Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Trial and Tissue Collection
2.3. RNA Isolation
2.4. Quantitative Reverse Transcription PCR (RT-qPCR)
2.5. Relative Quantification of Gene Expression and Statistical Analysis
3. Results
3.1. Effects of in ovo Treatment and Thermal Challenge on Gene Expression
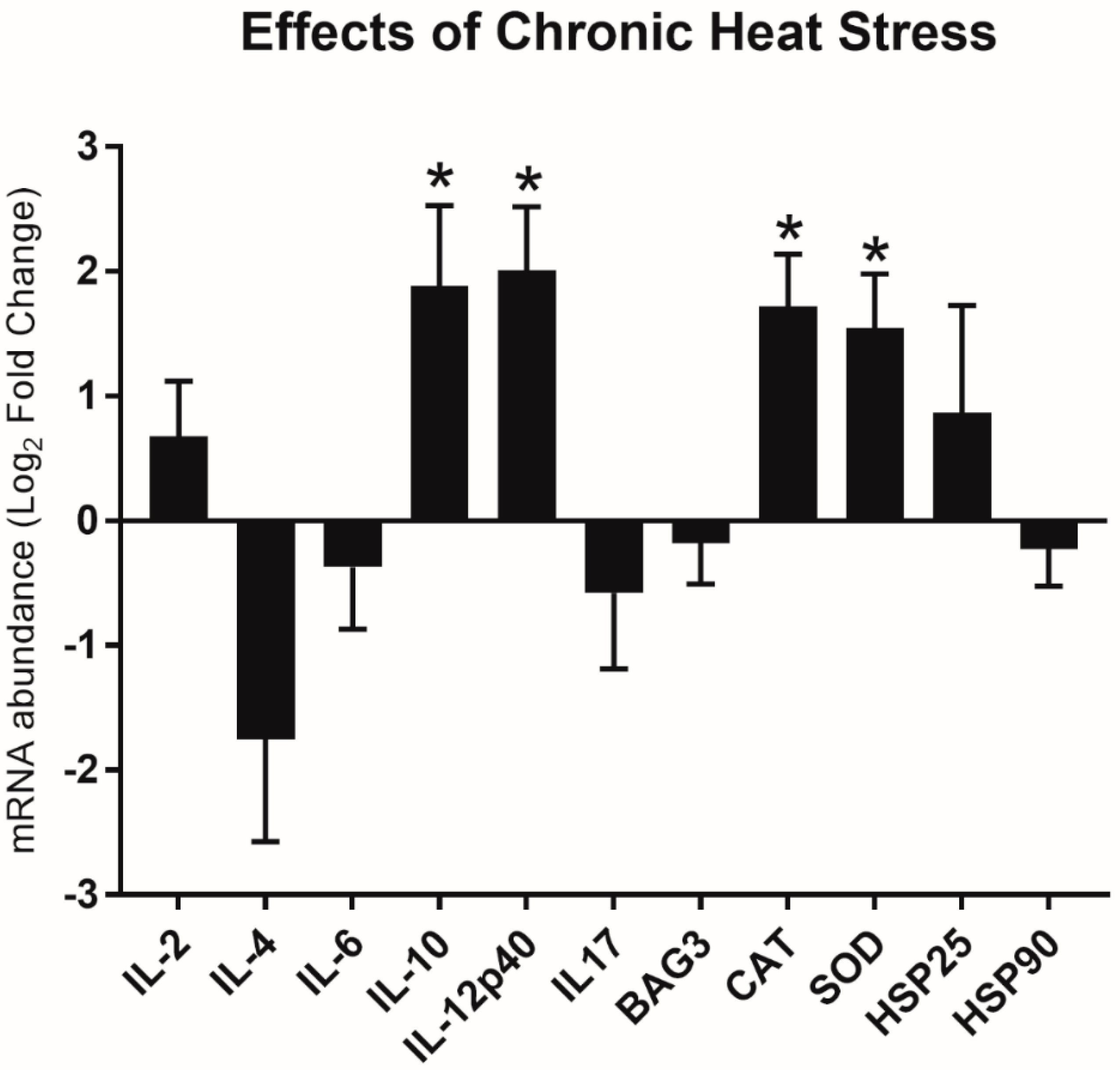
3.2. Relative Gene Expression Changes in Heat Stress
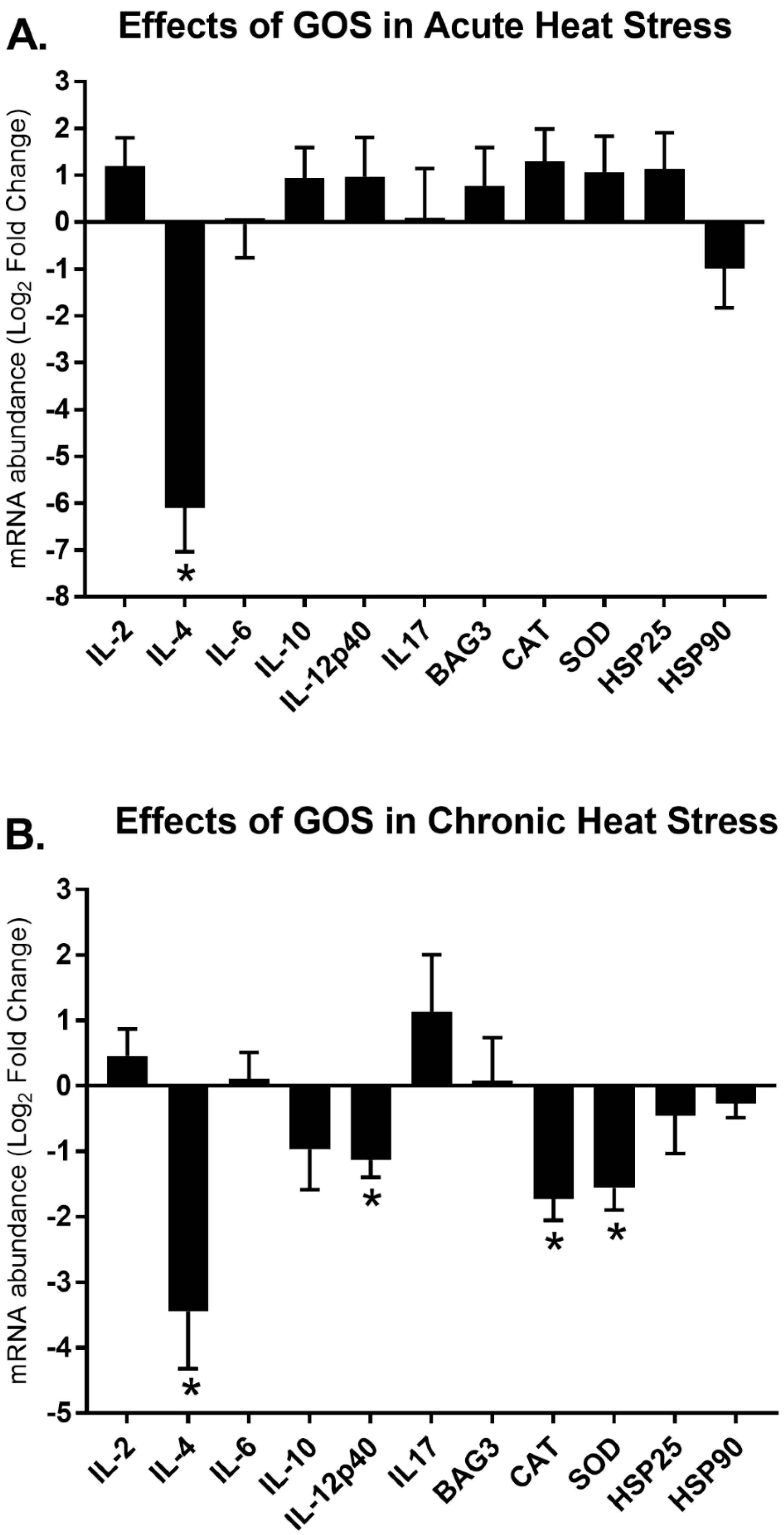
3.3. Effects of GOS Delivered in ovo on Gene Expression Modulation during Acute and Chronic Heat Stress
4. Discussion
4.1. Immune-related Gene Expression Signatures
4.2. Stress-related Gene Expression Signatures
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roushdy, E.M.; Zaglool, A.W.; El-Tarabany, M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018, 74, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Suk, Y.O.; Washburn, K.W. Effects of environment on growth, efficiency of feed utilization, carcass fatness, and their association. Poult. Sci. 1995, 74, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Reay, D.; Reay, D. Climate-Smart Chicken. In Climate-Smart Food; Springer International Publishing: New York City, NY, USA, 2019; pp. 107–120. [Google Scholar]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef]
- Washburn, K.W.; Peavey, R.; Renwick, G.M. Relationship of strain variation and feed restriction to variation in blood pressure and response to heat stress. Poult. Sci. 1980, 59, 2586–2588. [Google Scholar] [CrossRef]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef]
- Sohail, M.U.; Hume, M.E.; Byrd, J.A.; Nisbet, D.J.; Ijaz, A.; Sohail, A.; Shabbir, M.Z.; Rehman, H. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012, 91, 2235–2240. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Liu, F.Z.; Yan, Q.L.; Li, W.C. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult. Sci. 2009, 88, 2101–2107. [Google Scholar] [CrossRef]
- Deeb, N.; Cahaner, A. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult. Sci. 2002, 81, 293–301. [Google Scholar] [CrossRef]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Nienabar, J.A.; Hahn, G.L. Livestock production system management responses to thermal challenges. Int. J. Biometeorol. 2007, 52, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Nyoni, N.M.B.; Grab, S.; Archer, E.R.M. Heat stress and chickens: Climate risk effects on rural poultry farming in low-income countries. Clim. Dev. 2019, 11, 83–90. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; p. 1535. [Google Scholar]
- Taylor, N.A.S. Human heat adaptation. Compr. Physiol. 2014, 4, 325–365. [Google Scholar]
- Song, J.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H.; Diao, Q.Y.; Shi, B.; Zou, X.T. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult. Sci. 2014, 93, 581–588. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Garcia, A.A.P.; Farias Filho, R.V.; Souza, T.M.; De Oliveira, E.R.; De Oliveira, E.B.; Do Nascimento, C.S.; Meneghetti, C.; Wenceslau, A.A. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS ONE 2017, 12, e0186083. [Google Scholar] [CrossRef]
- Duangjinda, M.; Tunim, S.; Duangdaen, C.; Boonkum, W. Hsp70 genotypes and heat tolerance of commercial and native chickens reared in hot and humid conditions. Rev. Bras. Cienc. Avic. 2017, 19, 7–18. [Google Scholar] [CrossRef]
- Horst, P. Native fowl as reservoir for genomes and major genes with direct and indirect effects on the adaptability and their potential for tropically oriented breeding plans. Arch. Anim. Breed 1989, 53, 93–101. [Google Scholar]
- Galal, A.; Radwan, L.M.; Rezik, H.H.; Ayoub, H. Expression levels of HSP70 and CPT-1 in three local breeds of chickens reared under normal or heat stress conditions after the introduction of the naked neck gene. J. Therm. Biol. 2019, 80, 113–118. [Google Scholar] [CrossRef]
- Aengwanich, W. Comparative ability to tolerate heat between Thai indigenous chickens, Thai indigenous chickens crossbred and broilers by using heterophil/lymphocyte ratio. Pakistan J. Biol. Sci. 2007, 10, 1840–1844. [Google Scholar]
- Suzuki, K.; Harasawa, R.; Yoshitake, Y.; Mitsuoka, T. Effects of crowding and heat stress on intestinal flora, body weight gain, and feed efficiency of growing rats and chicks. Nippon Juigaku Zasshi. Jpn. J. Vet. Sci. 1983, 45, 331–338. [Google Scholar] [CrossRef] [PubMed]
- He, J.; He, Y.; Pan, D.; Cao, J.; Sun, Y.; Zeng, X. Associations of gut microbiota with heat stress-induced changes of growth, fat deposition, intestinal morphology, and antioxidant capacity in ducks. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G., Jr.; Tellez-Isaias, G.; Dridi, S. Heat Stress and Gut Health in Broilers: Role of Tight Junction Proteins. Adv. Food Technol. Nutr. Sci. 2017, 3, e1–e4. [Google Scholar] [CrossRef]
- Wang, W.C.; Yan, F.F.; Hu, J.Y.; Amen, O.A.; Cheng, H.W. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J. Anim. Sci. 2018, 96, 1654–1666. [Google Scholar] [CrossRef]
- Sohail, M.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Zaneb, H.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, I.; Abdulllah, N.; Azrin, N.M.; Ho, Y.W. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br. Poult. Sci. 2000, 41, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef]
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298. [Google Scholar] [CrossRef]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics - In ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018, 14. [Google Scholar] [CrossRef]
- Slawinska, A.; Dunislawska, A.; Plowiec, A.; Radomska, M.; Lachmanska, J.; Siwek, M.; Tavaniello, S.; Maiorano, G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery In Ovo. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for Broiler Chickens—In Vitro Design and Evaluation of the Influence on Host and Selected Microbiota Populations following In Ovo Delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, M.; Stadnicka, K.; Kozłowska, I.; Abiuso, C.; Tavaniello, S.; Dankowiakowska, A.; Sławińska, A.; Maiorano, G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal 2016, 10, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Sławinska, A.; Siwek, M.; Zylinska, J.; Bardowski, J.; Brzezinska, J.; Gulewicz, K.A.; Nowak, M.; Urbanowski, M.; Płowiec, A.; Bednarczyk, M. Influence of synbiotics delivered in ovo on immune organs development and structure. Folia Biol. 2014, 62, 277–285. [Google Scholar] [CrossRef]
- Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Łukaszewicz, E.; Kowalczyk, A.; Sieńczyk, M.; Bednarczyk, M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019, 15, 105. [Google Scholar] [CrossRef]
- Bogucka, J.; Dankowiakowska, A.; Elminowska-Wenda, G.; Sobolewska, A.; Szczerba, A.; Bednarczyk, M. Effects of prebiotics and synbiotics delivered in ovo on broiler small intestine histomorphology during the first days after hatching. Folia Biol. 2016, 64, 131–143. [Google Scholar] [CrossRef]
- Hughes, R.A.; Ali, R.A.; Mendoza, M.A.; Hassan, H.M.; Koci, M.D. Impact of dietary galacto-oligosaccharide (GOS) on chicken’s gut microbiota, mucosal gene expression, and Salmonella colonization. Front. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Slawinska, A.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Bertocchi, M.; Tavaniello, S.; Maiorano, G. Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult. Sci. 2020, 99, 407–415. [Google Scholar] [CrossRef]
- Slawinska, A.; Plowiec, A.; Siwek, M.; Jaroszewski, M.; Bednarczyk, M. Long-Term Transcriptomic Effects of Prebiotics and Synbiotics Delivered In Ovo in Broiler Chickens. PLoS ONE 2016, 11, e0168899. [Google Scholar] [CrossRef]
- Slawinska, A.; Mendes, S.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems 2019, 178, 10–15. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sławinska, A.; Siwek, M.Z.; Bednarczyk, M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014, 75, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.I.; Berghman, L.R.; Zhou, H. Inhibition of NF-kB 1 (NF-kBp50) by RNA interference in chicken macrophage HD11 cell line challenged with Salmonella enteritidis. Genet. Mol. Biol. 2009, 32, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, L.; Young, J.R.; Zoorob, R.; Whittaker, C.A.; Hesketh, P.; Archer, A.; Smith, A.L.; Kaiser, P. Cloning and Characterization of Chicken IL-10 and Its Role in the Immune Response to Eimeria maxima. J. Immunol. 2004, 173, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Cheng, C.Y.; Tang, P.C.; Chen, C.F.; Chen, H.H.; Lee, Y.P.; Huang, S.Y. Differential gene expressions in testes of L2 strain Taiwan country chicken in response to acute heat stress. Theriogenology 2013, 79. [Google Scholar] [CrossRef]
- El-Deep, M.H.; Ijiri, D.; Eid, Y.Z.; Yamanaka, H.; Ohtsuka, A. Effects of dietary supplementation with Aspergillus Awamorion growth performance and antioxidative status of broiler chickens exposed to high ambient temperature. Egypt. J. Neurol. Psychiatry Neurosurg. 2014, 51, 281–288. [Google Scholar]
- De Boever, S.; Vangestel, C.; De Backer, P.; Croubels, S.; Sys, S.U. Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet. Immunol. Immunopathol. 2008, 122, 312–317. [Google Scholar] [CrossRef]
- Zhang, Q.; Waqas, Y.; Yang, P.; Sun, X.; Liu, Y.; Ahmed, N.; Chen, B.; Li, Q.; Hu, L.; Huang, Y.; et al. Cytological study on the regulation of lymphocyte homing in the chicken spleen during LPS stimulation. Oncotarget 2017, 8, 7405–7419. [Google Scholar] [CrossRef]
- Ohtsu, H.; Yamazaki, M.; Abe, H.; Murakami, H.; Toyomizu, M. Heat Stress Modulates Cytokine Gene Expression in the Spleen of Broiler Chickens. J. Poult. Sci. 2015, 52, 282–287. [Google Scholar] [CrossRef]
- Liu, F.; Yin, J.; Du, M.; Yan, P.; Xu, J.; Zhu, X.; Yu, J. Heat-stress-induced damage to porcine small intestinal epithelium associated with downregulation of epithelial growth factor signaling1. J. Anim. Sci. 2009, 87, 1941–1949. [Google Scholar] [CrossRef]
- Varasteh, S.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Differences in Susceptibility to Heat Stress along the Chicken Intestine and the Protective Effects of Galacto-Oligosaccharides. PLoS ONE 2015, 10, e0138975. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Karnati, H.K.; Pasupuleti, S.R.; Kandi, R.; Undi, R.B.; Sahu, I.; Kannaki, T.R.; Subbiah, M.; Gutti, R.K. TLR-4 signalling pathway: MyD88 independent pathway up-regulation in chicken breeds upon LPS treatment. Vet. Res. Commun. 2015, 39, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Heled, Y.; Fleischmann, C.; Epstein, Y. Cytokines and their role in hyperthermia and heat stroke. J. Basic Clin. Physiol. Pharmacol. 2013, 24, 85–96. [Google Scholar] [CrossRef]
- Heufler, C.; Koch, F.; Stanzl, U.; Topar, G.; Wysocka, M.; Trinchieri, G.; Enk, A.; Steinman, R.M.; Romani, N.; Schuler, G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-γ production by T helper 1 cells. Eur. J. Immunol. 1996, 26, 659–668. [Google Scholar] [CrossRef]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Chau, T.A.; McCully, M.L.; Brintnell, W.; An, G.; Kasper, K.J.; Vinés, E.D.; Kubes, P.; Haeryfar, S.M.M.; McCormick, J.K.; Cairns, E.; et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat. Med. 2009, 15, 641–648. [Google Scholar] [CrossRef]
- Duell, B.L.; Tan, C.K.; Carey, A.J.; Wu, F.; Cripps, A.W.; Ulett, G.C. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol. Med. Microbiol. 2012, 64, 295–313. [Google Scholar] [CrossRef]
- De Waal Malefyt, R.; Haanen, J.; Spits, H.; Koncarolo, M.G.; Te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; De Vries, J.E. Interleukin 10 (il-10) and viral il-10 strongly reduce antigen-specific human t cell proliferation by diminishing the antigen-presenting capacity of monocytes via dowm’egulation of class h major histocompatibility complex expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Silva-Filho, J.L.; Caruso-Neves, C.; Pinheiro, A.A.S. IL-4: An important cytokine in determining the fate of T cells. Biophys. Rev. 2014, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Keegan, A.D. Interleukin 4 Receptor. In Encyclopedia of Immunology; Academic Press: Cambridge, MA, USA, 1998; pp. 1453–1455. [Google Scholar]
- Quinteiro-Filho, W.M.; Calefi, A.S.; Cruz, D.S.G.; Aloia, T.P.A.; Zager, A.; Astolfi-Ferreira, C.S.; Piantino Ferreira, J.A.; Sharif, S.; Palermo-Neto, J. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis. Vet. Immunol. Immunopathol. 2017, 186, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.E.; El-Kassas, S.; El-Nahas, A.F.; Mahmoud, S. Modulatory Effect of Monochromatic Blue Light on Heat Stress Response in Commercial Broilers. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Habashy, W.S.; Milfort, M.C.; Rekaya, R.; Aggrey, S.E. Expression of genes that encode cellular oxidant/antioxidant systems are affected by heat stress. Mol. Biol. Rep. 2018, 45, 389–394. [Google Scholar] [CrossRef]
- Yan, L.J. Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2014, 2, 165–169. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From subclinical condition to pathological biomarker. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Jones, R.M.; Mercante, J.W.; Neish, A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic implications. Curr. Med. Chem. 2012, 19, 1519–1529. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, H.; Collier-Hyams, L.S.; Hansen, J.M.; Li, T.; Yamoah, K.; Pan, Z.Q.; Jones, D.P.; Neish, A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007, 26, 4457–4466. [Google Scholar] [CrossRef]
- Kaźmierczuk, A.; Kiliańska, Z.M. The pleiotropic activity of heat-shock proteins. Postep. Hig Med Dosw 2009, 63, 502–521. (In Polish) [Google Scholar]
| Ingredient | Starter (0–14 d) | Grower (15–36 d) | Finisher (37–50 d) |
|---|---|---|---|
| Corn | 42.17 | 34.96 | 12.73 |
| White corn | 0.00 | 0.00 | 15.00 |
| Wheat | 10.00 | 20.00 | 25.01 |
| Sorghum | 0.00 | 0.00 | 5.00 |
| Soybean meal | 23.11 | 20.63 | 17.60 |
| Expanded soybean | 10.00 | 10.00 | 13.00 |
| Sunflower | 3.00 | 3.00 | 3.00 |
| Corn gluten | 4.00 | 3.00 | 0.00 |
| Soybean oil | 3.08 | 4.43 | 5.48 |
| Dicalcium phosphate | 1.52 | 1.20 | 0.57 |
| Calcium carbonate | 0.91 | 0.65 | 0.52 |
| Sodium bicarbonate | 0.15 | 0.10 | 0.15 |
| Salt | 0.27 | 0.27 | 0.25 |
| Coline cloride | 0.10 | 0.10 | 0.10 |
| Lysine solfate | 0.59 | 0.55 | 0.46 |
| Dl-methionine | 0.27 | 0.29 | 0.30 |
| Threonine | 0.15 | 0.14 | 0.14 |
| Enzyme-roxazyme g2g | 0.08 | 0.08 | 0.08 |
| Phytase 0.1% | 0.10 | 0.10 | 0.10 |
| Coccidiostat | |||
| Vit-min premix 1 | 0.50 | 0.50 | 0.50 |
| Dry matter, % | 88.57 | 88.65 | 88.64 |
| Protein, % | 22.70 | 21.49 | 19.74 |
| Lipid, % | 7.06 | 8.24 | 9.74 |
| Fiber, % | 3.08 | 3.04 | 3.07 |
| Ash, % | 5.85 | 5.17 | 4.49 |
| Lys, % | 1.38 | 1.29 | 1.21 |
| Met, % | 0.67 | 0.62 | 0.59 |
| Met + Cys, % | 1.03 | 0.97 | 0.91 |
| Calcium, % | 0.91 | 0.80 | 0.59 |
| Phosphate, % | 0.63 | 0.57 | 0.46 |
| Metabolizable energy (kcal/kg) | 3.076 | 3.168 | 3.264 |
| Gene a | NCBI Gene ID | Primer Sequences (5′-3′) | Function b | Ref. |
|---|---|---|---|---|
| Panel 1. Immune-related genes | ||||
| IL-2 | 373958 | F: GCTTATGGAGCATCTCTATCATCA R: GGTGCACTCCTGGGTCTC | Cytokine important for the proliferation of T and B lymphocytes. Important role in the immune response to antigenic stimuli. | [41] |
| IL-4 | 416330 | F: GCTCTCAGTGCCGCTGATG R: GGAAACCTCTCCCTGGATGTC | Pleiotropic cytokine produced by activated T cells. B-cell stimulatory factor. | [43] |
| IL-6 | 395337 | F: AGGACGAGATGTGCAAGAAGTTC R: TTGGGCAGGTTGAGGTTGTT | Cytokine that plays a role in inflammation and the maturation of B cells. Produced at sites of acute and chronic inflammation. | [44] |
| IL-10 | 428264 | F: CATGCTGCTGGGCCTGAA R: CGTCTCCTTGATCTGCTTGATG | Pleiotropic effects in immunoregulation and inflammation. Inhibits synthesis of cytokines. | [45] |
| IL-12p40 | 404671 | F: TTGCCGAAGAGCACCAGCCG R: CGGTGTGCTCCAGGTCTTGGG | Can act as a growth factor for activated T and Natural Killer cells. Stimulates production of IFN-gamma. | [46] |
| IL-17 | 395111 | F: GGGATTACAGGATCGATGAGGA R: GAGTTCACGCACCTGGAATG | Cytotoxic T-lymphocyte-associated protein 8. Proinflammatory cytokine produced by activated T cells. | [41] |
| Panel 2. Stress response genes | ||||
| HSP25 | 428310 | F: CCGTCTTCTGCTGAGAGGAGTG R: ACCGTTGTTCCGTCCCATCAC | Heat shock protein family B (small) member 9. Response to various cellular stresses. Molecular chaperones which bind to and inhibit irreversible protein aggregation or misfolding under stressful conditions. | [47] |
| HSP90AA1 | 423463 | F: GGTGTTGGTTCCTACTCTGCTTAC R: ACTGCTCATCATCATTGTGCTTGG | Heat shock protein family class A member 1. Is a molecular chaperone that aids protein folding and quality control for a large proteins. | [47] |
| BAG3 | 423931 | F: AGGGTCGTGCGGATGTGC R: TGTGGTGGCTTAGGCTCTGC | BAG family molecular chaperone regulator 3. Cellular response to stress. | [47] |
| CAT | 423600 | F: GGGGAGCTGTTTACTGCAAG R: CTTCCATTGGCTATGGCATT | Catalase a key antioxidant enzyme in the bodies defense against oxidative stress. | [48] |
| SOD1 | 395938 | F: AGGGGGTCATCCACTTCC R: CCCATTTGTGTTGTCTCCAA | Superoxide Dismutase binds copper and zinc ions. Responsible for destroying free superoxide radicals. | [48] |
| Reference genes | ||||
| ACTB | 396526 | F: CACAGATCATGTTTGAGACCTT R: CATCACAATACCAGTGGTACG | Beta-actin is highly conserved protein involved in cell motility, structure, integrity and intercellular signaling. Ubiquitously expressed in all eukaryotic cells. | [49] |
| UB | 101747587F | F: GGGATGCAGATCTTCGTGAAA R: CTTGCCAGCAAAGATCAACCTT | Ubiquitin is associated with protein degradation, DNA repair, cell cycle regulation kinase modification, and regulation of other cell signals pathways. | [49] |
| Gene | Treatment 1 | Temperature 2 | Treatment × Temperature 3 |
|---|---|---|---|
| Acute HS | |||
| Immune-related panel | |||
| IL-2 | NS | NS | NS |
| IL-4 | <0.001 | <0.05 | <0.01 |
| IL-6 | NS | NS | NS |
| IL-10 | NS | NS | NS |
| IL-12p40 | NS | NS | NS |
| IL-17 | NS | NS | NS |
| Stress-related panel | |||
| BAG3 | <0.05 | NS | NS |
| CAT | <0.05 | NS | NS |
| SOD | <0.05 | NS | NS |
| HSP25 | NS | NS | NS |
| HSP90 | NS | NS | NS |
| Chronic HS | |||
| Immune-related panel | |||
| IL-2 | <0.05 | NS | NS |
| IL-4 | <0.001 | <0.01 | NS |
| IL-6 | NS | NS | NS |
| IL-10 | NS | NS | NS |
| IL-12p40 | NS | <0.05 | NS |
| IL-17 | NS | NS | NS |
| Stress-related panel | |||
| BAG3 | NS | NS | NS |
| CAT | NS | NS | <0.01 |
| SOD | NS | NS | <0.01 |
| HSP25 | NS | NS | NS |
| HSP90 | NS | NS | NS |
| Gene | Treatment 1 | HS 2 | Treatment × HS 3 |
|---|---|---|---|
| Immune-related panel | |||
| IL-2 | NS | <0.05 | NS |
| IL-4 | <0.001 | NS | NS |
| IL-6 | NS | NS | NS |
| IL-10 | NS | NS | NS |
| IL-12p40 | NS | NS | NS |
| IL-17 | NS | NS | NS |
| Stress-related panel | |||
| BAG3 | NS | NS | NS |
| CAT | NS | NS | <0.05 |
| SOD | NS | NS | <0.05 |
| HSP25 | NS | NS | NS |
| HSP90 | NS | <0.05 | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, E.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G.; Slawinska, A. Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat. Animals 2020, 10, 474. https://doi.org/10.3390/ani10030474
Pietrzak E, Dunislawska A, Siwek M, Zampiga M, Sirri F, Meluzzi A, Tavaniello S, Maiorano G, Slawinska A. Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat. Animals. 2020; 10(3):474. https://doi.org/10.3390/ani10030474
Chicago/Turabian StylePietrzak, Elzbieta, Aleksandra Dunislawska, Maria Siwek, Marco Zampiga, Federico Sirri, Adele Meluzzi, Siria Tavaniello, Giuseppe Maiorano, and Anna Slawinska. 2020. "Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat" Animals 10, no. 3: 474. https://doi.org/10.3390/ani10030474
APA StylePietrzak, E., Dunislawska, A., Siwek, M., Zampiga, M., Sirri, F., Meluzzi, A., Tavaniello, S., Maiorano, G., & Slawinska, A. (2020). Splenic Gene Expression Signatures in Slow-Growing Chickens Stimulated in Ovo with Galactooligosaccharides and Challenged with Heat. Animals, 10(3), 474. https://doi.org/10.3390/ani10030474






