Antimicrobial Resistance in Farm Animals in Brazil: An Update Overview
Simple Summary
Abstract
1. Introduction
2. Legal Aspects Related to Animal Antimicrobial Control and Monitoring Programs in Brazil
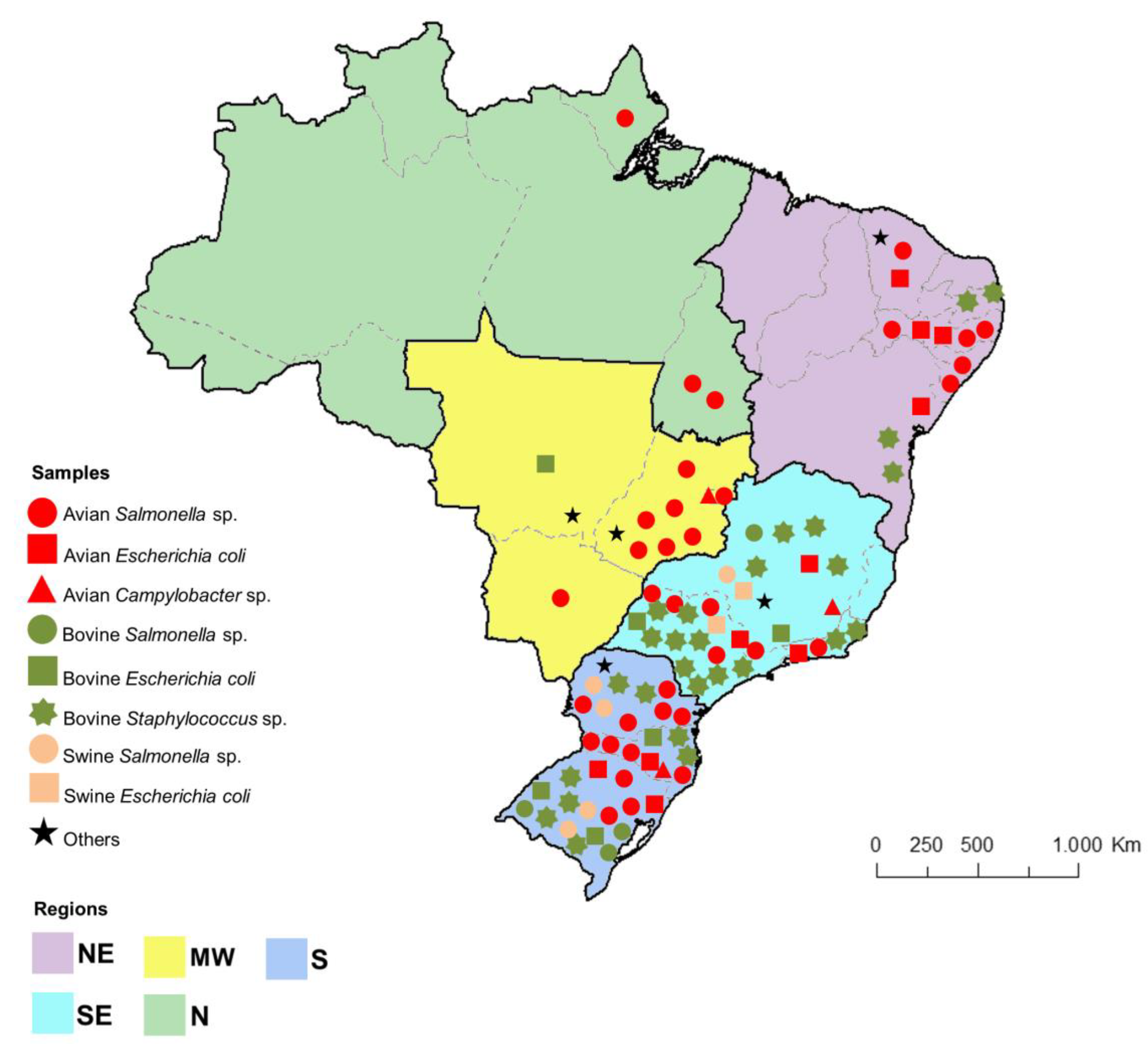
3. Antimicrobial Resistance in Poultry Farming
3.1. Salmonella sp.
3.2. E. coli
3.3. Campylobacter spp.
4. Antimicrobial Resistance in Pig Breeding
4.1. Salmonella spp.
4.2. E. coli
4.3. Yersinia enterocolitica
5. Antimicrobial Resistance in Dairy and Beef Cattle Breeding
5.1. Staphylococcus aureus and other Staphylococcus spp.
5.2. Streptococcus spp.
5.3. E. coli, Salmonella sp., and Listeria monocytogenes
6. Mobile Genetic Elements Associated with Emergent Antimicrobial Resistance Mechanisms Detected in Isolates from Farm Animals and Animal-Derived Foods Produced in Brazil
6.1. β-Lactams Resistance-ESBL and Plasmid-Mediated AmpC (pAmpC)
6.2. β-Lactams Resistance-mecA and Van
6.3. Colistin Resistance-Mcr
6.4. Quinolones Resistance-Qnr
7. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Legislation | Public Agency * | Year | Objective | Reference |
|---|---|---|---|---|
| Circular Letter nº 047/1998 | MAPA | 1998 | Prohibits the use of avoparcin for growth promoter or animal performance enhancer purposes. | [11] |
| Normative Instruction N.º 42 | MAPA | 1999 | Change the National Plan for the Control of Residues in Products of Animal Origin - PNCR and the Programs for the Control of Residues in Meat - PCRC, Honey - PCRM, Milk - PCRL and Fish - PCRP. | [12] |
| Ordinance Nº 31 | MAPA | 2002 | Prohibits the use of arsenicals and antimonial active ingredients in the manufacture of products intended for animal feed, for growth promoters or animal performance improvers. | [13] |
| Normative Instruction N.º 09 | MAPA | 2003 | Prohibits the use of chloramphenicol and nitrofurans, and products containing these active ingredients for veterinary use, and susceptible to feeding to all animals and insects. | [14] |
| Normative Instruction N.º 11 | MAPA | 2004 | Prohibits the manufacture, import, sale, and use of the chemical called olaquindox, as a growth-promoting additive in food-producing animals. | [15] |
| Normative Instruction N.º 35 | MAPA | 2005 | Prohibits the use of feed products containing the chemical called carbadox. | [16] |
| Normative Instruction Nº 26 | MAPA | 2009 | Approves the technical regulation for the manufacture, quality control, marketing and employment of veterinary antimicrobial products, and determines that amphenicols, tetracyclines, beta-lactams (systemic benzyl penicillamines and cephalosporins), quinolones, and systemic sulfonamides are for use exclusively in veterinary antimicrobial products, and are prohibited for use as performance-enhancing zootechnical additives, or as food preservatives. | [17] |
| Ordinance N.º 396 | MAPA | 2009 | Establishes responsibilities of the units of the Secretariat of Agricultural Defense (SDA) involved in the PNCRC/MAPA research subprogram. | [183] |
| Normative Instruction Nº 14 | MAPA | 2012 | Prohibits the import, manufacture, and use of antimicrobial substances spiramycin and erythromycin throughout the national territory for zootechnical additive to improve performance in animal feed. | [18] |
| ANVISA—Resolution Nº 53 - Internalize the Resolution Mercosul N.º 54/2000 | MS | 2012 | Approves the maximum residue levels of veterinary medicines in animal food. | [184] |
| Codex Alimentarius—N.º 02/2015 | FAO-OMS/ANVISA | 2015 | Updates maximum residue limits for veterinary food products. | [185] |
| Normative Instruction Nº 45 | MAPA | 2016 | Prohibits, throughout the national territory, the import and the manufacture of the antimicrobial substance colistin sulfate, with the purpose of a performance-enhancing feed animal additive. | [19] |
| Normative Instruction Nº 54 | MAPA | 2018 | Approves the technical regulation for the registration of performance-enhancing antimicrobial additives and anticoccidial feed additives. | [186] |
| ANVISANormative Instruction Nº 51 | MS | 2019 | Establishes the list of maximum residue limits (LMR), acceptable daily intake (IDA) and acute reference dose (DRfA) for active pharmaceutical ingredients (IFA) of veterinary drugs in foods of animal origin. | [187] |
| Normative Instruction No 1 | MAPA | 2020 | Prohibits, throughout the national territory, the importation, manufacture, sale, and use of performance-enhancing additives containing the antimicrobial agents tylosin, lincomycin, and tiamulin, classified as important in human medicine. | [20] |
References
- Alexandratos, N.; Bruinsma, J. World Agriculture Towards 2030/2050: The 2012 Revision; ESA Working Paper No. 12-03; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2012. [Google Scholar]
- Tomley, F.M.; Shirley, M.W. Livestock infectious diseases and zoonoses. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; et al. Comparison of different approaches to antibiotic restriction in food-producing animals: Stratified results from a systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001710. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (OIE). Annual Report on Antimicrobial Agents Intended for Use in Animals: Better Understand the Global Situation; Third Report; World Organization for Animal Health (OIE): Paris, France, 2018; p. 131. [Google Scholar]
- Word Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/ (accessed on 19 December 2019).
- World Organization for Animal Health (OIE). The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. 2016. Available online: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf (accessed on 19 December 2019).
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). Timeline of FDA Action on Antimicrobial Resistance. Available online: https://www.fda.gov/animal-veterinary/antimicrobial-resistance/timeline-fda-action-antimicrobial-resistance (accessed on 19 December 2019).
- Antimicrobial Resistance and Animals—Actions. Available online: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/actions.html (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Substâncias Proibidas na Alimentação Animal Com a Finalidade de Aditivo e Legislação Correspondente. Available online: http://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-pecuarios/arquivos-de-insumos-pecuarios/PortalMAPASubstanciasproibidasmaio2012.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa SDA No 42, de 20 de Dezembro de 1999. Available online: https://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/instrucao-normativa-sda-n-o-42-de-20-de-dezembro-de-1999.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Portaria no 31, de 29 de Janeiro de 2002. Available online: https://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/portaria-no-31-de-29-de-janeiro-de-2002.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa SDA No 09, de 27 de Junho de 2003. Available online: https://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/instrucao-normativa-sda-n-o-09-de-27-de-junho-de-2003.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa SDA No 11, de 24 de Novembro de 2004. Available online: https://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/instrucao-normativa-sda-n-o-11-de-24-de-novembro-de-2004.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa SDA No 35, de 14 de Novembro de 2005. Available online: https://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/instrucao-normativa-sda-n-o-35-de-14-de-novembro-de-2005.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa No 26, de 9 de Julho de 2009. Available online: https://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/instrucao-normativa-no-26-de-9-de-julho-de-2009.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa No 14, de 17 de Maio de 2012. Available online: https://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/instrucao-normativa-no-14-de-17-de-maio-de-2012.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa No 45, de 22 de Novembro de 2016. Available online: https://www.agricultura.gov.br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/instrucao-normativa-no-45-de-22-de-novembro-de-2016.pdf/view (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa No 01, de 13 de Janeiro de 2020. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-n-1-de-13-de-janeiro-de-2020-239402385 (accessed on 27 January 2010).
- World Health Organization (WHO). Critically Important Antimicrobials for Human Medicine, 5th Revision. Available online: https://www.who.int/foodsafety/publications/antimicrobials-fifth/en/ (accessed on 19 December 2019).
- Brazil Agência Nacional de Vigilância Sanitária (ANVISA). Limites Máximos de Resíduos de Medicamentos Veterinários em Alimentos de Origem Animal: Documento de Base para Discussão Regulatória; ANVISA: Brasília, Brazil, 2018; p. 136. [Google Scholar]
- Brazil Agência Nacional de Vigilância Sanitária (ANVISA). Programa de Análise de Resíduos de Medicamentos Veterinários em Alimentos de Origem Animal. Available online: https://portal.anvisa.gov.br/documents/33916/395364/PAMVet-+Monitoramento+de+Res%C3%ADduos+em+Leite+Exposto+ao+Consumo+-+Relatório+2006-2007/4777c371-e5b5-42e0-9c3f-43670009a802 (accessed on 19 December 2019).
- Brazil Ministério da Agricultura, Pecuária e Abastecimento (MAPA). Instrução Normativa No 5, de 23 de Abril de 2019. Available online: https://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/InstruoNormativaN05.2019PNCRC2019.pdf/view (accessed on 19 December 2019).
- Brazil Agência Nacional de Vigilância Sanitária (ANVISA). Relatório do Monitoramento da Prevalência e do Perfil de Suscetibilidade Aos Antimicrobianos em Enterococos e Salmonelas Isolados de Carcaças de Frango Congeladas Comercializadas No Brasil. Available online: http://portal.anvisa.gov.br/documents/33916/395481/Relatório+Prebaf+-+Programa+Nacional+de+Monitoramento+da+Prevalência+e+da+Resistência+Bacteriana+em+Frango/f6bb5296-e633-4f7b-b81f-48a99430da6a?version=1.1&download=true (accessed on 19 December 2019).
- Food and Agriculture Organization (FAO). Monitoring Global Progress on Addressing Antimicrobial Resistance: Analysis Report of the Second Round of Results of AMR Country Self-Assessment Survey 2018; Food and Agriculture Organization (FAO): Rome, Italy; World Organisation for Animal Health (OIE): Paris, France; World Health Organization (WHO): Geneva, Switzerland, 2018. [Google Scholar]
- Wall, B.A.; Mateus, A.; Marshall, L.; Pfeiffer, D.; Lubroth, J.; Ormel, H.J.; Otto, P.; Patriarchi, A.; Food and Agriculture Organization of the United Nations (FAO). Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; Food and Agriculture Organization (FAO): Rome, Italy, 2016; ISBN 978-92-5-109441-9. [Google Scholar]
- Associação Brasileira de Proteína animal (ABPA). Relatório Anual. 2018. Available online: https://abpa-br.com.br/storage/files/relatorio-anual-2018.pdf (accessed on 19 December 2019).
- Minafra e Rezende, C.S.; de Mesquita, A.J.; Andrade, A.M.; Linhares, G.F.C.; de Mesquita, A.Q.; Minafra, C.S. Serovars of Salmonella isolated from carcasses of broilers slaughtered in the State of Goiás, Brazil, and their resistance profiles to antibioctics. Rev. Port. Cien. Vet. 2005, 100, 199–203. [Google Scholar]
- Dias de Oliveira, S.; Siqueira Flores, F.; dos Santos, L.R.; Brandelli, A. Antimicrobial resistance in Salmonella enteritidis strains isolated from broiler carcasses, food, human and poultry-related samples. Int. J. Food Microbiol. 2005, 97, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.O.; Ribeiro, A.R.; dos Santos, L.R.; Pilotto, F.; de Moraes, H.L.S.; Salle, C.T.P.; da Silveira Rocha, S.L.; Nascimento, V.P. Do antibiotic resistance in Salmonella enteritidis isolated from broiler carcasses. Braz. J. Microbiol. 2006, 37, 368–371. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Kellermann, A. Resistência antimicrobiana em Salmonella enterica subsp. enterica sorovar Hadar isoladas de carcaças de frango. Arq. Inst. Biol. 2006, 4, 357–360. [Google Scholar]
- Ribeiro, A.R.; Kellermann, A.; Santos, L.R.; Nascimento, V.P. Resistência antimicrobiana em Salmonella enteritidis isoladas de amostras clínicas e ambientais de frangos de corte e matrizes pesadas. Arq. Bras. Med. Vet. Zootech. 2008, 60, 1259–1262. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Cardoso, W.M.; Salles, R.P.R.; Romão, J.M.; Teixeira, R.S.C.; Câmara, S.R.; Siqueira, A.A.; Marques, L.C.L. Initial identification and sensitivity to antimicrobial agents of Salmonella sp. isolated from poultry products in the state of Ceara, Brazil. Braz. J. Poult. Sci. 2006, 8, 193–199. [Google Scholar] [CrossRef]
- Duarte, D.A.M.; Ribeiro, A.R.; Vasconcelos, A.M.M.; Santos, S.B.; Silva, J.V.D.; de Andrade, P.L.A.; da Costa de Arruda Falcão, L.S.P. Occurrence of Salmonella spp. in broiler chicken carcasses and their susceptibility to antimicrobial agents. Braz. J. Microbiol. 2009, 40, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Penha Filho, R.A.C.; Ferreira, J.C.; Kanashiro, A.M.I.; Berchieri, A., Jr.; Darini, A.L.C. Emergent multidrug-resistant nontyphoidal Salmonella serovars isolated from poultry in Brazil coharboring blaCTX-M-2 and qnrB or blaCMY-2 in large plasmids. Diagn. Microbiol. Infect. Dis. 2019, 95, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.S.L.; Streck, A.F.; Michael, G.B.; Marks, F.S.; Rodrigues, D.P.; dos Reis, E.M.F.; Cardoso, M.R.; Canal, C.W. Antimicrobial resistance and subtyping of Salmonella enterica subspecies enterica serovar Enteritidis isolated from human outbreaks and poultry in southern Brazil. Poult. Sci. 2010, 89, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.A.N.; de Oliveira, D.C.N.; Rodrigues, D.P.; de Freitas, D.R.C. Prevalence and antimicrobial resistance of Salmonella in chicken carcasses at retail in 15 Brazilian cities. Rev. Panam. Salud Publica Pan Am. J. Public Health 2011, 30, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Kottwitz, L.B.M.; Scheffer, M.C.; Costa, L.M.D.; Leitao, J.A.; Back, A.; Rodrigues, D.P.; Magnani, M.; de Oliveira, T.C.R.M. Perfil de resistência a antimicrobianos, fagotipagem e caracterização molecular de cepas de Salmonella enteritidis de origem avícola. Semin. Ciênc. Agrár. 2012, 33, 705–712. [Google Scholar] [CrossRef][Green Version]
- Kottwitz, L.B.M.; Leão, J.A.; Back, A.; Rodrigues, D.P.; Magnani, M.; de Oliveira, T.C.R.M. Commercially laid eggs vs. discarded hatching eggs: Contamination by Salmonella spp. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2013, 44, 367–370. [Google Scholar] [CrossRef][Green Version]
- Campioni, F.; Zoldan, M.M.; Falcão, J.P. Characterization of Salmonella enteritidis strains isolated from poultry and farm environments in Brazil. Epidemiol. Infect. 2014, 142, 1403–1410. [Google Scholar] [CrossRef]
- Pandini, J.A.; da Pinto, F.G.S.; Muller, J.M.; Weber, L.D.; de Moura, A.C. Ocorrência e perfil de resistencia antimicrobiana de sorotipos de Salmonella spp. isolados de aviários do Paraná, Brasil. Arq. Inst. Biol. 2015, 82, 00200. [Google Scholar] [CrossRef]
- Palmeira, A.; dos Santos, L.R.; Borsoi, A.; Rodrigues, L.B.; Calasans, M.; do Nascimento, V.P. Serovars and antimicrobial resistance of Salmonella spp. isolated from turkey and broiler carcasses in southern Brazil between 2004 and 2006. Rev. Inst. Med. Trop. São Paulo 2016, 58, 19. [Google Scholar] [CrossRef][Green Version]
- Borges, K.A.; Furian, T.Q.; de Souza, S.N.; Menezes, R.; Salle, C.T.P.; de Souza Moraes, H.L.; Tondo, E.C.; do Nascimento, V.P. Phenotypic and molecular characterization of Salmonella enteritidis SE86 isolated from poultry and Salmonellosis outbreaks. Foodborne Pathog. Dis. 2017, 14, 742–754. [Google Scholar] [CrossRef]
- Koerich, P.K.V.; Fonseca, B.B.; Balestrin, E.; Tagliari, V.; Hoepers, P.G.; Ueira-Vieira, C.; Oldoni, I.; Rauber, R.H.; Ruschel, L.; Nascimento, V.P. Salmonella gallinarum field isolates and its relationship to vaccine strain SG9R. Br. Poult. Sci. 2018, 59, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Jajere, S.M. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 2019, 12, 504–521. [Google Scholar] [CrossRef] [PubMed]
- Baptista, D.Q.; Santos, A.F.M.; Aquino, M.H.C.; Abreu, D.L.C.; Rodrigues, D.P.; Nascimento, E.R.; Pereira, V.L.A. Prevalência e susceptibilidade antimicrobiana de sorotipos de Salmonella spp. isolados de frangos vivos e carcaças no estado do Rio de Janeiro. Pesqui. Vet. Bras. 2018, 38, 1278–1285. [Google Scholar] [CrossRef]
- Public Health Agency of Canadá. Salmonella Heidelberg—Ceftiofur-Related Resistance in Human and Retail Chicken Isolates. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/notice-stakeholders-collaborative-efforts-promote-judicious-use-medically-important-antimicrobial-drugs-food-animal-production.html (accessed on 6 February 2020).
- Webber, B.; Borges, K.A.; Furian, T.Q.; Rizzo, N.N.; Tondo, E.C.; dos Santos, L.R.; Rodrigues, L.B.; do Nascimento, V.P.; Webber, B.; Borges, K.A.; et al. Detection of virulence genes in Salmonella Heidelberg isolated from chicken carcasses. Rev. Inst. Med. Trop. São Paulo 2019, 61, e36. [Google Scholar] [CrossRef]
- Voss-Rech, D.; Vaz, C.S.L.; Alves, L.; Coldebella, A.; Leão, J.A.; Rodrigues, D.P.; Back, A. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poult. Sci. 2015, 94, 433–441. [Google Scholar] [CrossRef]
- Voss-Rech, D.; Kramer, B.; Silva, V.S.; Rebelatto, R.; Abreu, P.G.; Coldebella, A.; Vaz, C.S.L. Longitudinal study reveals persistent environmental Salmonella Heidelberg in Brazilian broiler farms. Vet. Microbiol. 2019, 233, 118–123. [Google Scholar] [CrossRef]
- Costa, R.G.; Festivo, M.L.; Araujo, M.S.; Reis, E.M.F.; Lázaro, N.S.; Rodrigues, D.P. Antimicrobial susceptibility and serovars of salmonella circulating in commercial poultry carcasses and poultry products in Brazil. J. Food Prot. 2013, 76, 2011–2017. [Google Scholar] [CrossRef]
- Voss-Rech, D.; Potter, L.; Vaz, C.S.L.; Pereira, D.I.B.; Sangioni, L.A.; Vargas, Á.C.; de Avila Botton, S. Antimicrobial resistance in nontyphoidal Salmonella isolated from human and poultry-related samples in Brazil: 20-Year meta-analysis. Foodborne Pathog. Dis. 2017, 14, 116–124. [Google Scholar] [CrossRef]
- da Cunha-Neto, A.; Carvalho, L.A.; Carvalho, R.C.T.; dos Prazeres Rodrigues, D.; Mano, S.B.; de Figueiredo, E.E.S.; Conte, C.A., Jr. Salmonella isolated from chicken carcasses from a slaughterhouse in the state of Mato Grosso, Brazil: Antibiotic resistance profile, serotyping, and characterization by repetitive sequence-based PCR system. Poult. Sci. 2018, 97, 1373–1381. [Google Scholar] [CrossRef]
- Perin, A.P.; Martins, B.T.F.; Barreiros, M.A.B.; Yamatogi, R.S.; Nero, L.A.; Dos Santos Bersot, L. Occurrence, quantification, pulse types, and antimicrobial susceptibility of Salmonella sp. isolated from chicken meat in the state of Paraná, Brazil. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2020, 51, 335–345. [Google Scholar] [CrossRef]
- Borges, K.A.; Furian, T.Q.; Souza, S.N.; Salle, C.T.P.; Moraes, H.L.S.; Nascimento, V.P.; Borges, K.A.; Furian, T.Q.; Souza, S.N.; Salle, C.T.P.; et al. Antimicrobial resistance and molecular characterization of Salmonella enterica serotypes isolated from poultry sources in Brazil. Braz. J. Poult. Sci. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Monte, D.F.; Lincopan, N.; Berman, H.; Cerdeira, L.; Keelara, S.; Thakur, S.; Fedorka-Cray, P.J.; Landgraf, M. Genomic features of high-priority Salmonella enterica serovars circulating in the food production chain, Brazil, 2000–2016. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gazal, L.E.S.; Puño-Sarmiento, J.J.; Medeiros, L.P.; Cyoia, P.S.; da Silveira, W.D.; Kobayashi, R.K.T.; Nakazato, G. Presence of pathogenicity islands and virulence genes of extraintestinal pathogenic Escherichia coli (ExPEC) in isolates from avian organic fertilizer. Poult. Sci. 2015, 94, 3025–3033. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, W.G.A.; da Silva, I.N.G.; Vasconcelos, R.H.; Machado, D.N.; Lopes, E.D.S.; Lima, S.V.G.; de Teixeira, R.S.C.; Lima, J.B.; Oliveira, F.R.; Maciel, W.C. Isolation and antimicrobial resistance of Escherichia coli and Salmonella enterica subsp. enterica (O:6,8) in broiler chickens. Acta Sci. Vet. 2016, 44, 7. [Google Scholar]
- Maciel, J.F.; Matter, L.B.; Trindade, M.M.; Camillo, G.; Lovato, M.; de Ávila Botton, S.; Castagna de Vargas, A. Virulence factors and antimicrobial susceptibility profile of extraintestinal Escherichia coli isolated from an avian colisepticemia outbreak. Microb. Pathog. 2017, 103, 119–122. [Google Scholar] [CrossRef]
- Stella, A.E.; Oliveira, M.C.D.; da Fontana, V.L.D.S.; Maluta, R.P.; Borges, C.A.; de Ávila, F.A. Characterization and antimicrobial resistance patterns of Escherichia coli isolated from feces of healthy broiler chickens. Arq. Inst. Biol. 2016, 83, e0392014. [Google Scholar] [CrossRef]
- Borzi, M.M.; Cardozo, M.V.; de Oliveira, E.S.; de Pollo, A.S.; Guastalli, E.A.L.; dos Santos, L.F.; de Ávila, F.A. Characterization of avian pathogenic Escherichia coli isolated from free-range helmeted guineafowl. Braz. J. Microbiol. 2018, 49, 107–112. [Google Scholar] [CrossRef]
- Lima-Filho, J.V.; Martins, L.V.; de Nascimento, D.C.O.; Ventura, R.F.; Batista, J.E.C.; Silva, A.F.B.; Ralph, M.T.; Vaz, R.V.; Rabello, C.B.-V.; de Matos Mendes da Silva, I.; et al. Zoonotic potential of multidrug-resistant extraintestinal pathogenic Escherichia coli obtained from healthy poultry carcasses in Salvador, Brazil. Braz. J. Infect. Dis. 2013, 17, 54–61. [Google Scholar] [CrossRef]
- Ferro, I.D.; Benetti, T.M.; Oliveira, T.C.R.M.; Abrahão, W.M.; Farah, S.M.S.S.; Luciano, F.B.; Macedo, R.E.F. Evaluation of antimicrobial resistance of Campylobacter spp. isolated from broiler carcasses. Br. Poult. Sci. 2015, 56, 66–71. [Google Scholar] [CrossRef]
- Braga, J.F.V.; Chanteloup, N.K.; Trotereau, A.; Baucheron, S.; Guabiraba, R.; Ecco, R.; Schouler, C. Diversity of Escherichia coli strains involved in vertebral osteomyelitis and arthritis in broilers in Brazil. BMC Vet. Res. 2016, 12, 140. [Google Scholar] [CrossRef]
- Carvalho, D.; Finkler, F.; Grassotti, T.T.; Kunert Filho, H.C.; de Lima, F.E.S.; Soares, B.D.; Rossato, J.M.; da Cunha, A.C.; de Brito, K.C.T.; de Brito, B.G. Antimicrobial susceptibility and pathogenicity of Escherichia coli strains of environmental origin. Ciênc. Rural 2015, 45, 1249–1255. [Google Scholar] [CrossRef]
- Vaz, R.V.; Gouveia, G.V.; Andrade, N.M.J.; da Costa, M.M.; Lima-Filho, J.V. Phylogenetic characterization of serum plus antibiotic-resistant extraintestinal Escherichia coli obtained from the liver of poultry carcasses in Pernambuco. Pesqui. Vet. Bras. 2017, 37, 1069–1073. [Google Scholar] [CrossRef][Green Version]
- Barros, M.R. Resistência antimicrobiana e perfil plasmidial de Escherichia coli isolada de frangos de corte e poedeiras comerciais no estado de pernambuco. Pesq. Vet. Bras. 2012, 6, 405–410. [Google Scholar] [CrossRef]
- Koga, V.L.; Scandorieiro, S.; Vespero, E.C.; Oba, A.; de Brito, B.G.; de Brito, K.C.T.; Nakazato, G.; Kobayashi, R.K.T. Comparison of antibiotic resistance and virulence factors among Escherichia coli isolated from conventional and free-range poultry. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Moura Oliveira, K.A.; Mendonca, R.C.S.; De Oliveira, G.V.; Sodre, A.F. Antibiotic resistance of Campylobacter isolated from automated broiler farms. J. Food Saf. 2006, 26, 82–91. [Google Scholar] [CrossRef]
- Kuana, S.L.; dos Santos, L.R.; Rodrigues, L.B.; Borsoi, A.; do Moraes, H.L.S.; Salle, C.T.P.; do Nascimento, V.P. Antimicrobial resistance in Campylobacter spp isolated from broiler flocks. Braz. J. Microbiol. 2008, 39, 738–740. [Google Scholar] [CrossRef]
- Ku, B.K.; Kim, H.J.; Lee, Y.J.; Kim, Y.I.; Choi, J.S.; Park, M.Y.; Kwon, J.W.; Nam, H.-M.; Kim, Y.H.; Jung, S.-C.; et al. Genetic characterization and antimicrobial susceptibility of Campylobacter spp. isolated from domestic and imported chicken meats and humans in Korea. Foodborne Pathog. Dis. 2011, 8, 381–386. [Google Scholar] [CrossRef]
- Melo, R.T.; Grazziotin, A.L.; Júnior, E.C.V.; Prado, R.R.; Mendonça, E.P.; Monteiro, G.P.; Peres, P.A.B.M.; Rossi, D.A. Evolution of Campylobacter jejuni of poultry origin in Brazil. Food Microbiol. 2019, 82, 489–496. [Google Scholar] [CrossRef]
- Moraes, D.M.C.; Andrade, M.A.; Minafra-Rezende, C.S.; de Barnabé, A.C.S.; de Jayme, V.S.; Nunes, I.A.; de Batista, D.A. Fontes de infecção e perfil de suscetibilidade aos antimicrobianos de Salmonella sp. isoladas no fluxo de produção de frangos de corte. Arq. Inst. Biol. 2014, 81, 195–201. [Google Scholar] [CrossRef]
- Minharro, S.; Nascimento, C.A.; Galletti, J.P.; Merisse, T.J.; Feitosa, A.C.F.; Santos, H.D.; Dias, F.E.F.; Santana, E.S.; Baldani, C.D.; Andrade, M.A. Antimicrobial susceptibility of Salmonella serovars isolated from edible offal and carcasses of slaughtered poultry in the state of Tocantins, Brazil. Semina Ciênc. Agrár. 2015, 36, 2661. [Google Scholar] [CrossRef][Green Version]
- de Moura, H.M.; Silva, P.R.; da Silva, P.H.C.; Souza, N.R.; Racanicci, A.M.C.; Santana, Â.P. Antimicrobial resistance of Campylobacter jejuni isolated from chicken carcasses in the federal district, Brazil. J. Food Prot. 2013, 76, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Brazil Ministério da Súde (MS). Surtos de Doenças Transmitidas por Alimentos no Brasil. 2018. Available online: https://portalarquivos2.saude.gov.br/images/pdf/2018/janeiro/17/Apresentacao-Surtos-DTA-2018.pdf (accessed on 19 December 2019).
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 5500. [Google Scholar]
- Arguello, H.; Álvarez-Ordoñez, A.; Carvajal, A.; Rubio, P.; Prieto, M. Role of slaughtering in Salmonella spreading and control in pork production. J. Food Prot. 2013, 76, 899–911. [Google Scholar] [CrossRef]
- Lopes, G.V.; Pissetti, C.; da Cruz Payão Pellegrini, D.; da Silva, L.E.; Cardoso, M. Resistance phenotypes and genotypes of Salmonella enterica subsp. enterica isolates from feed, pigs, and carcasses in Brazil. J. Food Prot. 2015, 78, 407–413. [Google Scholar]
- Kich, J.D.; Coldebella, A.; Morés, N.; Nogueira, M.G.; Cardoso, M.; Fratamico, P.M.; Call, J.E.; Fedorka-Cray, P.; Luchansky, J.B. Prevalence, distribution, and molecular characterization of Salmonella recovered from swine finishing herds and a slaughter facility in Santa Catarina, Brazil. Int. J. Food Microbiol. 2011, 151, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Advice to Clinicians—Multistate Outbreak of Salmonella Infections Linked to Raw Chicken Products, October 2018. Available online: https://www.cdc.gov/salmonella/infantis-10-18/advice.html (accessed on 21 December 2019).
- Almeida, F.; Medeiros, M.I.C.; Kich, J.D.; Falcão, J.P. Virulence-Associated genes, antimicrobial resistance and molecular typing of Salmonella typhimurium strains isolated from swine from 2000 to 2012 in Brazil. J. Appl. Microbiol. 2016, 120, 1677–1690. [Google Scholar] [CrossRef]
- Souto, M.S.M.; Coura, F.M.; Dorneles, E.M.S.; Stynen, A.P.R.; Alves, T.M.; Santana, J.A.; Pauletti, R.B.; Guedes, R.M.C.; Viott, A.M.; Heinemann, M.B.; et al. Antimicrobial susceptibility and phylotyping profile of pathogenic Escherichia coli and Salmonella enterica isolates from calves and pigs in Minas Gerais, Brazil. Trop. Anim. Health Prod. 2017, 49, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Viana, C.; Sereno, M.J.; Pegoraro, K.; Yamatogi, R.S.; Call, D.R.; dos Santos Bersot, L.; Nero, L.A. Distribution, diversity, virulence genotypes and antibiotic resistance for Salmonella isolated from a Brazilian pork production chain. Int. J. Food Microbiol. 2019, 310, 108310. [Google Scholar] [CrossRef] [PubMed]
- Spindola, M.G.; Cunha, M.P.V.; Moreno, L.Z.; Amigo, C.R.; Silva, A.P.S.; Parra, B.M.; Poor, A.P.; de Oliveira, C.H.; Perez, B.P.; Knöbl, T.; et al. Genetic diversity, virulence genotype and antimicrobial resistance of uropathogenic Escherichia coli (UPEC) isolated from sows. Vet. Q. 2018, 38, 79–87. [Google Scholar] [CrossRef]
- Silva, K.C.; Moreno, M.; Cabrera, C.; Spira, B.; Cerdeira, L.; Lincopan, N.; Moreno, A.M. First characterization of CTX-M-15-producing Escherichia coli strains belonging to sequence type (ST) 410, ST224, and ST1284 from commercial swine in South America. Antimicrob. Agents Chemother. 2016, 60, 2505–2508. [Google Scholar] [CrossRef]
- Morales, A.S.; Fragoso de Araújo, J.; de Moura Gomes, V.T.; Reis Costa, A.T.; dos Prazeres Rodrigues, D.; Porfida Ferreira, T.S.; de Lima Filsner, P.H.N.; Felizardo, M.R.; Micke Moreno, A. Colistin resistance in Escherichia coli and Salmonella enterica strains isolated from swine in Brazil. Sci. World J. 2012, 2012, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, N.; Nordmann, P.; Moreno, A.M.; Zanolli Moreno, L.; Chaby, R.; Breton, A.; Tissières, P.; Poirel, L. Genetic and functional characterization of an MCR-3-Like enzyme-producing Escherichia coli isolate recovered from swine in Brazil. Antimicrob. Agents Chemother. 2018, 62, e00278-18. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, I.; Berkvens, D.; Vanantwerpen, G.; Baré, J.; Houf, K.; Wauters, G.; De Zutter, L. Contamination of freshly slaughtered pig carcasses with enteropathogenic Yersinia spp.: Distribution, quantification and identification of risk factors. Int. J. Food Microbiol. 2015, 204, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.T.F.; Botelho, C.V.; Silva, D.A.L.; Lanna, F.G.P.A.; Grossi, J.L.; Campos-Galvão, M.E.M.; Yamatogi, R.S.; Falcão, J.P.; dos Bersot, L.S.; Nero, L.A. Yersinia enterocolitica in a Brazilian pork production chain: Tracking of contamination routes, virulence and antimicrobial resistance. Int. J. Food Microbiol. 2018, 276, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Rusak, L.A.; Dos Reis, C.M.F.; Barbosa, A.V.; Santos, A.F.M.; Paixão, R.; Hofer, E.; Vallim, D.C.; Asensi, M.D. Phenotypic and genotypic analysis of bio-serotypes of Yersinia enterocolitica from various sources in Brazil. J. Infect. Dev. Ctries. 2014, 8, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Frazão, M.R.; Andrade, L.N.; Darini, A.L.C.; Falcão, J.P. Antimicrobial resistance and plasmid replicons in Yersinia enterocolitica strains isolated in Brazil in 30 years. Braz. J. Infect. Dis. 2017, 21, 477–480. [Google Scholar] [CrossRef]
- Fàbrega, A.; Roca, I.; Vila, J. Fluoroquinolone and multidrug resistance phenotypes associated with the overexpression of AcrAB and an orthologue of MarA in Yersinia enterocolitica. Int. J. Med. Microbiol. 2010, 300, 457–463. [Google Scholar] [CrossRef]
- Rau, R.B.; de Lima-Morales, D.; Wink, P.L.; Ribeiro, A.R.; Martins, A.F.; Barth, A.L. Emergence of mcr-1 producing Salmonella enterica serovar Typhimurium from retail meat: First detection in Brazil. Foodborne Pathog. Dis. 2018, 15, 58–59. [Google Scholar] [CrossRef]
- Brazil Companhia Nacional de Abastecimento (CONAB). Compêndio de Estudos da Conab-v16—Pecuária Leiteira—Análise dos Custos. 2018. Available online: https://www.conab.gov.br/institucional/publicacoes/compendio-de-estudos-da-conab (accessed on 21 December 2019).
- Sindicado Nacional da Indústria de Produtos para Saúde Animal (SINDAM). Compêndio de Produtos Veterinários. Available online: https://sistemas.sindan.org.br/cpvs/ (accessed on 21 December 2019).
- Keane, O.M. Symposium review: Intramammary infections—Major pathogens and strain-associated complexity. J. Dairy Sci. 2019, 102, 4713–4726. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.C.; Guimarães, F.F.; Manzi, M.P.; Júnior, A.F.; Gómez-Sanz, E.; Gómez, P.; Langoni, H.; Rall, V.L.M.; Torres, C. Methicillin-Resistant Staphylococcus aureus of lineage ST398 as cause of mastitis in cows. Lett. Appl. Microbiol. 2014, 59, 665–669. [Google Scholar] [CrossRef]
- da Costa Krewer, C.; Santos Amanso, E.; Veneroni Gouveia, G.; de Lima Souza, R.; da Costa, M.M.; Aparecido Mota, R. Resistance to antimicrobials and biofilm formation in Staphylococcus spp. isolated from bovine mastitis in the Northeast of Brazil. Trop. Anim. Health Prod. 2015, 47, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Rossi, B.F.; Camargo, C.H.; Dantas, S.T.A.; Langoni, H.; Guimarães, F.F.; Lima, F.S.; Fitzgerald, J.R.; Fernandes, A.; et al. Molecular epidemiology of methicillin-susceptible Staphylococcus aureus (MSSA) isolated from milk of cows with subclinical mastitis. Microb. Pathog. 2018, 124, 130–135. [Google Scholar] [CrossRef]
- Lange, C.; Cardoso, M.; Senczek, D.; Schwarz, S. Molecular subtyping of Staphylococcus aureus isolates from cases of bovine mastitis in Brazil. Vet. Microbiol. 1999, 67, 127–141. [Google Scholar] [CrossRef]
- Cabral, K.G.; Lämmler, C.; Zschöck, M.; Langoni, H.; de Sá, M.E.P.; Victória, C.; Da Silva, A.V. Pheno and genotyping of Staphylococcus aureus, isolated from bovine milk samples from São Paulo State, Brazil. Can. J. Microbiol. 2004, 50, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Rabello, R.F.; Souza, C.R.V.M.; Duarte, R.S.; Lopes, R.M.M.; Teixeira, L.M.; Castro, A.C.D. Characterization of Staphylococcus aureus isolates recovered from bovine mastitis in Rio de Janeiro, Brazil. J. Dairy Sci. 2005, 88, 3211–3219. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M.; Parente, C.E.S.R.; Vieira-da-Motta, O.; Bonna, I.C.F.; Silva, D.A.; de Lencastre, H. Characterization of Staphylococcus aureus isolates from buffalo, bovine, ovine, and caprine milk samples collected in Rio de Janeiro State, Brazil. Appl. Environ. Microbiol. 2007, 73, 3845–3849. [Google Scholar] [CrossRef]
- Ceotto, H.; dos Nascimento, J.S.; de Paiva Brito, M.A.V.; do Carmo de Freire Bastos, M. Bacteriocin production by Staphylococcus aureus involved in bovine mastitis in Brazil. Res. Microbiol. 2009, 160, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.S.; Marinho, P.R.; da Santos, O.C.S.; de Almeida, P.; Romanos, M.T.V.; Muricy, G.; Brito, M.A.V.P.; Giambiagi-deMarval, M. Antimicrobial activity of marine sponges against coagulase-negative staphylococci isolated from bovine mastitis. Vet. Microbiol. 2012, 155, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.L.; Lange, C.C.; Brito, M.A.; Ribeiro, J.B.; Mendonça, L.C.; Vaz, E.K. Characterisation of penicillin and tetracycline resistance in Staphylococcus aureus isolated from bovine milk samples in Minas Gerais, Brazil. J. Dairy Res. 2017, 84, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Fernandes dos Santos, F.; Mendonça, L.C.; de Reis, D.R.L.; de Guimarães, A.S.; Lange, C.C.; Ribeiro, J.B.; Machado, M.A.; Brito, M.A.V.P. Presence of mecA-positive multidrug-resistant Staphylococcus epidermidis in bovine milk samples in Brazil. J. Dairy Sci. 2016, 99, 1374–1382. [Google Scholar] [CrossRef]
- Marques, V.F.; da Motta, C.C.; da Soares, B.S.; de Melo, D.A.; Coelho, S.M.; da Silva Coelho, I.; Barbosa, H.; Souza, M.S. Biofilm production and beta-lactamic resistance in Brazilian Staphylococcus aureus isolates from bovine mastitis. Braz. J. Microbiol. 2017, 48, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Castelani, L.; Pilon, L.E.; Martins, T.; Pozzi, C.R.; Arcaro, J.R.P. Investigation of biofilm production and icaA and icaD genes in Staphylococcus aureus isolated from heifers and cows with mastitis: Mastitis. Anim. Sci. J. 2014, 86, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Haubert, L.; Kroning, I.S.; Iglesias, M.A.; da Silva, W.P. First report of the Staphylococcus aureus isolate from subclinical bovine mastitis in the South of Brazil harboring resistance gene dfrG and transposon family Tn 916-1545. Microb. Pathog. 2017, 113, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.H.; Mendes, J.F.; Villarreal, P.V.; Santos, P.R.; Gonçalves, C.L.; Gonzales, H.L.; Nascente, P.S. Identification and antimicrobial suceptibility profile of bacteria causing bovine mastitis from dairy farms in Pelotas, Rio Grande do Sul. Braz. J. Biol. 2018, 78, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.M.; Paiva, L.V.; Figueiredo, H.C.P.; Figueira, A.R.; Pereira, U.P.; Silva, N. Population diversity of Staphylococcus aureus isolated from bovine mastitis in Brazilian dairy herds. Res. Vet. Sci. 2012, 93, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Community-Associated MRSA: What makes them special? Int. J. Med. Microbiol. 2013, 303, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Carnelutti, A.; Castaldo, N.; Peghin, M. Important new therapies for methicillin-resistant Staphylococcus aureus. Expert Opin. Pharmacother. 2019, 20, 2317–2334. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.C.; Guimarães, F.F.; Manzi, M.P.; Budri, P.E.; Gómez-Sanz, E.; Benito, D.; Langoni, H.; Rall, V.L.M.; Torres, C. Molecular characterization and clonal diversity of methicillin-susceptible Staphylococcus aureus in milk of cows with mastitis in Brazil. J. Dairy Sci. 2013, 96, 6856–6862. [Google Scholar] [CrossRef]
- Mello, P.L.; Pinheiro, L.; de Martins, L.A.; Brito, M.A.V.P.; de Lourdes Ribeiro de Souza da Cunha, M. Short communication: β-Lactam resistance and vancomycin heteroresistance in Staphylococcus spp. isolated from bovine subclinical mastitis. J. Dairy Sci. 2017, 100, 6567–6571. [Google Scholar] [CrossRef]
- Guimarães, F.F.; Manzi, M.P.; Joaquim, S.F.; Richini-Pereira, V.B.; Langoni, H. Short communication: Outbreak of methicillin-resistant Staphylococcus aureus (MRSA)-associated mastitis in a closed dairy herd. J. Dairy Sci. 2017, 100, 726–730. [Google Scholar] [CrossRef]
- Miranda, P.S.D.; Lannes-Costa, P.S.; Pimentel, B.A.S.; Silva, L.G.; Ferreira-Carvalho, B.T.; Menezes, G.C.; Mattos-Guaraldi, A.L.; Hirata, R.; Mota, R.A.; Nagao, P.E. Biofilm formation on different pH conditions by Streptococcus agalactiae isolated from bovine mastitic milk. Lett. Appl. Microbiol. 2018, 67, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.C.A.; Costa, N.S.; Vianna Souza, A.R.; da Silva, L.G.; de Corrêa, A.B.A.; Fernandes, F.G.; Oliveira, I.C.M.; de Mattos, M.C.; Rosado, A.S.; Benchetrit, L.C. Distribution of serotypes and evaluation of antimicrobial susceptibility among human and bovine Streptococcus agalactiae strains isolated in Brazil between 1980 and 2006. Braz. J. Infect. Dis. 2013, 17, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.B.C.; Zanardo, L.G.; Galvão, N.N.; Carvalho, I.A.; Nero, L.A.; Moreira, M.A.S. Escherichia coli from clinical mastitis: Serotypes and virulence factors. J. Vet. Diagn. Invest. 2011, 23, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Zanella, G.N.; Mikcha, J.M.G.; Bando, E.; Siqueira, V.L.D.; Machinski, M. Occurrence and antibiotic resistance of coliform bacteria and antimicrobial residues in pasteurized cow’s milk from Brazil. J. Food Prot. 2010, 73, 1684–1687. [Google Scholar] [CrossRef]
- dos Alves, T.S.; Lara, G.H.B.; Maluta, R.P.; Ribeiro, M.G.; da Leite, D.S. Carrier flies of multidrug-resistant Escherichia coli as potential dissemination agent in dairy farm environment. Sci. Total Environ. 2018, 633, 1345–1351. [Google Scholar] [CrossRef]
- United States of America Department of Agriculture (USDA); Economic Research Service (ERS). Brazil Once Again Becomes the World’s Largest Beef Exporter. Available online: https://www.ers.usda.gov/amber-waves/2019/july/brazil-once-again-becomes-the-world-s-largest-beef-exporter (accessed on 22 December 2019).
- dos Santos, E.C.C.; Castro, V.S.; Cunha-Neto, A.; dos Santos, L.F.; Vallim, D.C.; de Lisbôa, R.C.; Carvalho, R.C.T.; Junior, C.A.C.; de Figueiredo, E.E.S. Escherichia coli O26 and O113:H21 on carcasses and beef from a slaughterhouse located in Mato Grosso, Brazil. Foodborne Pathog. Dis. 2018, 15, 653–659. [Google Scholar] [CrossRef]
- Fernandes, F.P.; Voloski, F.L.S.; Ramires, T.; Haubert, L.; Reta, G.G.; Mondadori, R.G.; da Silva, W.P.; de Cássia dos Santos da Conceição, R.; Duval, E.H. Virulence and antimicrobial resistance of Salmonella spp. and Escherichia coli in the beef jerky production line. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Loiko, M.R.; de Paula, C.M.D.; Langone, A.C.J.; Rodrigues, R.Q.; Cibulski, S.; de Rodrigues, R.O.; Camargo, A.C.; Nero, L.A.; Mayer, F.Q.; Tondo, E.C. Genotypic and antimicrobial characterization of pathogenic bacteria at different stages of cattle slaughtering in southern Brazil. Meat Sci. 2016, 116, 193–200. [Google Scholar] [CrossRef]
- Cossi, M.V.C.; Burin, R.C.K.; Lopes, D.A.; Dias, M.R.; de Castilho, N.P.A.; de Arruda Pinto, P.S.; Nero, L.A. Antimicrobial resistance and virulence profiles of Salmonella isolated from butcher shops in Minas Gerais, Brazil. J. Food Prot. 2013, 76, 1633–1637. [Google Scholar] [CrossRef]
- da Silva, F.F.P.; Horvath, M.B.; Silveira, J.G.; Pieta, L.; Tondo, E.C. Occurrence of Salmonella spp. and generic Escherichia coli on beef carcasses sampled at a Brazilian slaughterhouse. Braz. J. Microbiol. 2014, 45, 17–24. [Google Scholar] [CrossRef]
- Camargo, A.C.; de Castilho, N.P.A.; da Silva, D.A.L.; Vallim, D.C.; Hofer, E.; Nero, L.A. Antibiotic resistance of Listeria monocytogenes isolated from meat-processing environments, beef products, and clinical cases in Brazil. Microb. Drug Resist. 2015, 21, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.C.C.; Guimarães, F.F.; de P. Manzi, M.; Gómez-Sanz, E.; Gómez, P.; Araújo, J.P., Jr.; Langoni, H.; Rall, V.L.M.; Torres, C. Characterization of methicillin-resistant coagulase-negative Staphylococci in milk from cows with mastitis in Brazil. Antonie Van Leeuwenhoek 2014, 106, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; Lafisca, A.; Cossi, M.V.C.; Lanna, F.G.P.A.; Dias, M.R.; de Arruda Pinto, P.S.; Nero, L.A. Low occurrence of Listeria monocytogenes on bovine hides and carcasses in Minas Gerais State, Brazil: Molecular characterization and antimicrobial resistance. J. Food Prot. 2014, 77, 1148–1152. [Google Scholar] [CrossRef]
- Warren, R.E.; Ensor, V.M.; O’Neill, P.; Butler, V.; Taylor, J.; Nye, K.; Harvey, M.; Livermore, D.M.; Woodford, N.; Hawkey, P.M. Imported chicken meat as a potential source of quinolone-resistant Escherichia coli producing extended-spectrum beta-lactamases in the UK. J. Antimicrob. Chemother. 2008, 61, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Dhanji, H.; Murphy, N.M.; Doumith, M.; Durmus, S.; Surman Lee, S.; Hope, R.; Woodford, N.; Livermore, D.M. Cephalosporin resistance mechanisms in Escherichia coli isolated from raw chicken imported into the UK. J. Antimicrob. Chemother. 2010, 65, 2534–2537. [Google Scholar] [CrossRef]
- Casella, T.; Rodríguez, M.M.; Takahashi, J.T.; Ghiglione, B.; Dropa, M.; Assunção, E.; Nogueira, M.L.; Lincopan, N.; Gutkind, G.; Nogueira, M.C.L. Detection of blaCTX-M-type genes in complex class 1 integrons carried by Enterobacteriaceae isolated from retail chicken meat in Brazil. Int. J. Food Microbiol. 2015, 197, 88–91. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Penha Filho, R.A.C.; Andrade, L.N.; Berchieri, A., Jr.; Darini, A.L.C. Evaluation and characterization of plasmids carrying CTX-M genes in a non-clonal population of multidrug-resistant Enterobacteriaceae isolated from poultry in Brazil. Diagn. Microbiol. Infect. Dis. 2016, 85, 444–448. [Google Scholar] [CrossRef]
- Moura, Q.; Fernandes, M.R.; Silva, K.C.; Monte, D.F.; Esposito, F.; Dropa, M.; Noronha, C.; Moreno, A.M.; Landgraf, M.; Negrão, F.J.; et al. Virulent nontyphoidal Salmonella producing CTX-M and CMY-2 β-lactamases from livestock, food and human infection, Brazil. Virulence 2018, 9, 281–286. [Google Scholar] [CrossRef]
- Galetti, R.; Antonio Casarin Penha Filho, R.; Ferreira, J.C.; Varani, M.A.; Costa Darini, A.L. Antibiotic resistance and heavy metal tolerance plasmids: The antimicrobial bulletproof properties of Escherichia fergusonii isolated from poultry. Infect. Drug Resist. 2019, 12, 1029–1033. [Google Scholar] [CrossRef]
- Botelho, L.A.B.; Kraychete, G.B.; Costa e Silva, J.L.; Regis, D.V.V.; Picão, R.C.; Moreira, B.M.; Bonelli, R.R. Widespread distribution of CTX-M and plasmid-mediated AmpC β-lactamases in Escherichia coli from Brazilian chicken meat. Mem. Inst. Oswaldo Cruz 2015, 110, 249–254. [Google Scholar] [CrossRef]
- Fitch, F.M.; Carmo-Rodrigues, M.S.; Oliveira, V.G.S.; Gaspari, M.V.; dos Santos, A.; de Freitas, J.B.; Pignatari, A.C.C. β-Lactam resistance genes: Characterization, epidemiology, and first detection of blaCTX-M-1 and blaCTX-M-14 in Salmonella spp. isolated from poultry in Brazil—Brazil Ministry of Agriculture’s Pathogen Reduction Program. Microb. Drug Resist. 2016, 22, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Ibbe, R.; Ferreira, K.F.S.; Silva, R.L.; Machado, S.A.; Nascimento, E.R.; Rodrigues, D.P.; Aquino, M.H.C.; de Almeida Pereira, V.L. Amoxicillin/Clavulanic acid and cefotaxime resistance in Salmonella Minnesota and Salmonella Heidelberg from broiler chickens. Poult. Sci. J. 2017, 5, 123–129. [Google Scholar]
- Ferreira, J.C.; Penha Filho, R.A.C.; Andrade, L.N.; Berchieri, A., Jr.; Darini, A.L.C. Diversity of plasmids harboring blaCMY-2 in multidrug-resistant Escherichia coli isolated from poultry in Brazil. Diagn. Microbiol. Infect. Dis. 2017, 88, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Tiba-Casas, M.R.; Camargo, C.H.; Soares, F.B.; Doi, Y.; Fernandes, S.A. Emergence of CMY-2-Producing Salmonella Heidelberg associated with IncI1 plasmids isolated from poultry in Brazil. Microb. Drug Resist. 2019, 25, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.A.; Camargo, C.H.; Francisco, G.R.; Bueno, M.F.C.; Garcia, D.O.; Doi, Y.; Casas, M.R.T. Prevalence of extended-spectrum β-lactamases CTX-M-8 and CTX-M-2-producing Salmonella serotypes from clinical and nonhuman isolates in Brazil. Microb. Drug Resist. 2017, 23, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Casella, T.; Haenni, M.; Madela, N.K.; de Andrade, L.K.; Pradela, L.K.; de Andrade, L.N.; da Darini, A.L.C.; Madec, J.-Y.; Nogueira, M.C.L. Extended-Spectrum cephalosporin-resistant Escherichia coli isolated from chickens and chicken meat in Brazil is associated with rare and complex resistance plasmids and pandemic ST lineages. J. Antimicrob. Chemother. 2018, 73, 3293–3297. [Google Scholar] [CrossRef]
- Cunha, M.P.V.; Lincopan, N.; Cerdeira, L.; Esposito, F.; Dropa, M.; Franco, L.S.; Moreno, A.M.; Knöbl, T. Coexistence of CTX-M-2, CTX-M-55, CMY-2, FosA3, and QnrB19 in extraintestinal pathogenic Escherichia coli from poultry in Brazil. Antimicrob. Agents Chemother. 2017, 61, e02474-16. [Google Scholar] [CrossRef]
- Koga, V.L.; Rodrigues, G.R.; Scandorieiro, S.; Vespero, E.C.; Oba, A.; de Brito, B.G.; de Brito, K.C.T.; Nakazato, G.; Kobayashi, R.K.T. Evaluation of the antibiotic resistance and virulence of Escherichia coli strains isolated from chicken carcasses in 2007 and 2013 from Paraná, Brazil. Foodborne Pathog. Dis. 2015, 12, 479–485. [Google Scholar] [CrossRef]
- Cyoia, P.S.; Koga, V.L.; Nishio, E.K.; Houle, S.; Dozois, C.M.; de Brito, K.C.T.; de Brito, B.G.; Nakazato, G.; Kobayashi, R.K.T. Distribution of ExPEC virulence factors, blaCTX-M, fosA3, and mcr-1 in Escherichia coli isolated from commercialized chicken carcasses. Front. Microbiol. 2019, 9, 3254. [Google Scholar] [CrossRef]
- Zogg, A.L.; Zurfluh, K.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of ESBL-producing Enterobacteriaceae and Methicillinresistant Staphylococcus aureus (MRSA) isolated from Swiss and imported raw poultry meat collected at retail level. Schweiz. Arch. Tierheilkd. 2016, 158, 451–456. [Google Scholar] [CrossRef]
- Nahar, A.; Awasthi, S.P.; Hatanaka, N.; Okuno, K.; Hoang, P.H.; Hassan, J.; Hinenoya, A.; Yamasaki, S. Prevalence and characteristics of extended-spectrum β-lactamase-producing Escherichia coli in domestic and imported chicken meats in Japan. J. Vet. Med. Sci. 2018, 80, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Moon, J.-S.; Oh, D.-H.; Chon, J.-W.; Song, B.-R.; Lim, J.-S.; Heo, E.-J.; Park, H.-J.; Wee, S.-H.; Sung, K. Genotypic characterization of ESBL-producing E. coli from imported meat in South Korea. Food Res. Int. 2018, 107, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hoepers, P.G.; Silva, P.L.; Rossi, D.A.; Valadares, E.C., Jr.; Ferreira, B.C.; Zuffo, J.P.; Koerich, P.K.; Fonseca, B.B. The association between extended spectrum beta-lactamase (ESBL) and ampicillin C (AmpC) beta-lactamase genes with multidrug resistance in Escherichia coli isolates recovered from turkeys in Brazil. Br. Poult. Sci. 2018, 59, 396–401. [Google Scholar] [CrossRef]
- Saraiva, M.M.S.; Moreira Filho, A.L.B.; Freitas Neto, O.C.; Silva, N.M.V.; Givisiez, P.E.N.; Gebreyes, W.A.; Oliveira, C.J.B. Off-Label use of ceftiofur in one-day chicks triggers a short-term increase of ESBL-producing E. coli in the gut. PLoS ONE 2018, 13, e0203158. [Google Scholar] [CrossRef]
- Fernandes, S.A.; Paterson, D.L.; Ghilardi-Rodrigues, Â.C.; Adams-Haduch, J.M.; Tavechio, A.T.; Doi, Y. CTX-M-2–producing Salmonella typhimurium isolated from pediatric patients and poultry in Brazil. Microb. Drug Resist. 2009, 15, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Moura, Q.; Fernandes, M.R.; Cerdeira, L.; Ienne, S.; Souza, T.A.; Negrão, F.J.; Lincopan, N. Draft genome sequence of a multidrug-resistant CMY-2-producing Salmonella enterica subsp. enterica serovar Minnesota ST3088 isolated from chicken meat. J. Glob. Antimicrob. Resist. 2017, 8, 67–69. [Google Scholar] [PubMed]
- Botelho, L.A.B.; Kraychete, G.B.; Rocha, P.B.; da Silva, A.P.S.; Picão, R.C.; Moreira, B.M.; Bonelli, R.R. CTX-M-and pAmpC-encoding genes are associated with similar mobile genetic elements in Escherichia coli isolated from different brands of Brazilian chicken meat. Microb. Drug Resist. 2020, 26, 14–20. [Google Scholar] [CrossRef]
- Martins, P.D.; de Almeida, T.T.; Basso, A.P.; de Moura, T.M.; Frazzon, J.; Tondo, E.C.; Frazzon, A.P.G. Coagulase-Positive Staphylococci isolated from chicken meat: Pathogenic potential and vancomycin resistance. Foodborne Pathog. Dis. 2013, 10, 771–776. [Google Scholar] [CrossRef]
- Rodrigues, M.X.; Silva, N.C.C.; Trevilin, J.H.; Cruzado, M.M.B.; Mui, T.S.; Duarte, F.R.S.; Castillo, C.J.C.; Canniatti-Brazaca, S.G.; Porto, E. Molecular characterization and antibiotic resistance of Staphylococcus spp. isolated from cheese processing plants. J. Dairy Sci. 2017, 100, 5167–5175. [Google Scholar] [CrossRef]
- Moreno, L.Z.; Dutra, M.C.; Moreno, M.; Ferreira, T.S.; da Silva, G.F.; Matajira, C.E.; Silva, A.P.S.; Moreno, A.M. Vancomycin-Intermediate livestock-associated methicillin-resistant Staphylococcus aureus ST398/t9538 from swine in Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 659–661. [Google Scholar] [CrossRef]
- Xavier, D.B.; Bernal, F.E.M.; Titze-de-Almeida, R. Absence of VanA-and VanB-containing Enterococci in poultry raised on nonintensive production farms in Brazil. Appl. Environ. Microbiol. 2006, 72, 3072–3073. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.M.; Perin, L.M.; Todorov, S.D.; Silva, A.; Franco, B.D.G.M.; Nero, L.A. Bacteriocinogenic and virulence potential of Enterococcus isolates obtained from raw milk and cheese. J. Appl. Microbiol. 2012, 113, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Perin, L.M.; Miranda, R.O.; Todorov, S.D.; de Franco, B.D.G.M.; Nero, L.A. Virulence, antibiotic resistance and biogenic amines of bacteriocinogenic lactococci and Enterococci isolated from goat milk. Int. J. Food Microbiol. 2014, 185, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sacramento, A.G.; Zanella, R.C.; de Almeida, L.M.; Pires, C.; Popazoglo, C.; Costa, E.A.S.; Cerdeira, L.T.; Mamizuka, E.M.; Lincopan, N. Identification of new sequence types among Enterococcus faecium and Enterococcus faecalis carrying the vanA gene in retail chicken meat. J. Glob. Antimicrob. Resist. 2016, 4, 72–73. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Moura, Q.; Sartori, L.; Silva, K.C.; Cunha, M.P.; Esposito, F.; Lopes, R.; Otutumi, L.K.; Gonçalves, D.D.; Dropa, M.; et al. Silent dissemination of colistin-resistant Escherichia coli in South America could contribute to the global spread of the mcr-1 gene. Eurosurveillance 2016, 21, 30214. [Google Scholar] [CrossRef]
- Lentz, S.A.; de Lima-Morales, D.; Cuppertino, V.M.; de Nunes, L.S.; da Motta, A.S.; Zavascki, A.P.; Barth, A.L.; Martins, A.F. Letter to the editor: Escherichia coli harbouring mcr-1 gene isolated from poultry not exposed to polymyxins in Brazil. Eurosurveillance 2016, 21, 30267. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Moura, Q.; Esposito, F.; Lincopan, N. Authors’ reply: Escherichia coli harbouring mcr-1 gene isolated from poultry not exposed to polymyxins in Brazil. Eurosurveillance 2016, 21, 30268. [Google Scholar] [CrossRef]
- Oliveira, C.C.; Lopes, E.S.; Barbosa, D.R.; Pimenta, R.L.; Sobrinho, N.M.B.A.; Coelho, S.M.O.; Souza, M.M.S.; Coelho, I.S. Occurrence of the colistin resistance mcr-1 gene in soils from intensive vegetable production and native vegetation. Eur. J. Soil Sci. 2019, 70, 876–881. [Google Scholar] [CrossRef]
- Monte, D.F.; Mem, A.; Fernandes, M.R.; Cerdeira, L.; Esposito, F.; Galvão, J.A.; Franco, B.D.G.M.; Lincopan, N.; Landgraf, M. Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 genes in South America. Antimicrob. Agents Chemother. 2017, 61, e02718-16. [Google Scholar] [CrossRef]
- Moreno, L.Z.; Gomes, V.T.M.; Moreira, J.; de Oliveira, C.H.; Peres, B.P.; Silva, A.P.S.; Thakur, S.; La Ragione, R.M.; Moreno, A.M. First report of mcr-1-harboring Salmonella enterica serovar Schwarzengrund isolated from poultry meat in Brazil. Diagn. Microbiol. Infect. Dis. 2019, 93, 376–379. [Google Scholar] [CrossRef]
- Aires, C.A.M.; da Conceição-Neto, O.C.; Tavares, E.; Oliveira, T.R.; Dias, C.F.; Montezzi, L.F.; Picão, R.C.; Albano, R.M.; Asensi, M.D.; Carvalho-Assef, A.P.D. Emergence of the plasmid-mediated mcr-1 gene in clinical KPC-2-producing Klebsiella pneumoniae sequence type 392 in Brazil. Antimicrob. Agents Chemother. 2017, 61, e00317-17. [Google Scholar] [CrossRef] [PubMed]
- Dalmolin, T.V.; Castro, L.; Mayer, F.Q.; Zavascki, A.P.; Martins, A.F.; de Lima-Morales, D.; Barth, A.L. Co-Occurrence of mcr-1 and blaKPC-2 in a clinical isolate of Escherichia coli in Brazil. J. Antimicrob. Chemother. 2017, 72, 2404–2406. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R.; McCulloch, J.A.; Vianello, M.A.; Moura, Q.; Pérez-Chaparro, P.J.; Esposito, F.; Sartori, L.; Dropa, M.; Matté, M.H.; Lira, D.P.A.; et al. First report of the globally disseminated IncX4 plasmid carrying the mcr-1 gene in a colistin-resistant Escherichia coli sequence type 101 isolate from a human infection in Brazil. Antimicrob. Agents Chemother. 2016, 60, 6415–6417. [Google Scholar] [CrossRef] [PubMed]
- Sellera, F.P.; Fernandes, M.R.; Sartori, L.; Carvalho, M.P.N.; Esposito, F.; Nascimento, C.L.; Dutra, G.H.P.; Mamizuka, E.M.; Pérez-Chaparro, P.J.; McCulloch, J.A.; et al. Escherichia coli carrying IncX4 plasmid-mediated mcr-1 and blaCTX-M genes in infected migratory Magellanic penguins (Spheniscus magellanicus). J. Antimicrob. Chemother. 2017, 72, 1255–1256. [Google Scholar]
- Ferrari, R.; Galiana, A.; Cremades, R.; Rodríguez, J.C.; Magnani, M.; Tognim, M.C.B.; Oliveira, T.C.R.M.; Royo, G. Plasmid-Mediated quinolone resistance (PMQR) and mutations in the topoisomerase genes of Salmonella enterica strains from Brazil. Braz. J. Microbiol. 2013, 44, 657–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campioni, F.; Souza, R.A.; Martins, V.V.; Stehling, E.G.; Bergamini, A.M.M.; Falcão, J.P. Prevalence of gyrA mutations in nalidixic acid-resistant strains of Salmonella enteritidis isolated from humans, food, chickens, and the farm environment in Brazil. Microb. Drug Resist. 2017, 23, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.; Penha Filho, R.A.C.; Kuaye, A.P.Y.; Andrade, L.N.; Berchieri, A., Jr.; da Darini, A.L.C. Identification and characterization of plasmid-mediated quinolone resistance determinants in Enterobacteriaceae isolated from healthy poultry in Brazil. Infect. Genet. Evol. 2018, 60, 66–70. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Filho, R.A.C.P.; Andrade, L.N.; Berchieri, A.; Darini, A.L.C. Detection of chromosomal blaCTX-M-2 in diverse Escherichia coli isolates from healthy broiler chickens. Clin. Microbiol. Infect. 2014, 20, O623–O626. [Google Scholar] [CrossRef]
- Brisola, M.C.; Crecencio, R.B.; Bitner, D.S.; Frigo, A.; Rampazzo, L.; Stefani, L.M.; Faria, G.A. Escherichia coli used as a biomarker of antimicrobial resistance in pig farms of Southern Brazil. Sci. Total Environ. 2019, 647, 362–368. [Google Scholar] [CrossRef]
- Panzenhagen, P.H.N.; Cabral, C.C.; Suffys, P.N.; Franco, R.M.; Rodrigues, D.P.; Conte, C. A, Jr. Comparative genome analysis and characterization of the Salmonella typhimurium strain CCRJ_26 isolated from swine carcasses using whole-genome sequencing approach. Lett. Appl. Microbiol. 2018, 66, 352–359. [Google Scholar] [CrossRef]
- Mattiello, S.P.; Drescher, G.; Barth, V.C.; Ferreira, C.A.S.; Oliveira, S.D. Characterization of antimicrobial resistance in Salmonella enterica strains isolated from Brazilian poultry production. Antonie Van Leeuwenhoek 2015, 108, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low-and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef] [PubMed]
- Brazil, Ministério da Agricultura, Pecuária e Abastecimento (MAPA) Portaria no 396 de 23 de novembro de 2009. Available online: http://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/portaria-sda-n-o-396-de-23-de-novembro-de-2009.pdf/view (accessed on 19 December 2019).
- Brazil, Agência Nacional de Vigilância Sanitária (ANVISA) RDC no 54 de 12 de novembro de 2012. Available online: http://portal.anvisa.gov.br/documents/%2033880/2568070/rdc0054_12_11_2012.pdf/c5ac23fd-974e-4f2c-9fbc-48f7e0a31864 (accessed on 11 November 2019).
- Food and Agriculture Organization (FAO) Codex Alimentarius- Maximum residue limits (mrls) and risk management recommendations (rmrs) for residues of veterinary drugs in foods cac/mrl 2-2015. Available online: http://www.agricultura.gov.br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes/documentos-da-pncrc/codex-alimentarius-cac-mrl-n-o-02-2015-de-julho-2015.pdf/view (accessed on 25 November 2019).
- Brazil, Ministério da Agricultura, Pecuária e Abastecimento (MAPA) Instrução normativa no 54, de 17 de dezembro de 2018. Available online: http://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/57733217/do1-2019-01-03-instrucao-normativa-n-54-de-17-de-dezembro-de-2018-57733055 (accessed on 11 November 2019).
- Brazil, Agência Nacional de Vigilancia Sanitária (ANVISA) Instrução normativa no 51, de 19 de dezembro de 2019. Available online: https://alimentusconsultoria.com.br/instrucao-normativa-n-51-de-19-de-dezembro-de-2019-anvisa/ (accessed on 27 December 2019).
| Reference | Sampling Period | Geographic Region a | Local (n) | Isolates (n) | Antimicrobial Resistance b | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactam | Tetracycline | Quinolone | Sulfonamide | Aminoglycoside | Others | |||||
| Salmonella sp. | ||||||||||
| Duarte et al., 2009 [35] | 2004 | NE | poultry carcasses (260) | 11 serotypes (19) | Amp: 10.5% | Tet: 31.6% | Cip, Eno: 5.2% Nal: 21.0% Nor: 2.5% | Sut: 5.2% | Kn: 15.8% Str: 73.7% | Clo: 5.2% Nit: 52.6% |
| Vaz et al., 2010 [37] | 1995–2003 | S | – | S. Enteritidis (96) | Amp, Caz: 0.0% | Tet: 1.0% | Nal: 14.6% | Sul: 34.4% Sut: 25.0% | Gen: 1.0% Str: 2.1% | – |
| Medeiros et al., 2011 [38] | 2004–2006 | N, NE, MW, SE, S | poultry carcasses (2679) | 18 serotypes (250) | Amp: 38.0% Atm: 19.2% Cfl: 12.0% Cfo: 13.2% Cro: 6.0% Ctf: 28.0% | Tet: 12.0% | Cip: 4.0% Eno: 19.2% Nal: 40.0% | Sul: 58.0% Sut, Tri: 10.0% | Gen: 12.0% Str: 78.0% | Clo: 6.0% Flo: 62.0% Nit: 8.0% |
| Kottwitz et al., 2012 [39] | 2002–2006 | S | breeding chickens | S. Enteritidis (38) | Amp, Ctx: 0.0% | – | Cip: 0.0% Nal: 26.3% | Sut: 0.0% | – | Clo: 2.6% |
| Kottwitz et al., 2013 [40] | 2003–2006 | S | discarded hatching eggs (1000) | 4 serotypes (26) | Amp, Ctx: 0.0% | – | Cip: 0.0% Nal: 23.1% | Sut: 0.0% | – | Clo: 0.0% |
| Costa et al., 2013 [52] | 2007–2011 | N, NE, MW, SE, S | broiler carcasses | 61 serotypes (1234) | Amp: 12.4%–18.9% | Tet: 15.2–18.9% | Nal: 15.5%-44.4% | Sut: 7.2%-11.7% | Gen: 7.0–10.6% | Nit: 9.2%–61.9% |
| Moraes et al., 2014 [74] | – | MW | one-day-old chicks and others | 12 serotypes (53) | Amp: 5.7% | Tet: 13.2% | Cip: 0.0% Eno: 5.7% | Sul: 73.6% Sut: 13.2% | Neo: 0.0% | Flo: 0.0% |
| Campioni et al., 2014 [41] | 2004-2010 | NE, MW, SE, S | – | S. Enteritidis (60) | Amp, Cfl, Cro: 0.0% | Tet: 0.0% | Nal: 73.3% | Sut: 0.0% | Ami, Str: 0.0% | Clo: 0.0% |
| Pandini et al., 2015 [42] | 2010–2011 | S | broiler farms (342 drag swabs) | 19 serotypes (39) | Amp: 20.5% Cfl: 23.0% Imp: 0.0% | Tet: 30.8% | Cip, Nor: 0.0% Nal: 28.2% | Sut: 12.8% | Gen: 2.6% Str: 10.2% Tob: 0.0% | Clo: 2.6% |
| Minharro et al., 2015 [75] | 2010–2011 | MW, SE | poultry carcasses (300), heart (600) and livers (600) | 9 serotypes (26) | Amc: 100% Amp: 0.0% Ctf: 3.8% | Dox, Tet: 0.0% | Cip: 0.0% Eno: 3.8% | Sul: 53.8% Sut: 0.0% | Gen: 3.8% | – |
| Voss-Rech et al., 2015 [50] | 2009–2010 | S, MW | broiler farms (1543 drag swabs) | 15 serotypes (82) | Amc: 6.1%; Ctf: 12.2% | Tet: 55.4% | Cip: 0%; Nor: 0%; Eno: 0% | Sut: 17.1% | Str: 24.4%; Gen: 6.1% | Fos: 0%; Col: 0% |
| Palmeira et al., 2016 [43] | 2004–2006 | S | broiler farms (18) and turkey carcasses | 25 serotypes (280) | Amp: 8.0% Amc: 0.0% Cfl: 5.0% Ctf: 1.0% | Tet: 35% | Cip, Nor: 0.0% Eno: 9.0% Nal: > 60% | Sul: 3% | Gen: 12% Kn, Str: 15% Neo: 30% | Clo: 2.5% Col: 15% Fos: 5% Nit: 35% Pol: 0.0% |
| Bezerra et al., 2016 [59] | 2014–2015 | NE | broiler farms (10/1000 samples) | O:6,8 (2) | Amp: 0.0% Ctf: 100% | Tet: 100% | – | Sut: 100% | Gen: 0.0% | Clo: 100% |
| Borges et al., 2017 [44] | – | S | various | S. Enteritidis (148) | Ctf: 4.1% | Tet: 2.7% | Cip: 41.9% | Sul: 75.0% Sut: 1.4% | Gen: 6.8% | – |
| Koerich et al. 2018 [45] | 2011–2014 | S | outbreaks of fowl typhoid | S. Gallinarum (60) | – | Tet: 33.0% | Eno: 83.0% Nor: 90.0% | Sut: 7.0% | Neo: 30.0% Str: 62.0% Spm: 100.0% | Col: 27.0% Fos: 0.0% |
| Cunha-Neto et al., 2018 [54] | 2014–2015 | MW | slaughterhouses (1) / carcasses (850) | 7 serotypes (31) | Amp, Cfl: 25.0% Atm: 21.9% Ctf: 6.3% Ctx: 18.8% | Tet: 9.4% | Cip, Eno, Nal: 0.0% Nor: 6.7% | Sul: 100% Sut: 75% Tri: 87.5% | Gen: 3.1% Str: 0.0% | Clo: 3.1% Flo, Nit: 0.0% |
| Baptista et al., 2018 [47] | 2016 | SE | slaughterhouses (6) | 7 serotypes (33) | Amc: 9.1% Amp, Cef, Ctx: 12.1% Ctf: 9.1% | – | Cip, Nor: 0.0% Eno: 3.0% | – | – | – |
| Borges et al., 2019 [56] | – | S | – | 11 serotypes (163) | Cft: 6.1% | Tet: 16% | Cip: 27%; Eno: 19% | Sox: 95.7%; Sut: 9.2% | Gen: 7.4%Spe: 12.3% | Clo: 6.1% |
| Penha-Filho et al., 2019 [36] | – | SE MW | chicken farms (6) and slaughterhouse (1) | 36 serotypes (83) | Amc, Caz, Ctf, Ctx: 13.5% Atm: 14.5% Cfo: 6.0% Cfp: 12.0% Etp: 0.0% | Tet: 28.0% | Cip: 52.0% Eno: 31.0% Nal: 41.0% | Sut: 20.5% | – | Clo:1.2% Flo: 0.0% |
| E. coli | ||||||||||
| Barros et al., 2012 [68] | – | NE | broiler farms (11) and laying hens farms (7) (120 samples) | E. coli (35) | Amo: 65.7% Cfx: 25.7% | Tet: 77.1% | Eno: 45.7% Nor: 40.0% | Sut: 65.7% | - | - |
| Lima-Filho et al.,2013 [63] | 2013 | NE | slaughterhouses (2/ 27 carcasses) | ExPEC | Amp: 81.5% Atm: 33.3% Caz: 14.8% Cfl: 88.8% Ipm: 0.0% | Tet: 100% | Cip: 44.4% Lev: 51.8% | – | Ami: 1.1% Gen: 33.3% Str: 100% | Clo: 18.5% |
| Gazal et al., 2015 [58] | 2011–2012 | S | 12 farms (40 samples of avian organic fertilizers) | E. coli (64) | Amo: 25.3% Amp: 18.7% Atm, Ctx, Ipm: 0.0% | Tet: 35.9% | Cip, Eno, Nor: 0.0% | Sut: 12.5% | Str:17.1% | Clo, Col, Pol: 0.0% |
| Carvalho et al., 2015 [66] | 2011–2012 | S | overshoe swab samples (109 broiler houses) | E. coli (109) | Amp: ~55.0% | Tet: ~75% | Cip: ~35.0% Eno: ~50.0% Nal: ~80.0% Nor: ~45.0% | Sul: ~70.0% Sut: ~50.0% | Gen: ~30.0% Neo: ~25.0% | Clo: ~20.0% Flo: ~5.0% Nit: ~30.0% |
| Bezerra et al., 2016 [59] | 2014–2015 | NE | 10 chicken farms (1000 samples) | E. coli (959) | Amp: 87.3% Ctf: 42.5% | Tet: 95.4% | Cip: 91.4% | Sut: 100% | Gen: 27.5% | Clo: 51.1% Fos: 33.3% Pol: 1.1% |
| Braga et al., 2016 [65] | 2011–2012 | SE | eight flocks from seven farms (osteomyelitis or arthritis) | APEC (15) | Amo: 73.3% Amc: 12.0% Cfl: 53.0% Cfo: 8.0% Ctf: 40.0% | Tet: 33.0% | Eno: 40.0% Nal: 68.0% | Sut: 33.0% | Gen: 20.0% Neo: 8.0% | Clo: 6.7% Pol: 0.0% |
| Stella et al., 2016 [61] | – | – | cloacal swabs from broilers (80) of 1 flock | APEC (15) | Amo, Amp, Cfl: 100% | Tet: 13.3% | Eno: 6.7% | Sut: 86.7% | Gen: 6.7% Neo, Str: 100% | Nit: 0.0% |
| – | – | non-APEC (76) | Amo: 80.3% Amp: 81.6% Cfl: 73.7 | Tet: 77.6% | Eno: 27.6% | Sut: 64.5% | Gen: 6.7% Neo: 42.1% Str: 88.2% | Nit: 5.3% | ||
| Maciel et al., 2017 [60] | – | S | avian colisepticemia outbreak (spleen and liver) | APEC (2) | Amp: 100% | Tet: 100% | Eno, Nor: 100% | Sut: 100% | Gen, Neo: 100% | – |
| Vaz et al., 2017 [67] | – | NE | liver of poultry carcasses (110) | E. coli (88) | Amc: 15.9% Atm: 19.1% Caz: 21.3% Cfl: 8.5% Ipm: 12.8% | Tet: 44.7% | Cip: 21.3% | – | Ami: 29.8% Gen:21.3% Str: 84% | |
| Borzi et al., 2018 [62] | – | SE | free range helmeted guineafowl (4 farms/56 cloaca, 56 oropharynges) | APEC (21) | Amc: 14.3% Amp: 71.4% Cfl: 100% Cfo: 9,5% Cro:14.3% Ctf: 4.8% | Tet: 61.9% | Cip: 23.8% Nor: 0.0% | Sut: 33.3% | Gen: 14.3% Kn: 33.3% Str: 90.5% | Clo: 9.5% Nit: 57.1% |
| Campylobacter sp. | ||||||||||
| Ku et al., 2011 [72] | – | – | Brazilian chicken meat imported by Korea | Campylobacter spp. (27) | Amp: 92.6% | Tet: 51.9% | Cip, Nal: 66.7% | – | Gen:18.5% | Azi, Ery: 29.6% Cli: 25.9% Flo:7.4% |
| Moura et al., 2013 [76] | – | MW | poultry carcasses (92) | Campylobacter spp. (16) | Amo: 87.5% | Tet: 93.8% | Cip: 100% Nal: 93.8% | – | Gen, Str: 93.8% | Clo: 37.5% Ery: 68.8% |
| Ferro et al., 2015 [64] | – | S | Campylobacter spp. (24) | Amc, Ctx, Mer: 0.0% Amp: 16.7% Cfl: 98.0%; | Tet: 75.0% | Cip, Nal: 75.0% | – | Gen, Tob: 0.0% | Clo: 4.16% Ery: 0.0% | |
| Melo et al., 2019 [73] | 2011–2012; 2015–2016 | SE | poultry carcasses (1070) | C. jejuni (2011-2012/55) (2015-2016/44) | 2011–2012 Amc: 65.5% 2015-2016 Amc: 43.2% | 2011–2012 Tet: 74.5% 2015-2016 Tet:81.8% | - | - | 2011–2012 Gen: 14.5% 2015–2016 Gen: 2.3% | 2011–2012 Ery: 38.2% 2015–2016 Ery: 9.1% |
| Ref. | Sampling Period | Geographic Region a | Local (n) | Isolate (n) | Antimicrobial Resistanceb | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactam | Tetracycline | Quinolone | Sulfonamide | Aminoglycoside | Others | |||||
| Salmonella sp. | ||||||||||
| Kich et al., 2011 [81] | 2007 | S | various | 8 serotypes (572) | Amc: 1.0% Amp: 46.6% Cfl: 5.0% Cfo: 1.0% | Tet: 79.0% | Nal: 5.0% | Sul: 23.0% Sut: 10.0% | Gen: 39.0% Kn: 41.0% Str: 35.0% | Clo: 10.0% |
| Morales et al., 2012 [88] | – | – | swine herds | S. enterica (124) | – | – | – | – | – | Col: 21.0% |
| Lopes et al., 2015 [80] | 2008–2011 | S | slaughterhouses (1)/ intestinal content and carcasses | 28 serotypes (225) | Amp: 29.8% | Tet: 54.5% | Cip: 0.9% Nal: 33.3% | Sul: 39.6% Str: 33.7% Tri: 8.0% | Gen: 10.7% Kn: 14.7% | Clo: 14.2% |
| Almeida et al., 2016 [83] | 2000–2012 | S | various | S. Typhimurium (22) | Amp: 81.4% | Tet: 62,9% | Cip, Lev: 3.0% Nal: 59.0% | Sut: 66.6% | – | Clo: 74.0% |
| Souto et al., 2017 [84] | 2011–2014 | SE | fecal samples | Salmonella sp. (39) | Amo: 89.7% Amp: 82.0% Cfo: 2.6 % | Tet: 97.4% | Nal: 33.3% Nor: 2.6 % | Sut: 53.8% | Gen: 87.1% | – |
| Rau et al., 2018 [95] | 2011–2017 | S | animal products (40) | Salmonella sp. (40) | – | – | v | – | Col: 1 isolate (mcr-1 positive) | |
| Viana et al., 2019 [85] | – | – | pork production chain | 25 serotypes (280) | Amp: 81.0% Caz, Cfo: 4.8% | Tet: 88.1% | Cip: 50.0% | Sut: 19.0% | Gen: 16.7%Str: 90.5% | Clo: 71.4% |
| E. coli | ||||||||||
| Morales et al., 2012 [88] | – | – | swine herds | ETEC (126) | – | – | – | – | – | Col: 6.3% |
| Silva et al., 2016 [87] | 2012 | – | swine herds | E. coli (267) | Ctf: eight isolates (CTX-M-15-producing) | – | – | – | – | – |
| Kiefer et al., 2018 [89] | – | – | swine herd (126) | colistin-resistant E. coli (8) | – | – | – | – | – | Col: colistin-resistant E. coli |
| Spindola et al., 2018 [86] | – | SE | swine urine (300) | E. coli (186) | Amc: 1.1% Amp: 80.1% Cfo: 1.1% Ctf: 2.6% | Tet: 91.9% | Cip: 22.5% Eno: 33.3% Nal: 66.1% Nor: 21.5% | Sul: 94.6% Sut: 54.6% | Gen: 2.6% Spe: 11.2% Str: 52.6% | Flo: 83.3% |
| Yersinia enterocolitica | ||||||||||
| Ruzak et al., 2014 [92] | 2005–2011 | SE, NE, S | various | Y. enterocolitica (60) | Amp: 100% Cfl: 97.0% Cfo: 13.0% | Tet: 8.0% | – | Sul: 68.0% Sut: 10.0% Tri: 12.0% | Ami: 2.0% | - |
| Frazão et al., 2017 [93] | 1979–2012 | – | various | Y. enterocolitica (39) | Amc: 55.8% Cfo, Cfz: 100% Amp, Tic: 94.0% | – | – | – | – | - |
| Martins et al., 2018 [91] | – | SE | Pig farm (2/20 samples); slaughterhouse (1/960 samples | Y. enterocolitica (16) | Amo, Amp, Ipm: 100% | Tet: 12.5% | Nal: 100.0% | Sul: 100.0% | Gen: 37.5 Neo: 100% Str: 100% | |
| Ref. | Sampling period | Region a | Local (n) | Isolate b(n) | Antimicrobial Resistance c | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactam | Tetracycline | Quinolone | Sulfonamide | Aminoglycoside | Others | |||||
| Staphylococcus sp. | ||||||||||
| Ceotto et al., 2009 [106] | – | SE | dairy herd | S. aureus (46) ** | Amp: 67.4% Oxa: 0.0% Pen G: 65,2% | Tet: 41.3% | Cip:10.9% | – | Gen:15.2% | Cli: 13.1% Ery: 58.7% |
| Laport et al., 2012 [107] | 1995–2003 | SE | dairy herd (21) | CNS (49) *** | Oxa: 6.1% Pen: 51.0% | Tet: 14.3% | Cip: 2,0% | Sut: 10.2% | Gen: 2,0% | Cli: 12.2% Ery: 18.4% Rif: 0.0% |
| Costa et al., 2012 [114] | – | – | dairy herd (38) | S. aureus (352) ** | Amp: 81.4% Oxa: 2.0% Pen: 82.3% | Tet: 16.7% | Eno: 0.3% | Sut: 6.3% | Gen: 1.7% Neo: 3.4% | Clo: 1.7% Flo: 0.3% Lin: 7.9% Nit: 0.0% Nov: 1.4% |
| Silva et al., 2013 [117] | – | SE | dairy herd (11) | S. aureus (56)*** | Cfl, Oxa: 0.0% | Tet: 3.5% | Cip: 0.0% | Sut: 0.0% | Gen, Tob: 0.0% | Cli, Ery: 0.0% Clo: 3,5% |
| Silva et al., 2014 [132] | – | SE | dairy herd | CNS (128) *** | Cfl, Oxa: 20.3% | – | – | – | – | – |
| da Costa Krewer et al., 2015 [100] | – | NE | dairy herd (8) | S. aureus (126) ** oCPS (61) CNS (31) | Amp: 67.0% Amo: 67.4% Oxa: 1.8% Pen: 66.0% | Dox: 11.4% Tet: 17.4% | Cip: 0.9% Eno: 0.5% | Sut: 2.2% | Gen: 0.5% Str: 11.9% | Ery, Lin: 1.8% Rif: 0.0% |
| Castelani et al., 2014 [111] | 2009-2010 | SE | dairy herd (2) | S. aureus * (110: 83 from heifers and 27 from cows) | Heifers Amp: 14.5% Oxa: 0.0% Pen: 39.6% Cows Amp: 40.7% Oxa: 0.0% Pen: 62.9% | – | – | v | Heifers Gen, Kn: 0% Neo: 8.4% Cows Gen, Kn: 0% Neo: 7.4% | Flo: 0.0% |
| Fernandes dos Santos et al., 2016 [109] | 2008–2010 | NE, S, SE | dairy herd (48) | S. aureus (79) * 91 CNS (91) | S. aureus Oxa: 0.0% Pen: 30.4% CoNS Oxa: 47.0% Pen: 34.1%; | S. aureus Tet: 8.9% CNS Tet: 24.2% | S. aureus Eno MIC90 0.06-0.5 CNS Eno MIC90 0.06-32 | S. aureus Sul: 1.3% Sut: 0.0% CNS Sul: 4.4% Sut: 2.2% | S. aureus Gen: 0% CNS Gen: 6.6% | S. aureus Ery: 1.3% Cli MIC90 0.125 CNS Ery: 13.2% Cli MIC90 0.25 |
| Marques et al., 2017 [110] | 2012 | SE | dairy herd (3) | S. aureus (20) *** | Amo: 5.0% Amp: 25.0% Oxa: 0.0% Pen: 100% | Tet: 5.0% | Cip: 25.0% Eno, Moxi: 20.0% | Sut: 35.0% | Neo: 15.0% Str: 25.0% | Azi, Clo: 20.0% Ery: 10.0% Nov: 30.0% |
| Mello et al., 2017 [118] | – | 6 states | dairy herd | S. aureus (82) *** others (99) | Oxa: 18.2% (1 S. aureus) S. aureus MIC50 0.094 MIC90 0.25 Others MIC50 0.25 MIC90 1.50 | Van: 0.0% S. aureus MIC50 0.5 MIC90 1.0 Others MIC50 1.0 MIC90 1.5 hR: 7.1% (1 S. aureus) | ||||
| Guimarães et al., 2017 [119] | – | SE | dairy herd (1) | S. aureus (60) ** | MRSA: 23.3% OS-MRSA: 25.0% MSSA: 51.7% | |||||
| Haubert et al., 2017 [112] | – | S | dairy herd | S. aureus (31) ** | Amp: 52.0% Cef: 19.0% Oxa: 42.0% Pen: 48.0% | Tet: 39.0% | Eno: 6.0% | Sul: 65.0% | Str: 16.1% Tob: 29.0% | Cli: 52.0% Ery: 35.0% Tri: 0.0% |
| Martini et al., 2017 [108] | – | SE | dairy herd (10) | S. aureus (266) * | Amp: 66.5% Oxa: 0.0% Pen: 70.7% | Tet: 27.4% | ||||
| Freitas et al., 2018 [113] | – | S | dairy herd | S. aureus (27) *** CNS (3) | Amo: 50.0% Amp: 43.3% Pen: 70.0% | Tet: 96.7% | Eno: 43.3% Nor: 6.7% | Gen: 86.7% Neo: 96.7% | B: 43.3% Tri: 100% | |
| E. coli | ||||||||||
| Fernandes et al., 2017 [127] | 2014 | - | industry (beef jerky) (1)/ processing surfaces | 2 | Amc, Ctx, Ipm: 0% Amp, Cef: 50% | Tet: 50% | Cip: 50% (I) | Sut: 50% | Ami, Gen: 0% Str: 50% (I) | Clo, Nal: 50.0% Tri: 0.0% |
| Santos et al., 2018 [126] | 2015 | SE | slaughterhouse (1)/carcasses | 18 STEC | Amp, Cef, Caz, Imp: 0% | Tet: 0% | Cip 0% | Sut 0% | Gen, Str: 0% | Clo, Nal, Nit: 0.0% |
| Salmonella spp. | ||||||||||
| Cossi et al., 2013 [129] | v | MW | butcher shops (3)/environment, equipment and employee hands | 7 (cutting board surfaces) | Ctx: 0% Cfo: 29% Cef: 29%, 14% (I) Ipm: 14% | Min: 71%, 14% (I) Tet: 86% | – | Sul, Sut: 86% | Ami: 0% Kn: 14% Tob: 29%, 14% (I) | – |
| da Silva et al., 2014 [130] | 2009–2010 | S | Slaughterhouse (1)/carcasses (120) | 6 | Amp, Cef, Cfo, Ctx, Ipm: 0% | Tet: 0% | Cip: 0% | Sul, Sut: 0% | Ami, Gen, Kn, Str: 0% | Clo, Nal: 0% |
| Loiko et al., 2016 [128] | 2010–2012 | S | Slaughterhouse (1)/carcasses (108) | 1 | Amp, Cef, Cfo: 100% Ctx, Ipm: 0% | Tet: 0% | Cip: 0% | Sul, Sut: 0% | Ami, Gen, Kn, Str: 0% | Clo: 0% Nal: 100% (I) |
| Fernandes et al., 2017 [127] | 2014 | - | industry (beef jerky) (1)/environment and food | 1 (processing surfaces) 3 (raw material) | Amp, Amc, Cfo, Cef, Ctx, Ipm: 0% | Tet: 0% | Cip: 0% | Sut: 25% | Ami, Gen, Str: 0% | Clo, Nal, Tri: 0% |
| Listeria monocytogenes | ||||||||||
| Camargo et al., 2014 [133] | – | SE | slaughterhouse (2)/animals and carcasses (209) | 5 | Amp: 0% | Tet: 0% | – | – | Gen: 0% | Ery, V: 0% |
| Camargo et al., 2015 [131] | 1978–2013 | 11 states | – | 69 (from carcass and food-processing environments), 43 (from beef food) and 25 (from clinical cases) | Imp, Pen: 0% Oxa: 57%, 17% (I) | Tet: 0% | – | Sut: 0% | Gen: 0% | Clo, Ery, Rif, V: 0% Cli: 53%, 36% (I) |
| Loiko et al., 2016 [128] | 2010–2012 | S | slaughterhouse (1)/carcasses (108) | 7 | Amp, Ipm: 0% Cef: 82%, Cfo: 91% Ctx: 100% Ipm: 0% | Tet, Min: 0% | Cip: 0% | Sul: 55% Sut: 0% | Ami, Gen, Kn: 20–10% Tob: ~30% | Clo, Ery, Tri, V: 0% Nal: 100% |
| Reference | Bacterial Species a | Year of Samples Isolation | Region b | Antimicrobial Resistance Gene | ||||
|---|---|---|---|---|---|---|---|---|
| Beta-lactam | Tetracycline | MLSBc | Aminoglycoside | Others | ||||
| Laport et al., 2012 [107] | S. chromogenes, S. sciuri, S. xylosus | – | SE | mecA | – | – | – | – |
| Silva et al., 2013 [117] | S. aureus | – | SE | – | tet(K) | – | – | fexA |
| Silva et al., 2014b [132] | CNS, oCNP | – | – | blaZ, mecA (S. epidermidis, S. chromogenes, S. warneri, S. hyicus, S. simulans) | tet(K) (S. epidermidis, S. chromogenes, S. warneri) | ermC (S. epidermidis); lnuB, lsaE (S. chromogenes) | ant(4’)-Ia (S. epidermidis, S. chromogenes, S. warneri); aac(6’)-aph(2”) (S. epidermidis, S. warneri); aadE (S. chromogenes); str (S. hyicus, S. warneri, S. epidermidis) | gyrA, grlA (mutation) |
| da Costa Krewer et al., 2015 [100] | S. aureus | 2004–2008 | NE | blaZ, mecA | – | – | – | – |
| Fernandes dos Santos et al., 2016 [109] | S. epidermidis | 2008–2010 | SE, S, NE | mecA | – | – | – | - |
| Martini et al., 2017 [108] | S. aureus | – | SE | blaZ | tet(K), tet(L), tet(M), tet(O) | – | – | – |
| Guimarães et al., 2017 [119] | S. aureus | – | SE | mecA | – | – | – | – |
| Haubert et al., 2017 [112] | S. aureus | – | S | blaZ | tet(B), tet(K), (tet)L, (tet)M | ermB, ermC, ereB | strA, strB | dfrA, dfrG |
| Marques et al., 2017 [110] | S. aureus | – | SE | blaZ, mecA | – | – | – | – |
| Mello et al., 2017 [118] | S. aureus, S. chromogenes, S. S. epidermidis, S. haemolyticus, S. saprophyticus, S. simulans, S. xylosus, S. warneri | – | 6 states | mecA | – | – | – | – |
| Ref. | Year of Isolation | Source | Country/ Regiona | Bacterial Species | β-Lactamase Genes or Group of Genes Found (bla) |
|---|---|---|---|---|---|
| Mattiello et al., 2015 [181] | 2002–2012 | Poultry producing environment and by-product meals | Brazil/SE | Salmonella Schwarzengrund, Salmonella enterica, Salmonella Infantis, Salmonella Senftenberg, Salmonella Montevideo, Salmonella Cerro, Salmonella Worthington, Salmonella Heidelberg | TEM, CTX-M, CMY |
| Fernandes et al., 2009 [155] | 2004 | Poultry | Brazil/SE | Salmonella Typhimurium | CTX-M-2 |
| Fitch et al., 2016 [141] | 2004–2011 | Poultry during slaughter | Brazil/MW, S | Salmonella Agona, Salmonella Brackenrindge, Salmonella Emek, Salmonella Enteritidis, Salmonella Gaminara, Salmonella Give, Salmonella GroupIII, Salmonella Hadar, Salmonella Heidelberg, S. Infantis, Samonella Minnesota, Salmonella Newport, Salmonella Panama, Salmonella Poona, Salmonella Rissen, Salmonella Saintpaul, Salmonella Schwarzengrund/, Bredeney, and S. Typhimurium, Salmonella Weslaco | TEM, CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-14, CMY-2 |
| Moura et al., 2018 [138] | 2008–2015 | Chicken and turkey meat; swine feces | Brazil/MW, SE, S | S. Agona, S. Typhimurium, S. Minnesota, S. Heidelberg, S. Infantis | CTX-M-2, CTX-M-8, CMY-2 |
| Penha Filho et al., 2019 [36] | 2009–2012 | Poultry at farms | Brazil/MW, SE | S. Schwarzengrund, S. Newport, S. Heidelberg | CTX-M-2, CMY-2 |
| Moura et al., 2017 [156] | 2010 | Chicken meat | Brazil/MW | S. Minnesota | CMY-2 |
| Botelho et al., 2015 [140] | 2010–2011 | Chicken carcasses (frozen) | Brazil/SE | Escherichia. coli | CTX-M-1, CTX-M-2, CTX-M-8, CMY-2 |
| Ferreira et al., 2014, 2016, 2017; Galetti, 2019 [137,139,143,178] | 2011–2012 | Poultry cloacal swabs | Brazil/SE | E. coli, E. fergusonii, K. pneumoniae | CTX-M-2, CTX-M-8, CTX-M-15, CMY-2 |
| Casella et al., 2015 [136] | 2011, 2013 | Chicken meat | Brazil/SE | Proteus mirabilis, Citrobacter diversus, Klebsiella pneumoniae, and E. coli | TEM, SHV, CTX-M-2, CTX-M-8 |
| Fernandes et al., 2017 [127] | 2012 | Poultry meat | Brazil/SE | Salmonella Muenchen, S.Typhimurium, Salmonella Corvallis | CTX-M-2, CTX-M-8 |
| Ibbe et al., 2017 [142] | 2012–2013 | Chicken (live and carcasses) | Brazil/MW, S | S. Minnesota, S. Hilderberg | CTX-M-8, ACC-1, CMY-2 |
| Koga et al., 2015a,b [69,148] | 2013 | Chicken carcasses (refrigerated) | Brazil/S | E. coli | SHV, CTX-M-1, CTX-M-2, CTX-M-8, CIT |
| Cyoia et al., 2019 [149] | 2013–2014 | Chicken carcasses | Brazil/S | E. coli | TEM, SHV, CTX-M-1, CTX-M-2, CTX-M-8 |
| Cunha et al., 2017 [147] | 2013–2016 | Poultry cloacal swabs | Brazil/S | E. coli | TEM, CTX-M-2, CTX-M-55, CMY-2 |
| Casella et al., 2018 [146] | 2014 | Chicken meat and cloacal swabs | Brazil/SE | E. coli | CTX-M-2, CTX-M-8, CTX-M-15, CTX-M-55, CMY-2 |
| Hoepers et al., 2018 [153] | 2014–2015 | Turkeys with clinical signs | Brazil/ MW, SE, S | E. coli | TEM, CTX-M-2, CTX-M-8, CMY-2 |
| Tiba Casas et al., 2019 [144] | 2014–2016 | Poultry and poultry meat | Brazil/SE | S. Heidelberg | CMY-2 |
| Zogg et al., 2016, [150] | 2015 | Chicken carcasses (frozen) | Swiss | E. coli | CTX-M-2, CTX-M-8 |
| Nahar et al., 2018 [151] | 2015 | Chicken meat | Japan | E. coli | TEM, CTX-M-1, CTX-M-2, CTX-M-8 |
| Brisola et al., 2019 [179] | 2016-2017 | Swine feces | Brazil/SC | E. coli | TEM, CMY-2 |
| Kim et al., 2018 [152] | n.d. | Chicken meat | South Korea | E. coli | TEM, CTX-M-2, CTX-M-94, OXA-1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabello, R.F.; Bonelli, R.R.; Penna, B.A.; Albuquerque, J.P.; Souza, R.M.; Cerqueira, A.M.F. Antimicrobial Resistance in Farm Animals in Brazil: An Update Overview. Animals 2020, 10, 552. https://doi.org/10.3390/ani10040552
Rabello RF, Bonelli RR, Penna BA, Albuquerque JP, Souza RM, Cerqueira AMF. Antimicrobial Resistance in Farm Animals in Brazil: An Update Overview. Animals. 2020; 10(4):552. https://doi.org/10.3390/ani10040552
Chicago/Turabian StyleRabello, Renata F., Raquel R. Bonelli, Bruno A. Penna, Julia P. Albuquerque, Rossiane M. Souza, and Aloysio M. F. Cerqueira. 2020. "Antimicrobial Resistance in Farm Animals in Brazil: An Update Overview" Animals 10, no. 4: 552. https://doi.org/10.3390/ani10040552
APA StyleRabello, R. F., Bonelli, R. R., Penna, B. A., Albuquerque, J. P., Souza, R. M., & Cerqueira, A. M. F. (2020). Antimicrobial Resistance in Farm Animals in Brazil: An Update Overview. Animals, 10(4), 552. https://doi.org/10.3390/ani10040552






