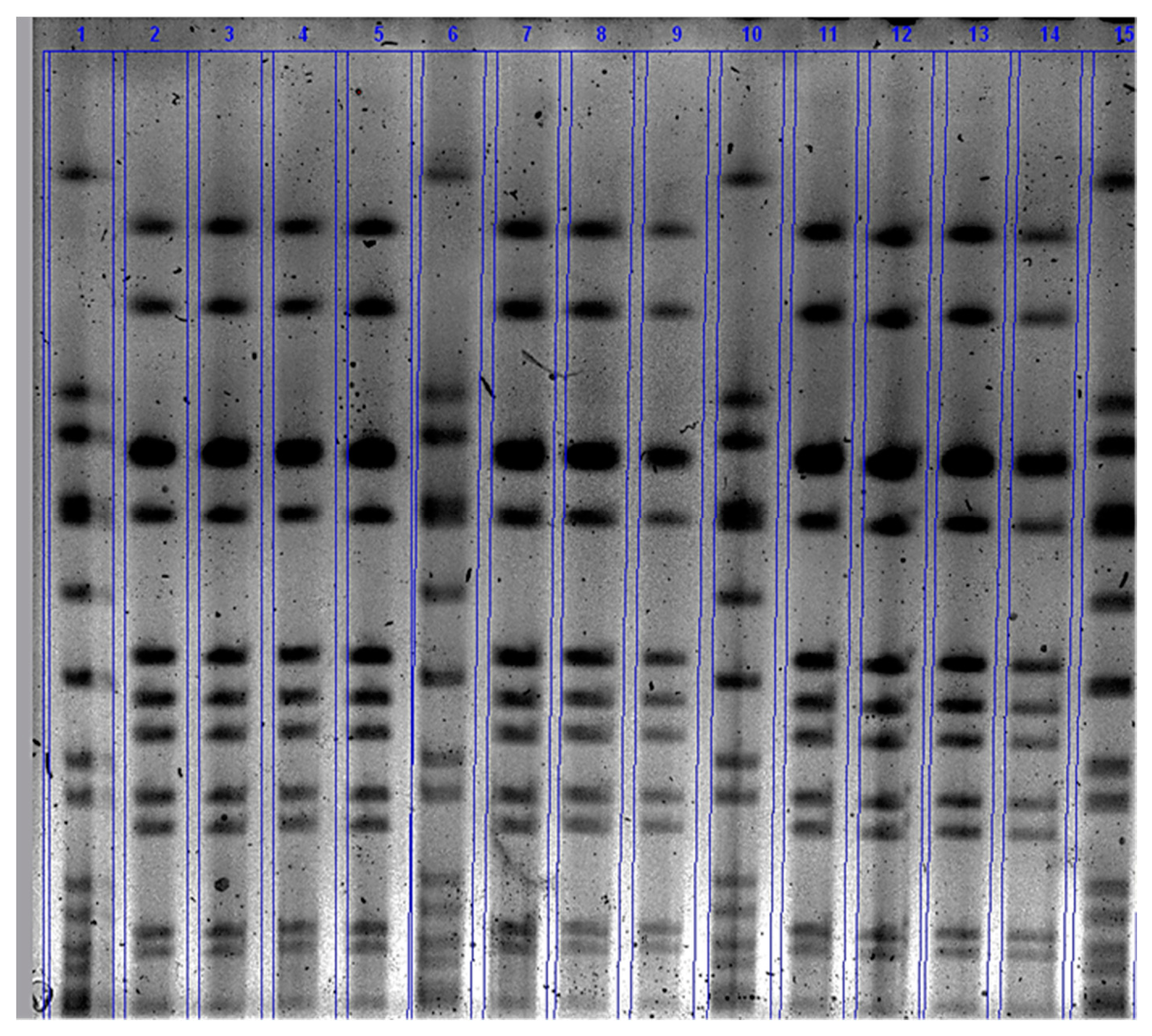
2.3.3. Pulsed-Field Gel Electrophoresis (PFGE)
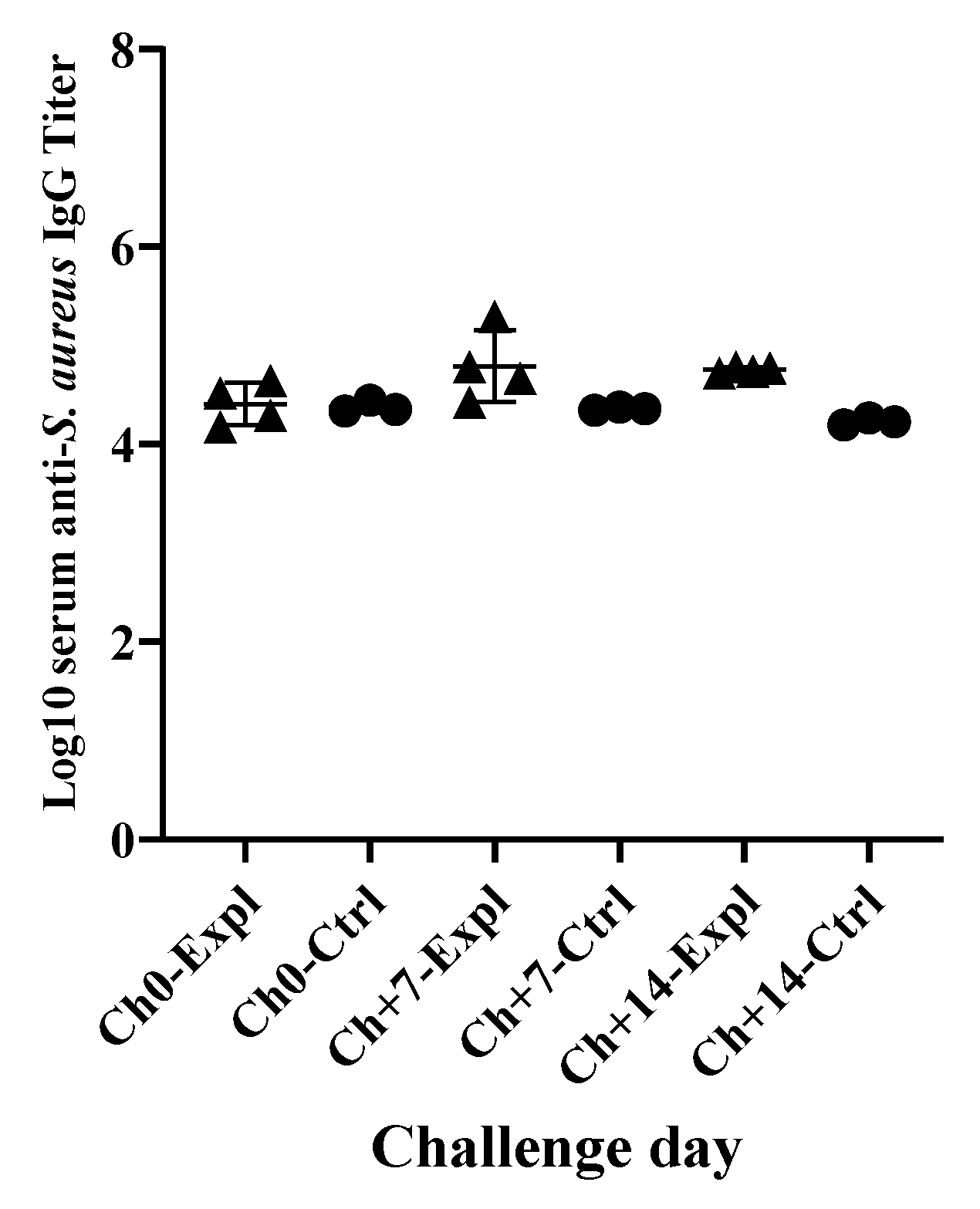
2.3.4. Evaluation of Systemic Humoral Immune Responses Against S. Aureus Infection by ELISA
2.4. Statistical Analysis
3. Results
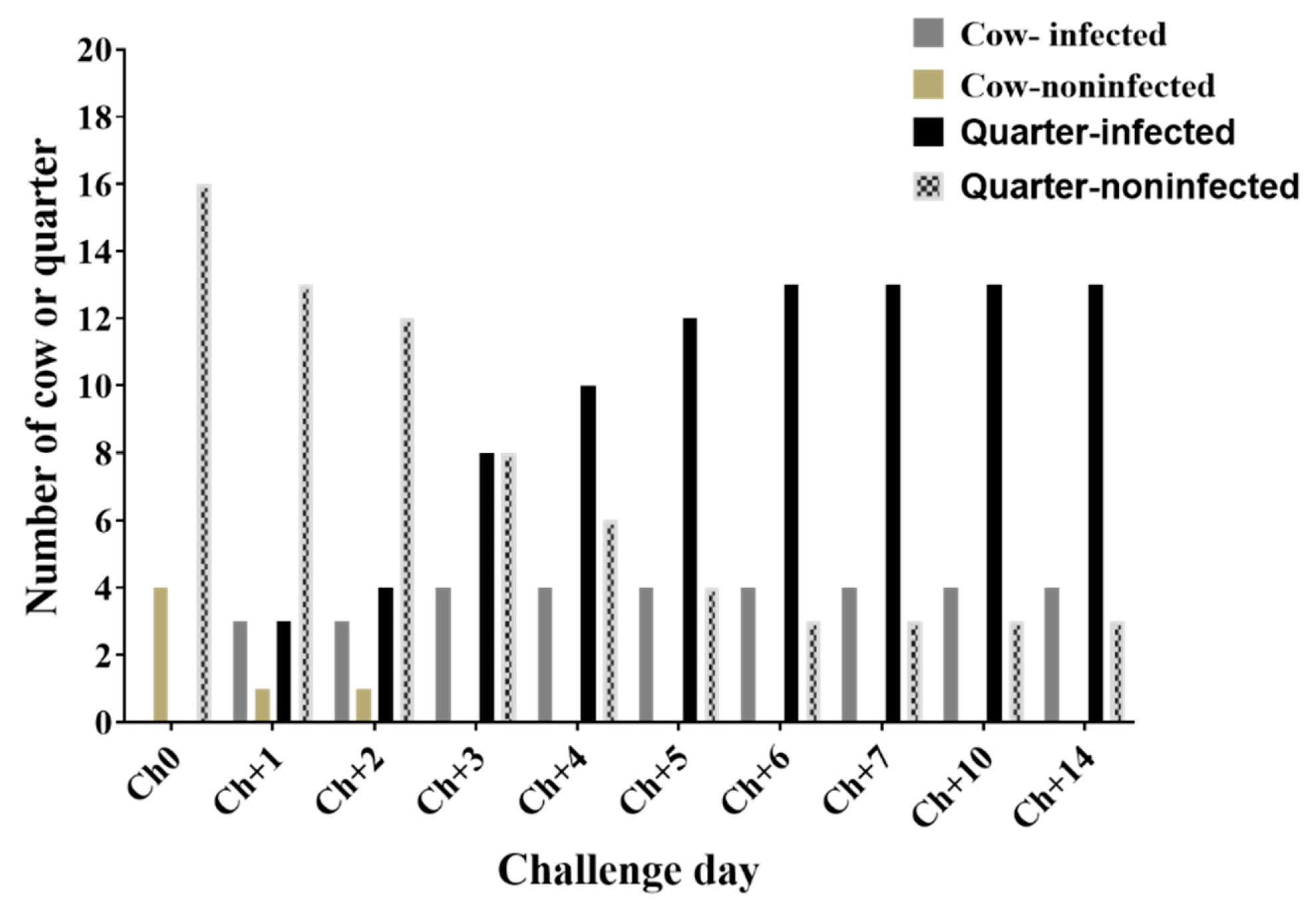
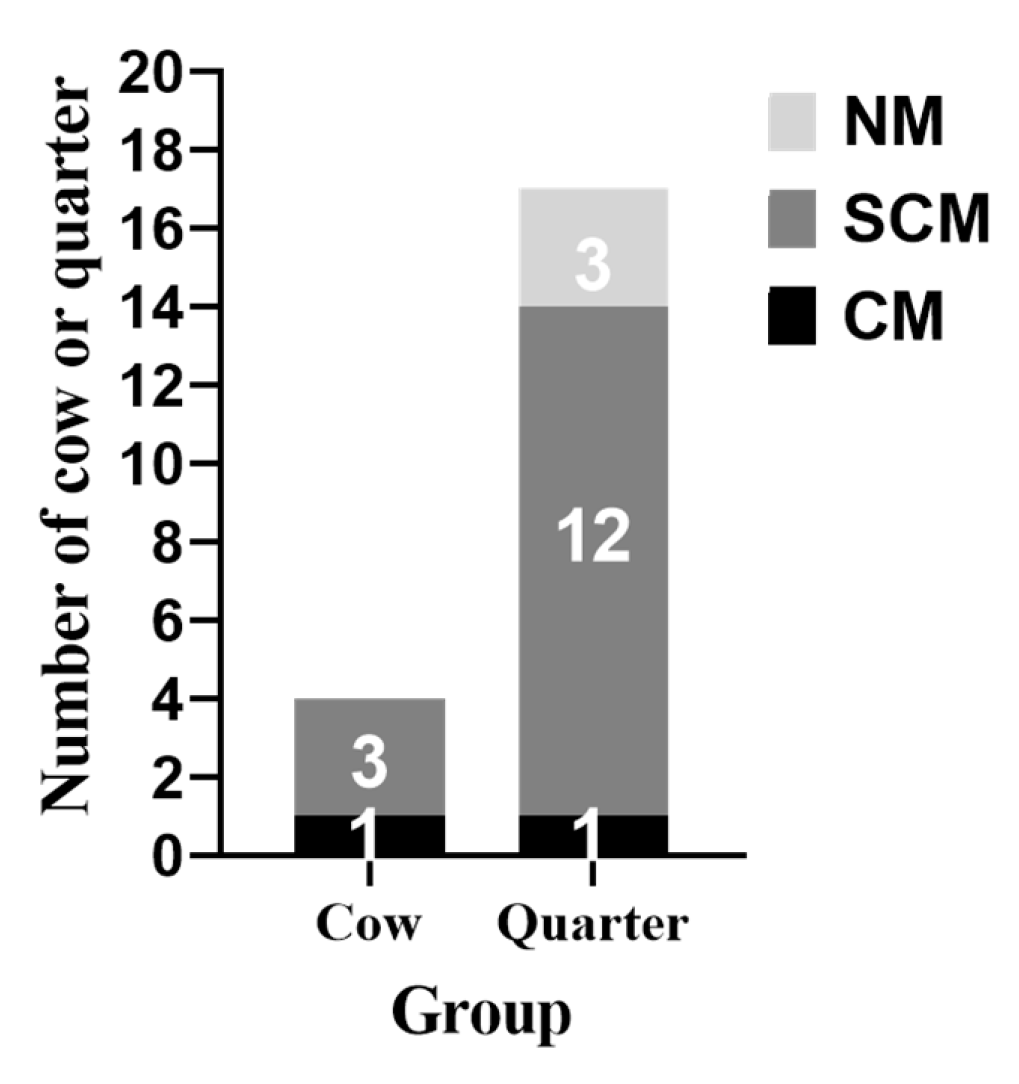
3.1. Clinical Examination of Results during Challenge Study
3.2. Number of S. Aureus Count (CFU/Ml) in the Mammary Secretion
3.3. Systemic Immunological Responses of Dairy Cows against S. Aureus Intramammary Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Group | Cow ID | Ch+0 | Ch+1 | Ch+2 | Ch+3 | Ch+4 | Ch+5 | Ch+6 | Ch+7 | Ch+88 | Ch+9 | Ch+10 | Ch+14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expl | −24 | 38.7 | 38.6 | 38.5 | 38.3 | 38.5 | 38.4 | 38.3 | 38.4 | 38.6 | 38.5 | 38.2 | 38.1 |
| −28 | 38.4 | 38.4 | 38.6 | 38.4 | 38.4 | 38.3 | 38.6 | 38.5 | 38.5 | 37.7 | 38.3 | 38.4 | |
| −98 | 38.5 | 38.2 | 38.1 | 38.4 | 38.5 | 38.3 | 38.8 | 38.4 | 38.8 | 39 | 39 | 39.3 | |
| −94 | 38.5 | 38.2 | 38.6 | 38.6 | 38.6 | 38.6 | 38.3 | 38.4 | 38.7 | 38.4 | 38.3 | 38.6 | |
| Ctrl | −58 | 38.6 | 38.5 | 38.8 | 38.7 | 38.6 | 38.6 | 38.5 | 38.7 | 38.6 | 38.7 | 38.5 | 38.6 |
| −71 | 38.6 | 38.3 | 38.6 | 38.4 | 38.4 | 38.5 | 38.1 | 38.4 | 38.3 | 38.5 | 38.5 | 38.7 | |
| −73 | 38.7 | 38.7 | 38.7 | 38.6 | 38.6 | 38.7 | 38.5 | 38.7 | 38.6 | 38.7 | 38.4 | 38.3 |
Appendix B
| G | CID | Qr. | Ch+0 | Ch+1 | Ch+2 | Ch+3 | Ch+4 | Ch+5 | Ch+6 | Ch+7 | Ch+8 | Ch+9 | Ch+10 | Ch+14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expl | −24 | RF | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 |
| RR | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| LR | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| LF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| −28 | RF | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| RR | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| LR | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| LF | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | ||
| −98 | RF | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | |
| RR | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| LR | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | |||
| LF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | ||
| −94 | RF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | |
| RR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| LR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | ||
| LF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| Ctrl | −58 | RF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| RR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| LR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| LF | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | ||
| −71 | RF | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| RR | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | ||
| LR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| LF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| −73 | RF | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| RR | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | ||
| LR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| LF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
References
- DeGraves, F.J.; Fetrow, J. Economics of mastitis and mastitis control. Vet. Clin. N. Am. Food Anim. Pract. 1993, 9, 421–434. [Google Scholar] [CrossRef]
- Ismail, Z.B. Mastitis vaccines in dairy cows: Recent developments and recommendations of application. Vet. World 2017, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- USDA APHIS. Antibiotic use on U.S. dairy operations, 2002 and 2007 (infosheet, 5p, October, 2008). 2008. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_is_AntibioticUse_1.pdf (accessed on 23 March 2020).
- USDA APHIS. United States Department of Agriculture, Animal Plant Health Inspection Service National Animal Health Monitoring System. Highlights of Dairy 2007 Part III: Reference of dairy cattle health and management practices in the United States, 2007 (Info Sheet 4p, October, 2008). 2008. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_ir_Food_safety.pdf (accessed on 23 March 2020).
- Vanderhaeghen, W.; Piepers, S.; Leroy, F.; Van Coillie, E.; Haesebrouck, F.; De Vliegher, S. Invited review: Effect, persistence, and virulence of coagulase-negative Staphylococcus species associated with ruminant udder health. J. Dairy Sci. 2014, 97, 5275–5293. [Google Scholar] [CrossRef] [PubMed]
- Taponen, S.; Pyorala, S. Coagulase-negative staphylococci as cause of bovine mastitis- not so different from Staphylococcus aureus? Vet Microbiol. 2009, 134, 29–36. [Google Scholar] [CrossRef]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited review: Mastitis in dairy heifers: Nature of the disease, potential impact, prevention, and control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef]
- Nyman, A.K.; Fasth, C.; Waller, K.P. Intramammary infections with different non-aureus staphylococci in dairy cows. J. Dairy Sci. 2018, 101, 1403–1418. [Google Scholar] [CrossRef]
- De Vliegher, S.; Opsomer, G.; Vanrolleghem, A.; Devriese, L.; Sampimon, O.; Sol, J.; Barkema, H.; Haesebrouck, F.; de Kruif, A. In vitro growth inhibition of major mastitis pathogens by Staphylococcus chromogenes originating from teat apices of dairy heifers. Vet. Microbiol. 2004, 101, 215–221. [Google Scholar] [CrossRef]
- Bradley, A.J. Bovine mastitis: An evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar] [CrossRef]
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- Pinedo, P.J.; Fleming, C.; Risco, C.A. Events occurring during the previous lactation, the dry period, and peripartum as risk factors for early lactation mastitis in cows receiving 2 different intramammary dry cow therapies. J. Dairy Sci. 2012, 95, 7015–7026. [Google Scholar] [CrossRef] [PubMed]
- Erskine, R.J.; Eberhart, R.J.; Scholz, R.W. Experimentally induced Staphylococcus aureus mastitis in selenium-deficient and selenium-supplemented dairy cows. Am. J. Vet. Res. 1990, 51, 1107–1111. [Google Scholar] [PubMed]
- Newbould, F.H.S. Evaluation of induced infections as a research method. J. Am. Vet. Med. Assoc. 1977, 170, 1208–1209. [Google Scholar] [PubMed]
- Schukken, Y.H.; Leslie, K.E.; Barnum, D.A.; Mallard, B.A.; Lumsden, J.H.; Dick, P.C.; Vessie, G.H.; Kehrli, M.E. Experimental Staphylococcus aureus intramammary challenge in late lactation dairy cows: Quarter and cow effects determining the probability of infection. J. Dairy Sci. 1999, 82, 2393–2401. [Google Scholar] [CrossRef]
- Enger, B.D.; Crutchfield, C.E.; Yohe, T.T.; Enger, K.M.; Nickerson, S.C.; Parsons, C.L.M.; Akers, R.M. Staphylococcus aureus intramammary challenge in non-lactating mammary glands stimulated to rapidly grow and develop with estradiol and progesterone. Vet. Res. 2018, 49, 47. [Google Scholar] [CrossRef] [PubMed]
- Kerro-Dego, O.; Prysliak, T.; Perez-Casal, J.; Potter, A.A. Role of GapC in the pathogenesis of Staphylococcus aureus. Vet. Microbiol. 2012, 156, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Concha, C.; Holmberg, O.; Morein, B. Proportion of B- and T-lymphocytes in normal bovine milk. J. Dairy Res. 1978, 45, 287–290. [Google Scholar] [CrossRef]
- Ezzat Alnakip, M.; Quintela-Baluja, M.; Bohme, K.; Fernandez-No, I.; Caamano-Antelo, S.; Calo-Mata, P.; Barros-Velazquez, J. The Immunology of Mammary Gland of Dairy Ruminants between Healthy and Inflammatory Conditions. J. Vet. Med. 2014, 2014, 659801. [Google Scholar] [CrossRef]
- Oliver, S.; Gonzalez, R.; Hogan, J.; Jayarao, B.; Owens, W. Microbiological Procedures for the Diagnosis of Bovine udder Infection and Determination of Milk Quality; National Mastitis Council: Verona WI, USA, 2004. [Google Scholar]
- McDougal, L.K.; Steward, C.D.; Killgore, G.E.; Chaitram, J.M.; McAllister, S.K.; Tenover, F.C. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: Establishing a national database. J. Clin. Microbiol. 2003, 41, 5113–5120. [Google Scholar] [CrossRef]
- Abdi, R.D.; Gillespie, B.E.; Vaughn, J.; Merrill, C.; Headrick, S.I.; Ensermu, D.B.; D’Souza, D.H.; Agga, G.E.; Almeida, R.A.; Oliver, S.P.; et al. Antimicrobial Resistance of Staphylococcus aureus Isolates from Dairy Cows and Genetic Diversity of Resistant Isolates. Foodborne Pathog. Dis. 2018, 15, 449–458. [Google Scholar] [CrossRef]
- Kerro Dego, O.; Prysliak, T.; Potter, A.A.; Perez-Casal, J. DNA-protein immunization against the GapB and GapC proteins of a mastitis isolate of Staphylococcus aureus. Vet. Immunol. Immunopathol. 2006, 113, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Boerhout, E.M.; Koets, A.P.; Vernooij, J.C.M.; Mols-Vorstermans, T.G.T.; Nuijten, P.J.M.; Rutten, V.; Bijlsma, J.J.E.; Eisenberg, S.W.F. Reisolation of Staphylococcus aureus from bovine milk following experimental inoculation is influenced by fat percentage and specific immunoglobulin G1 titer in milk. J. Dairy Sci. 2016, 99, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.D.; Paape, M.J.; Chockalingam, A. Staphylococcus aureus intramammary infection elicits increased production of transforming growth factor-alpha, beta1, and beta2. Vet. Immunol. Immunopathol. 2006, 112, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Eckersall, P.D.; Young, F.J.; Nolan, A.M.; Knight, C.H.; McComb, C.; Waterston, M.M.; Hogarth, C.J.; Scott, E.M.; Fitzpatrick, J.L. Acute phase proteins in bovine milk in an experimental model of Staphylococcus aureus subclinical mastitis. J. Dairy Sci. 2006, 89, 1488–1501. [Google Scholar] [CrossRef]
- Petzl, W.; Zerbe, H.; Gunther, J.; Yang, W.; Seyfert, H.M.; Nurnberg, G.; Schuberth, H.J. Escherichia coli, but not Staphylococcus aureus triggers an early increased expression of factors contributing to the innate immune defense in the udder of the cow. Vet. Res. 2008, 39, 18. [Google Scholar] [CrossRef]

| Group | No. of Cows | Challenge Strain | Challenge Dose (CFU/mL) | Challenge Route |
|---|---|---|---|---|
| 1 | 5 | SAUT2 * | 1 × 105 | Teat Dip ** |
| 2 | 3 | None | PBS (Control) | Teat Dip ** |
| Dry Secretion Appearance | Score | Mammary Gland Appearance |
|---|---|---|
| Normal | 0 | Normal; the udder is pliable. Heat, pain, redness, and/or swelling are not detectable. Cow exhibits no signs of discomfort. |
| Flakes | 1 | Slight swelling; the udder is less pliable with some firmness or heavier in weight. Redness, heat, and pain are generally not detectable. |
| Slugs/clots | 2 | Moderate swelling; the udder is firm, heavy, reddened and warm to the touch. The cow generally exhibits signs of discomfort (irritable, performs a stepping motion with feet and/or kicks) during evaluation. |
| Stringy/watery/bloody | 3 | Severe swelling; the udder is very hard, heavy, red and hot, and noticeably larger than other quarters. The cow is extremely uncomfortable, very irritable and manifests pain by kicking and stepping. |
| Sample Type | Sample Collection Time (Day) |
|---|---|
| Milk samples for bacteriological culture and somatic cell count | D−7, D0, C, C+3 |
| Mammary secretion for bacteriological culture and somatic cell count and flow cytometry | Ch0−Ch+7, Ch+10, Ch+14, |
| Blood samples to measure systemic antibody titers against challenge strain | Ch0, Ch+7 and Ch+14 |
| Rectal body temperature | D−7, D0, Ch0–Ch+14 |
| Recoding scores of inflammatory changes in the mammary secretion and mammary gland tissue | Ch0–Ch+7, Ch+10 and Ch+14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerro Dego, O.; Pacha, P.A.; Gillespie, B.E.; Pighetti, G.M. Experimental Staphylococcus aureus Mastitis Infection Model by Teat Dipping in Bacterial Culture Suspension in Dairy Cows. Animals 2020, 10, 751. https://doi.org/10.3390/ani10050751
Kerro Dego O, Pacha PA, Gillespie BE, Pighetti GM. Experimental Staphylococcus aureus Mastitis Infection Model by Teat Dipping in Bacterial Culture Suspension in Dairy Cows. Animals. 2020; 10(5):751. https://doi.org/10.3390/ani10050751
Chicago/Turabian StyleKerro Dego, Oudessa, Paulina A. Pacha, Barbara E. Gillespie, and Gina M. Pighetti. 2020. "Experimental Staphylococcus aureus Mastitis Infection Model by Teat Dipping in Bacterial Culture Suspension in Dairy Cows" Animals 10, no. 5: 751. https://doi.org/10.3390/ani10050751
APA StyleKerro Dego, O., Pacha, P. A., Gillespie, B. E., & Pighetti, G. M. (2020). Experimental Staphylococcus aureus Mastitis Infection Model by Teat Dipping in Bacterial Culture Suspension in Dairy Cows. Animals, 10(5), 751. https://doi.org/10.3390/ani10050751





