A Novel Therapeutic Reagent, KA-1002 for Alleviating Lysophosphatidic Acid-Mediated Inflammation Related Gene Expression in Swine Macrophages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
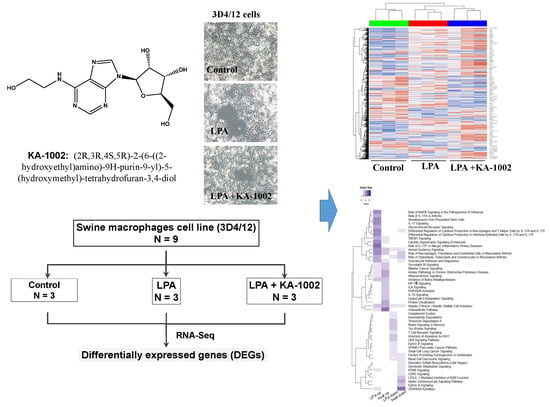
2.1. Drugs and Chemicals
2.2. Cell Culture
2.3. Sample Preparation form Illumina NovaSeq Sequencing
2.4. Genome Mapping and Identification of Paired-End Sequences
2.5. Gene Set Enrichment Test and Heat Map of Representative Gene Sets
2.6. Comparative Network Analysis
2.7. Statistical Analysis
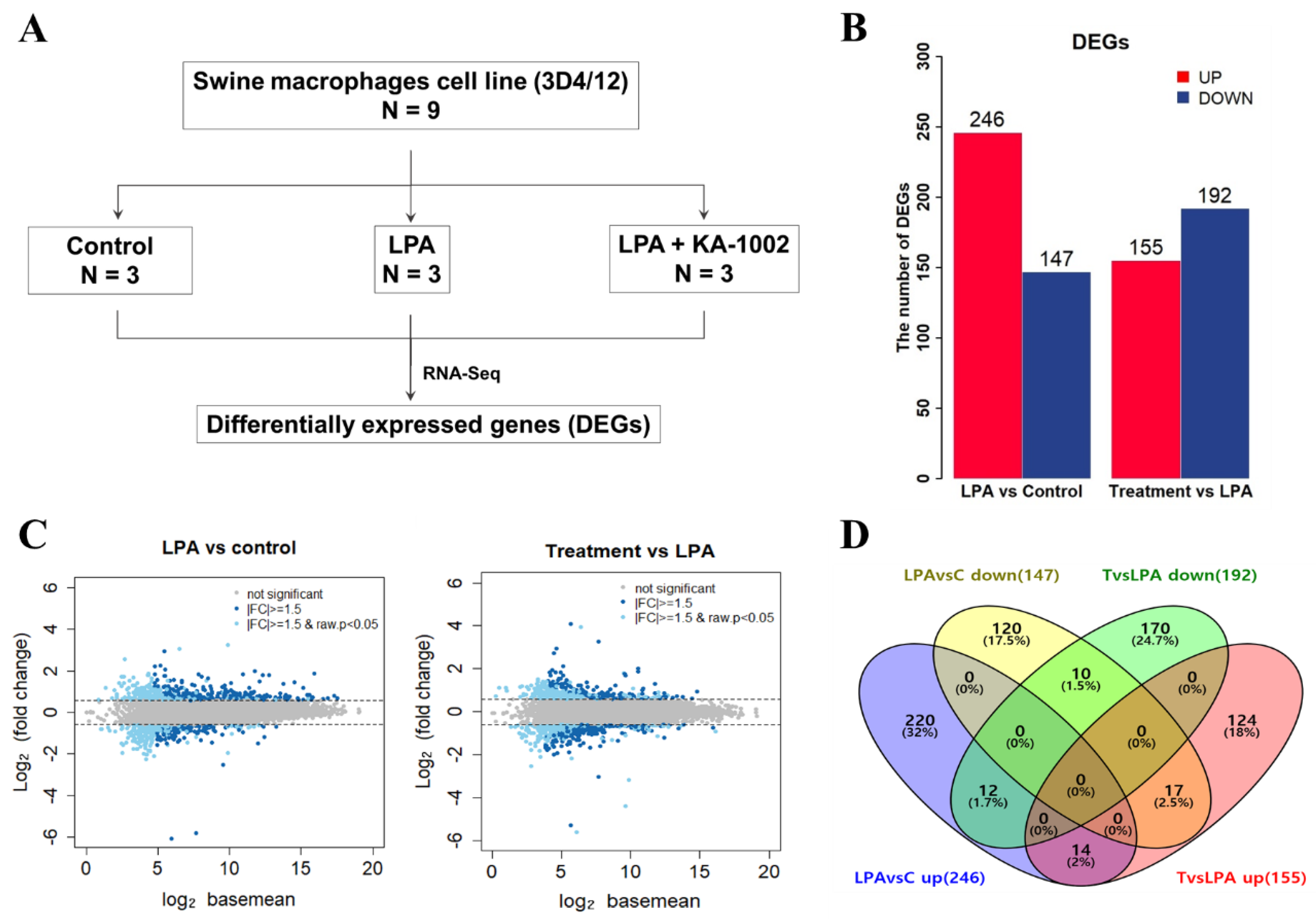
3. Results
3.1. The LPA-Antagonistic Novel Compound KA-1002 Differentially Regulates the Gene Expression Profiles of LPA Treated Swine Macrophage Cells
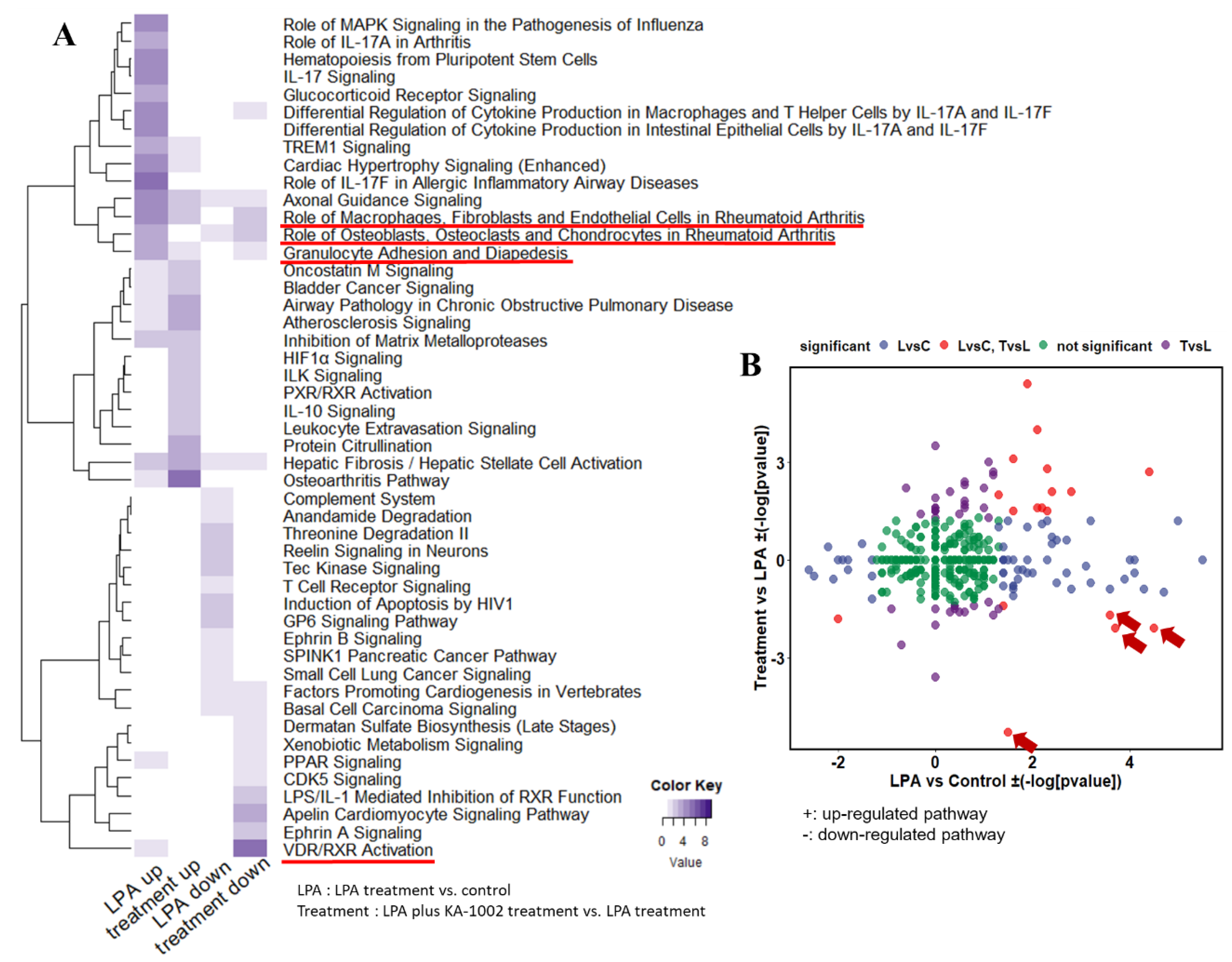
3.2. Gene Set Analysis among Control, LPA-Treated, and LPA plus KA-1002 Treated Swine Macrophages
3.3. Differential Biological Pathways among Control, LPA-Treated, and LPA plus KA-1002 Treated Swine Macrophages
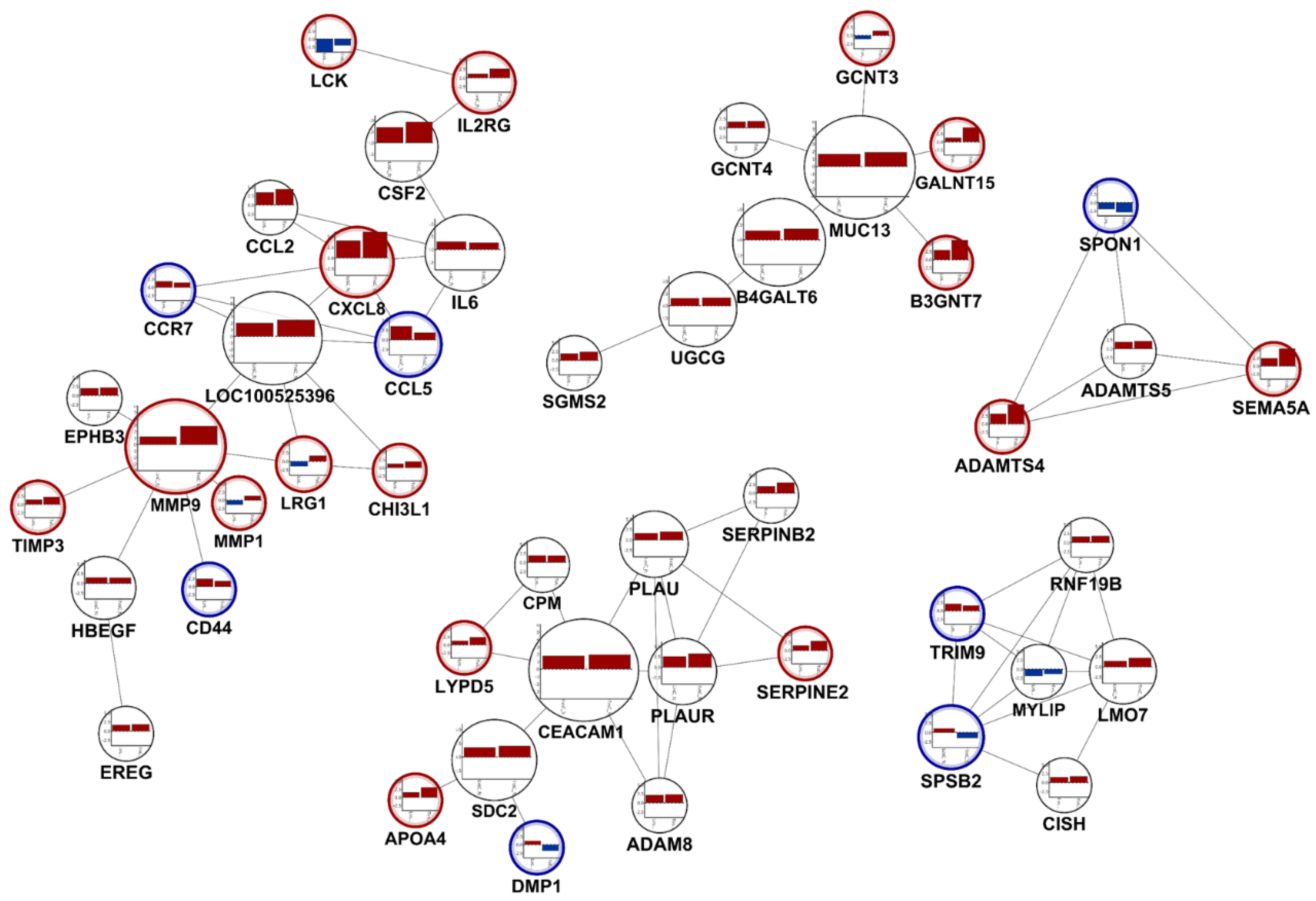
3.4. Selecting Distinguished Genes between LPA-Treated, and LPA plus KA-1002 Treated Swine Macrophages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Snowder, G.D.; Van Vleck, L.D.; Cundiff, L.V.; Bennett, G.L. Bovine respiratory disease in feedlot cattle: Environmental, genetic, and economic factors. J. Anim. Sci. 2006, 84, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, S.A. Epidemiology of bovine respiratory disease (BRD) in preweaned calves on California dairies: The BRD 10K study. J. Dairy Sci. 2019, 102, 7306–7319. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D. Economic impact associated with respiratory disease in beef cattle. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 367–377. [Google Scholar] [CrossRef]
- Seo, H.; Kim, M.; Choi, Y.; Lee, C.K.; Ka, H. Analysis of lysophosphatidic acid (LPA) receptor and LPA-induced endometrial prostaglandin-endoperoxide synthase 2 expression in the porcine uterus. Endocrinology 2008, 149, 6166–6175. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A.; Lehmkuhl, H.D.; Cutlip, R.C. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J. Biol. Chem. 2003, 278, 38875–38883. [Google Scholar]
- Opriessnig, T.; Giménez-Lirola, L.G.; Halbur, P.G. Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 2011, 12, 133–148. [Google Scholar] [CrossRef]
- Chu, X.; Wei, X.; Lu, S.; He, P. Autotoxin-LPA receptor axis in the pathogenesis of lung diseases. Int. J. Clin. Exp. Med. 2015, 8, 17117–17122. [Google Scholar]
- Zhao, Y.; Natarajan, V. Lysophosphatidic acid (LPA) and its receptors: Role in airway inflammation and remodeling. Biochim. Biophys. Acta. 2013, 192, 851–857. [Google Scholar] [CrossRef]
- Valdés-Rives, S.A.; González-Arenas, A. Autotaxin-Lysophosphatidic Acid: From Inflammation to Cancer Development. Mediat. Inflamm. 2017, 2017, 9173090. [Google Scholar] [CrossRef]
- Kim, S.J.; Moon, H.G.; Park, G.Y. The roles of autotaxin/lysophosphatidic acid in immune regulation and asthma. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 2020, 1865, 158641. [Google Scholar] [CrossRef]
- Plastira, I.; Joshi, L.; Bernhart, E.; Schoene, J.; Specker, E.; Nazare, M.; Sattler, W. Small-Molecule Lysophosphatidic Acid Receptor 5 (LPAR5) Antagonists: Versatile Pharmacological Tools to Regulate Inflammatory Signaling in BV-2 Microglia Cells. Front. Cell. Neurosci. 2019, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Herr, D.; Mutoh, T.; Chun, J. Lysophosphatidic acid (LPA) and its receptors. Curr. Opin. Pharmacol. 2009, 9, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Kim, M.; Kim, K.S.; Min, Y.K.; Lee, C.H. KA-1002, a Novel Lysophosphatidic Acid Signaling Antagonist, Alleviates Bovine Tracheal Cell Disruption and Inflammation. Animals 2020, 10, 295. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, S.; Georas, S.N. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J. Immunol. 2014, 192, 851–857. [Google Scholar] [CrossRef]
- Sengupta, S.; Wang, Z.; Tipps, R.; Xu, Y. Biology of LPA in health and disease. Semin. Cell Dev. Biol. 2004, 15, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Boruszewska, D.; Sinderewicz, E.; Kowalczyk-Zieba, I.; Skarzynski, D.J.; Woclawek-Potocka, I. Influence of lysophosphatidic acid on estradiol production and follicle stimulating hormone action in bovine granulosa cells. Reprod. Biol. 2013, 13, 344–347. [Google Scholar] [CrossRef]
- Panupinthu, N.; Lee, H.Y.; Mills, G.B. Lysophosphatidic acid production and action: Critical new players in breast cancer initiation and progression. Br. J. Cancer 2010, 102, 941–946. [Google Scholar] [CrossRef]
- Mathew, D.; Kremer, K.N.; Strauch, P.; Tigyi, G.; Pelanda, R.; Torres, R.M. LPA5 Is an Inhibitory Receptor That Suppresses CD8 T-Cell Cytotoxic Function via Disruption of Early TCR Signaling. Front. Immunol. 2019, 10, 1159. [Google Scholar] [CrossRef]
- Hul, W.; Zhao, C.; Bourgoin, S.G. LPA promotes T cell recruitment through synthesis of CXCL13. Mediat. Inflamm. 2015, 2015, 248491. [Google Scholar]
- Knowlden, S.A.; Capece, T.; Popovic, M.; Chapman, T.J.; Pezaee, F.; Kim, M.; Georas, S.N. Regulation of T cell motility in vitro and in vivo by LPA and LPA2. PLoS ONE 2014, 9, e101655. [Google Scholar] [CrossRef]
- Gustin, C.; Van Steenbrugge, M.; Raes, M. LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. Am. J. Physiol. Cell Physiol. 2008, 295, C905–C914. [Google Scholar] [CrossRef] [PubMed]
- Hosogaya, S.; Yatomi, Y.; Nakamura, K.; Ohkawa, R.; Okubo, S.; Yokota, H.; Ohta, M.; Yamazaki, H.; Koike, T.; Ozaki, Y. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: Strong correlation with lysophospholipase D activity. Ann. Clin. Biochem. 2008, 45, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Arima, N.; Ohgami, M.; Aoki, J. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr. Med. Chem. 2008, 15, 2122–2131. [Google Scholar] [CrossRef]
- Torres, C.; Boruszewska, D.; Batista, M.; Kowalczyk-Zieba, I.; Diniz, P.; Sinderewicz, E.; Saulnier-Blache, J.S.; Woclawek-Potocka, I.; Lopes-da-Costa, L. Lysophosphatidic acid signaling in late cleavage and blastocyst stage bovine embryos. Mediat. Inflamm. 2014, 2014, 678968. [Google Scholar] [CrossRef]
- Woclawek-Potocka, I. Lysophosphatitic acid action during early pregnancy in the cow: In vivo and in vitro studies. J. Reprod. Dev. 2010, 56, 411–420. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y.; Zhang, Q.; Li, X.; Zhou, J.; Wang, J.; Wei, L. ATX-LPA axis facilitates estrogen-induced endometrial cancer cell proliferation via MAPK/ERK signaling pathway. Mol. Med. Rep. 2019, 17, 4245–4252. [Google Scholar] [CrossRef]
- Wang, H.; Tu, S.; Yang, S.; Shen, P.; Huang, Y.; Ba, X.; Lin, W.; Huang, Y.; Wang, Y.; Qin, K.; et al. Berberine modulates LPA function to inhibit the proliferation and inflammation of FLS-RA via p38/ERK MAPK pathway mediated by LPA1. Evid. Based Complement Alternat. Med. 2019, 2019, 2580207. [Google Scholar] [CrossRef]
- Minciullo, P.L. Inflammaging and Anti-inflammating: The role of cytokines in extreme longevity. Arch. Immunol. Ther. Exp. 2016, 64, 1110126. [Google Scholar] [CrossRef]
| Gene Symbol | Gene Description | Fold-Change | Adjust p |
|---|---|---|---|
| NYAP2 | Neuronal tyrosine-phosphorylated phosphoinositide-3-kinase adaptor 2 | 7.57 | 2.5 × 10−4 |
| TMEM35A | Transmembrane protein 35A | 4.72 | 4.4 × 10−4 |
| LOC110255306 | N-acetyllactosaminide beta-1,6-N-acetylglucosaminyl-transferase-like | 4.69 | 3.3 × 10−3 |
| TIAM2 | T-cell lymphoma invasion and metastasis 2 | 4.16 | 3.2 × 10−3 |
| CXCL8 | C-X-C motif chemokine ligand 8 | 3.97 | 1.1 × 10−3 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | 3.62 | 5.6 × 10−4 |
| CCL5 | C-C motif chemokine ligand 5 | 3.53 | 3.8 × 10−5 |
| LOC110259478 | Uncharacterized LOC110259478 | 3.52 | 3.0 × 10−7 |
| CCL2 | Chemokine (C-C motif) ligand 2 | 3.47 | 2.6 × 10−4 |
| CSF2 | Colony stimulating factor 2 | 3.42 | 1.9 × 10−4 |
| LOC100515579 | Hydroxyacyl-thioester dehydratase type 2, mitochondrial | −55.60 | 1.0 × 10−2 |
| ADAP2 | ArfGAP with dual PH domains 2 | −2.93 | 4.4 × 10−2 |
| RTP4 | Receptor transporter protein 4 | −2.83 | 5.0 × 10−2 |
| NRARP | NOTCH-regulated ankyrin repeat protein | −2.75 | 4.0 × 10−5 |
| FAM110D | Family with sequence similarity 110 member D | −2.65 | 4.2 × 10−3 |
| MGP | Matrix Gla protein | −2.55 | 3.0 × 10−3 |
| AK5 | Adenylate kinase 5 | −2.51 | 2.5 × 10−3 |
| SLC34A1 | Solute carrier family 34 member 1 | −2.49 | 1.6 × 10−2 |
| KAZN | Kazrin, periplakin interacting protein | −2.38 | 3.7 × 10−2 |
| SMTNL2 | Smoothelin like 2 | −2.35 | 8.1 × 10−3 |
| Gene Symbol | Gene Description | Fold-Change | Adjust p |
|---|---|---|---|
| TGM3 | Transglutaminase 3 | 17.06 | 1.2 × 10−5 |
| RIPOR2 | RHO family interacting cell polarization regulator 2 | 7.63 | 2.6 × 10−3 |
| PTPRU | Protein tyrosine phosphatase, receptor type U | 4.36 | 2.5 × 10−2 |
| ARG1 | Arginase 1 | 4.00 | 7.8× 10−3 |
| GABRP | Gamma-aminobutyric acid type A receptor pi subunit | 3.39 | 3.6× 10−11 |
| FAM198B | Family with sequence similarity 198 member B | 2.59 | 3.3 × 10−2 |
| GPR157 | G protein-coupled receptor 157 | 2.33 | 4.5 × 10−2 |
| LRG1 | Leucine rich alpha-2-glycoprotein 1 | 2.32 | 4.2 × 10−2 |
| ESM1 | Endothelial cell specific molecule 1 | 2.25 | 9.9× 10−3 |
| SLC4A4 | Solute carrier family 4 member 4 | 2.08 | 2.5× 10−3 |
| HABP2 | Hyaluronan binding protein 2 | −3.15 | 2.1× 10−4 |
| CA8 | Carbonic anhydrase 8 | −3.06 | 3.7× 10−3 |
| POU2AF1 | POU class 2 associating factor 1 | −2.97 | 2.1 × 10−2 |
| PTPRR | Protein tyrosine phosphatase, receptor type R | −2.82 | 7.7× 10−3 |
| LOC100156231 | Gametogenetin-binding protein 1-like | −2.62 | 3.3 × 10−2 |
| CYP24A1 | Cytochrome P450, family 24, subfamily A, polypeptide 1 | −2.60 | 5.0× 10−5 |
| LOC100515166 | Keratin, type I cytoskeletal 13 | −2.52 | 2.5× 10−3 |
| LRRC25 | Leucine rich repeat containing 25 | −2.48 | 3.6 × 10−2 |
| ENPP4 | Ectonucleotide pyrophosphatase/phosphodiesterase 4 | −1.82 | 3.6 × 10−2 |
| PRR15L | Proline rich 15 like | −1.81 | 2.0 × 10−3 |
| Pathway | −log(p-value) | Number of Gene | Related Genes | |
|---|---|---|---|---|
| LPA vs. Control (Upregulated) | Treatment vs. LPA (Downregulated) | |||
| Role of Macrophages, Fibroblasts and Endothelial Cells in Rheumatoid Arthritis | 4.5 | −2.1 | 21 | HNF1A, RRAS2, ADAMTS4, DKK3, IL18R1, SOCS3, WNT9A, CXCL8, IL1RL1, CCL2, TRAF1, NGFR, PRKCE, MMP1, CSF2, IL6, CCL5, PGF, IL17RC, VEGFC, WNT11 |
| Role of Osteoblasts, Osteoclasts and Chondrocytes in Rheumatoid Arthritis | 3.7 | −2.1 | 15 | BMP7, DKK3, IL18R1, WNT9A, IL11, HNF1A, BCL2, MMP1, ADAMTS5, WNT11, IL1RL1, CSF2, IL6, NGFR, ADAMTS4 |
| Granulocyte Adhesion and Diapedesis | 3.6 | −1.7 | 13 | CCL5, CLDN7, SDC2, CLDN6, CXCL8, MMP1, ITGA1, MMP7, IL1RL1, CCL2, NGFR, PECAM1, MMP9 |
| VDR/RXR Activation | 1.5 | −5.3 | 10 | HES1, CCL5, HR, PRKCE, CYP24A1, COL13A1, KLF4, IL1RL1, MED1, CSF2 |
| STAT3 Pathway | 1.4 | −1.4 | 11 | IL18R1, SOCS3, IL2RG, TGFBR3, CISH, IL17RC, BCL2, IL1RL1, RRAS2, NGFR, IL10RA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-J.; Park, T.; Kim, M.; Shin, H.-s.; Hwang, W.; Min, Y.K.; Song, S.-g.; Park, D.; Lee, C.H. A Novel Therapeutic Reagent, KA-1002 for Alleviating Lysophosphatidic Acid-Mediated Inflammation Related Gene Expression in Swine Macrophages. Animals 2020, 10, 534. https://doi.org/10.3390/ani10030534
Hwang H-J, Park T, Kim M, Shin H-s, Hwang W, Min YK, Song S-g, Park D, Lee CH. A Novel Therapeutic Reagent, KA-1002 for Alleviating Lysophosphatidic Acid-Mediated Inflammation Related Gene Expression in Swine Macrophages. Animals. 2020; 10(3):534. https://doi.org/10.3390/ani10030534
Chicago/Turabian StyleHwang, Hyeon-Jeong, Tamina Park, Miok Kim, Hee-su Shin, Wooyeon Hwang, Yong Ki Min, Suk-gil Song, Daeui Park, and Chang Hoon Lee. 2020. "A Novel Therapeutic Reagent, KA-1002 for Alleviating Lysophosphatidic Acid-Mediated Inflammation Related Gene Expression in Swine Macrophages" Animals 10, no. 3: 534. https://doi.org/10.3390/ani10030534
APA StyleHwang, H.-J., Park, T., Kim, M., Shin, H.-s., Hwang, W., Min, Y. K., Song, S.-g., Park, D., & Lee, C. H. (2020). A Novel Therapeutic Reagent, KA-1002 for Alleviating Lysophosphatidic Acid-Mediated Inflammation Related Gene Expression in Swine Macrophages. Animals, 10(3), 534. https://doi.org/10.3390/ani10030534