Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Incubation and In Ovo Injections
2.2. Rearing Management and Performance Data
2.3. Gut Morphology and Microstructure
2.3.1. Histomorphological Samples
2.3.2. Histological Preparations
2.3.3. Staining Methods and Histomorphometry
2.4. Evaluation of the Oxidative Stability of The Meat
2.5. Blood Biochemical Analysis
2.6. Coccidia and Bacteria Quantification
2.6.1. Microbiological Sampling
2.6.2. Microbiological Analyses
2.7. Statistical Analyses
3. Results
3.1. Performance Parameters (Mortality, Body Weight (BW), Feed Conversion Ratio (FCR) and Feed Intake (FI)) after In Ovo Delivery of Raffinose Family Oligosaccharides (RFO) Prebiotic
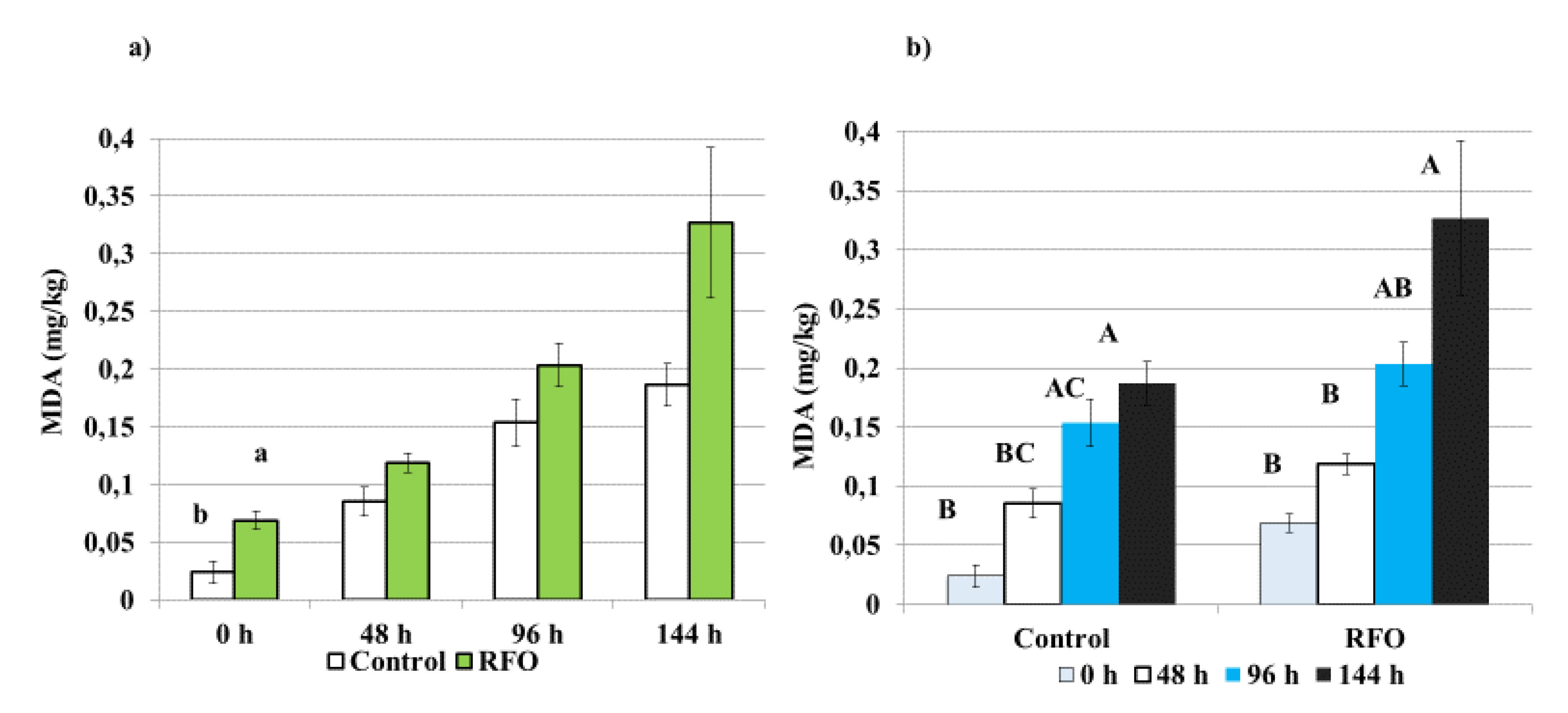
3.2. Oxidative Stability of Meat
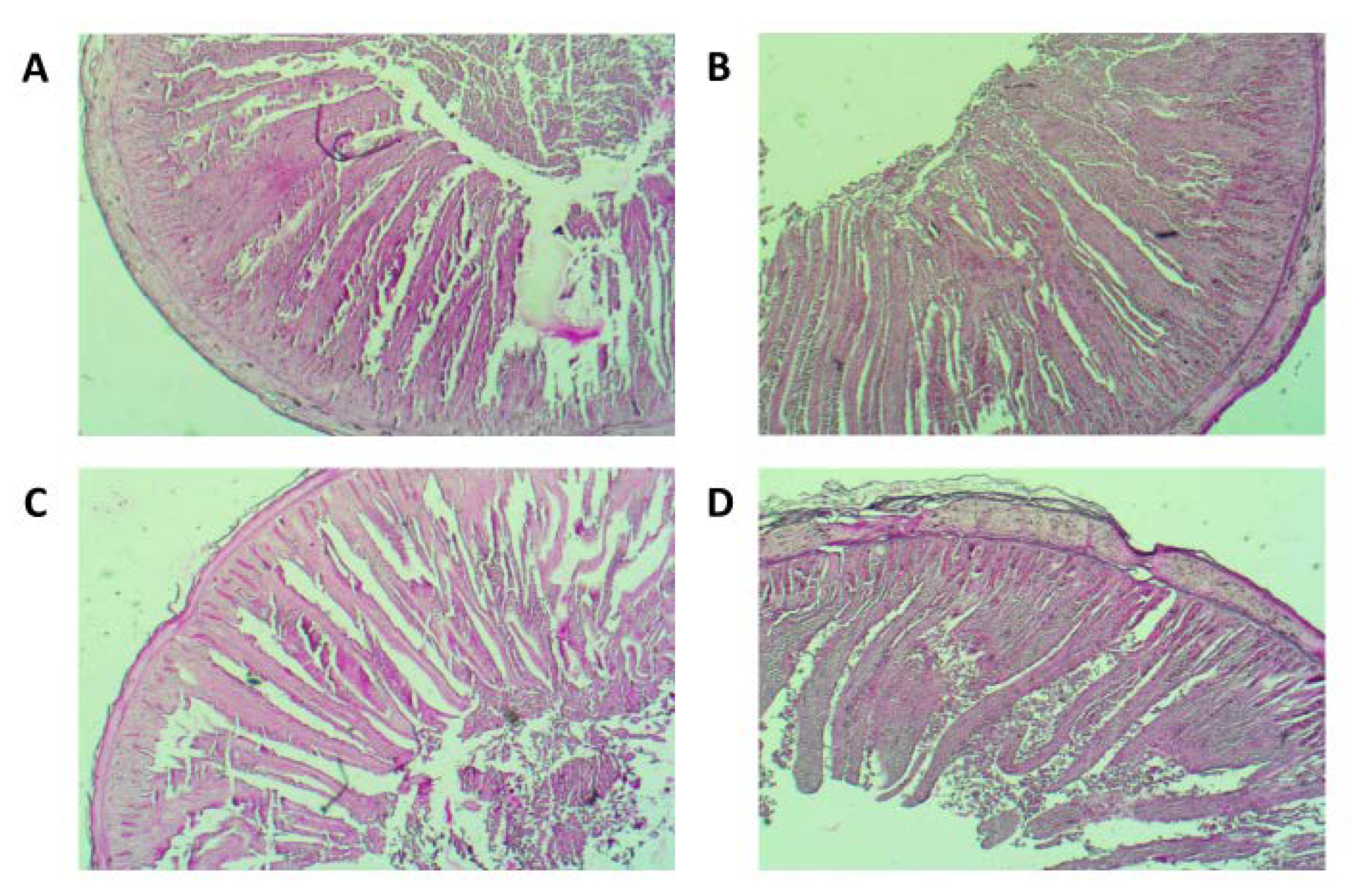
3.3. Gut Morphometry and Microstructure
3.4. Biochemical Profile in Blood
3.5. Bacteriological Counts
3.6. Oocyst Counts
4. Discussion
4.1. The Performance Was Not Affected by In Ovo Injected RFO
4.2. Structural Parameters of Gut Health
4.3. The Improved Biochemical Profile in Blood
4.4. The Mitigation of Natural Environmental Pathogenic Infections and Opportunistic Pathogens
4.5. ‘The Earlier The Better Effect’ of In Ovo Prebiotic Treatments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tavaniello, S.; Mucci, R.; Stadnicka, K.; Acaye, O.; Bednarczyk, M.; Maiorano, G. Effect of in ovo administration of different synbiotics on carcass and meat quality traits in broiler chickens. Poult. Sci. 2019, 98, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Łukaszewicz, E.; Kowalczyk, A.; Sieńczyk, M.; Bednarczyk, M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- Pruszynska-Oszmalek, E.; Kolodziejski, P.A.; Stadnicka, K.; Sassek, M.; Chalupka, D.; Kuston, B.; Nogowski, L.; Mackowiak, P.; Maiorano, G.; Jankowski, J.; et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015, 94, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Madej, J.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chicken. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Buzala, M.; Ponczek, M.B.; Slomka, A.; Roslewska, A.; Janicki, B.; Zekanowska, E.; Bednarczyk, M. A Pilot Study of Tissue Factor-Tissue Factor Pathway Inhibitor Axis and Other Selected Coagulation Parameters in Broiler Chickens Administered in Ovo with Selected Prebiotics. Folia Biol. 2016, 64, 213–224. [Google Scholar] [CrossRef]
- Teng, P.-Y.; Kim, W.K. Review: Roles of Prebiotics in Intestinal Ecosystem of Broilers. Front. Vet. Sci. 2018, 5, 245. [Google Scholar] [CrossRef]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics—in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018, 14, 402. [Google Scholar] [CrossRef]
- Sobolewska, A.; Elminowska-Wenda, G.; Bogucka, J.; Dankowiakowska, A.; Kułakowska, A.; Szczerba, A.; Stadnicka, K.; Szpinda, M.; Bednarczyk, M. The influence of in ovo injection with the prebiotic DiNovo on the development of histomorphological parameters of the duodenum, body mass and productivity in large-scale poultry production conditions. J. Anim. Sci. Biotechnol. 2017, 8, 45. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Bednarczyk, M.; Siwek, M. Transcriptome modulation by in ovo delivered Lactobacillus synbiotics in a range of chicken tissues. Gene 2019, 6, 27–33. [Google Scholar] [CrossRef]
- Angwech, H.; Tavaniello, S.; Ongwech, A.; Kaaya, A.M.G. Efficacy of in ovo delivered prebiotics on growth performance, meat quality and gut health of kuroiler chickens in the face of a natural coccidiosis challenge. Animals 2019, 9, 876. [Google Scholar] [CrossRef]
- Celebioglu, H.; Ejby, M.; Majumder, A.; Købler, C.; Goh, Y.; Thorsen, K.; Schmidt, B.; O’Flaherty, S.; Abou Hachem, M.; Lahtinen, S.; et al. Differential proteome and cellular adhesion analyses of the probiotic bacterium Lactobacillus acidophilus NCFM grown on raffinose—An emerging prebiotic. Proteomics 2016, 16, 1361–1375. [Google Scholar] [CrossRef] [PubMed]
- Villaluenga, C.M.; Frías, J. Production and Bioactivity of Oligosaccharides in Plant Foods. In Food Oligosaccharides: Production, Analysis and Bioactivity; Moreno, J.F., Sanz, M.L., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2014; pp. 35–54. [Google Scholar]
- Bednarczyk, M.; Urbanowski, M.; Gulewicz, P.; Kasperczyk, K.; Maiorano, G.; Szwaczkowski, T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. B. Vet. I. Pulawy 2011, 55, 465–469. [Google Scholar]
- Maiorano, G.; Stadnicka, K.; Tavaniello, S.; Abiuso, C.; Bogucka, J.; Bednarczyk, M. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 2017, 96, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Villaluenga, C.M.; Wardenska, M.; Pilarski, R.; Bednarczyk, M.; Gulewicz, K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia Biol. (Krakow) 2004, 52, 135–142. [Google Scholar] [CrossRef]
- Gulewicz, P.; Ciesiołka, D.; Frias, J.; Vidal-Valverde, C.; Frejnagel, S.; Trojanowska, K.; Gulewicz, K. Simple Method of Isolation and Purification of α-Galactosides from Legumes. J. Agric. Food Chem. 2000, 48, 3120–3123. [Google Scholar] [CrossRef]
- Sakamoto, K.; Hirose, H.; Onizuka, A.; Hayashi, M.; Futamura, N.; Kawamura, Y. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. JSR 2000, 94, 99–106. [Google Scholar] [CrossRef]
- Uni, Z.; Platin, R.; Sklan, D. Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. J. Comp. Physiol. B 1998, 168, 241–247. [Google Scholar] [CrossRef]
- Vyncke, W. Direct Determination of the Thiobarbituric Acid Value in Trichloracetic Acid Extracts of Fish as a Measure of Oxidative Rancidity. Fette, Seifen, Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 2nd ed.; Sparky House Publishing: Baltimore, MD, USA, 2009; pp. 1–319. [Google Scholar]
- Berthet, P.; Gerard, G. A Statistical Study of Microdistribution of Oribatei (Acari) Part I. The Distribution Pattern. Oikos 1965, 16, 214. [Google Scholar] [CrossRef]
- ROSS 308/ROSS 308 FF BROILER: Performance Objectives. Available online: http://eu.aviagen.com/tech-center/download/1339/Ross308-308FF-BroilerPO2019-EN.pdf (accessed on 31 March 2020).
- Ferket, P.; Gernat, A. Factors that affect feed intake of meat birds: A review. Int. J. Poult. Sci. 2006, 5, 905–911. [Google Scholar]
- Berrocoso, J.; Kida, R.; Singh, A.; Kim, Y.; Jha, R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017, 96, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Bednarczyk, M.; Lisowski, M.; Rutkowski, A.; Bernacki, Z.; Wardeńska, M.; Gulewicz, K. Assessment of the effect of galactosides injected during embryogenesis on selected chicken traits. Folia Biol. (Kraków) 2005, 53, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Kim, I.S.; Choi, Y.J.; Kim, B.G.; Hur, S.J. The development of imitation crab sticks containing chicken breast surimi. LWT-Food Sci. Technol. 2009, 42, 150–156. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hossain, M.M.; Rahman, S.M.E.; Amin, M.R.; Oh, D.H. Evaluation of physicochemical deterioration and lipid oxidation of beef muscle affected by freeze-thaw cycles. Korean J. Food Sci. An. 2015, 35, 772–782. [Google Scholar] [CrossRef]
- Craig, A.D.; Bedford, M.R.; Hastie, P.; Khattak, F.; Olukosi, O.A. The effect of carbohydrases or prebiotic oligosaccharides on growth performance, nutrient utilisation and development of small intestine and immune organs in broilers fed nutrient-adequate diets based on either wheat or barley. J. Sci. Food Agric. 2018, 99, 3246–3254. [Google Scholar] [CrossRef]
- Wu, Y.; Ravindran, V.; Thomas, D.; Birtles, M.; Hendriks, W. Influence of method of whole wheat inclusion and xylanase supplementation on the performance, apparent metabolisable energy, digestive tract measurements and gut morphology of broilers. Br. Poult. Sci. 2004, 45, 385–394. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.J.; Yu, S.H.; Wu, S.G.; Yoon, I.; Quigley, J.; Gao, Y.P.; Qi, G. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008, 87, 1377–1384. [Google Scholar] [CrossRef]
- Lamot, D.; Sapkota, D.; Wijtten, P.; Van Den Anker, I.; Heetkamp, M.; Kemp, B.; Van Den Brand, H. Diet density during the first week of life: Effects on growth performance, digestive organ weight, and nutrient digestion of broiler chickens. Poult. Sci. 2019, 98, 789–795. [Google Scholar] [CrossRef]
- Rebolé, A.; Ortiz, L.; Rodríguez, M.; Alzueta, C.; Treviño, J.; Velasco, S. Effects of inulin and enzyme complex, individually or in combination, on growth performance, intestinal microflora, cecal fermentation characteristics, and jejunal histomorphology in broiler chickens fed a wheat- and barley-based diet. Poult. Sci. 2010, 89, 276–286. [Google Scholar] [CrossRef]
- Pacifici, S.; Song, J.; Zhang, C.; Wang, Q.; Glahn, R.; Kolba, N.; Tako, E. Intra amniotic administration of raffinose and stachyose affects the intestinal brush border functionality and alters gut microflora populations. Nutrients 2017, 9, 304. [Google Scholar] [CrossRef]
- Tóthová, C.; Sesztáková, E.; Bielik, B.; Nagy, O. Changes of total protein and protein fractions in broiler chickens during the fattening period. Vet. World 2019, 12, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Alonge, E.O.; Eruvbetine, D.; Idowu, O.M.O.; Obadina, A.O.; Oukomaiya, O.O. Effect of dietary feed additives on haematological and serum biochemical parameters of broiler chickens. Online J. Anim. Feed Res. 2017, 7, 18–23. [Google Scholar]
- Biswas, A.; Mohan, N.; Raza, M.; Mir, N.A.; Mandal, A. Production performance, immune response and blood biochemical parameters in broiler chickens fed diet incorporated with prebiotics. J. Anim. Physiol. Anim. Nutr. (Berl.) 2019, 103, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Burlikowska, K.; Szymeczko, R. Changes in blood chemistry in broiler chickens during the fattening period. Folia Biol. 2011, 59, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Late, N.; Jameel, Y.; Abbas, R. Effects of dietary prebiotics (SafMannan) and local Iraqi prebiotic on some blood biochemical parameters of broiler chickens. Pakistan J. Nutr. 2019, 18, 80–85. [Google Scholar] [CrossRef]
- Das, O.; Patil, S.S.; Savsani, H.H.; Padodara, R.J.; Garg, D.D.; Marandi, S.; Barad, N. Effect of dietary prebiotics, probiotics and synbiotics as feed additives on blood profile and broiler performance. Int. J. Sci. Environ. Technol. 2016, 5, 3546–3552. [Google Scholar]
- Zhang, R.; Zhao, Y.; Sun, Y.; Lu, X.; Yang, X. Isolation, characterization, and hepatoprotective effects of the raffinose family oligosaccharides from rehmannia glutinosa libosch. J. Agric. Food Chem. 2013, 61, 7786–7793. [Google Scholar] [CrossRef]
- Krawczyk, M.; Mikulski, D.; Przywitowski, M.; Jankowski, J. The effect of dietary yellow lupine (L. Luteus cv. Baryt) on growth performance, carcass characteristics, meat quality and selected serum parameters of turkeys. J. Anim. Feed Sci. 2015, 24, 61–70. [Google Scholar] [CrossRef]
- Hatab, M.; Elsayed, M.; Ibrahim, N. Effect of some biological supplementation on productive performance, physiological and immunological response of layer chicks. J. Radiat. Res. Appl. Sci. 2016, 9, 185–192. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Aςil, Y.; Glüer, C.; Schrezenmeir, J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 2007, 137, 838S–846S. [Google Scholar] [CrossRef]
- Conway, D.P.; Mathis, G.F.; Lang, M. The use of diclazuril in extended withdrawal anticoccidial programmes: 1. Efficacy against Eimeria spp. In broiler chickens in floor pens. Poult. Sci. 2002, 81, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Peek, H.; Landman, W. Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011, 31, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Barberis, A.; Alloui, N.; Bennoune, O.; Ayachi, A.; Agabou, A. Effect of using an anticoccidial and a prebiotic on production performances, immunity status and coccidiosis in broiler chickens. Asian J. Poult. Sci. 2015, 9, 133–143. [Google Scholar] [CrossRef]
- Porter, R. Bacterial Enteritides of Poultry. Poult. Sci. 1998, 77, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Labbe, R.; Rey, D. Raffinose increases sporulation and enterotoxin production by Clostridium perfringens type A. Appl. Environ. Microbiol. 1979, 37, 1196–1200. [Google Scholar] [CrossRef]
- Zdunczyk, Z.; Jankowski, J.; Rutkowski, A.; Sosnowska, E.; Drazbo, A.; Zdunczyk, P.; Juskiewicz, J. The composition and enzymatic activity of gut microbiota in laying hens fed diets supplemented with blue lupine seeds. Anim. Feed Sci. Technol. 2014, 191, 57–66. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Benno, Y.; Endo, K.; Shiragami, N.; Sayama, K.; Mitsuokai, T. Effects of Raffinose Intake on Human Fecal Microflora. Bifidobacteria Microflora 1987, 6, 59–63. [Google Scholar] [CrossRef]
- Fernando, W.; Hill, J.; Zello, G.; Tyler, R.; Dahl, W.; Van Kessel, A. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef. Microbes 2010, 1, 197–207. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for Broiler Chickens-In Vitro Design and Evaluation of the Influence on Host and Selected Microbiota Populations following In Ovo Delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef]
- Slawinska, A.; Siwek, M.; Bednarczyk, M. Synbiotics injected in ovo regulate immune-related gene expression signatures in chicken. Am. J. Vet. Res. 2014, 75, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Ballou, A.; Ali, R.; Mendoza, M.; Ellis, J.; Hassan, H.; Croom, W.; Koci, M. Development of the chick microbiome: How early exposure influences future microbial diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Uni, Z.; Ferket, P. Enhancement of development of oviparous species by in ovo feeding. US Regular Patent 2003, US6592878B2. [Google Scholar]
- Roto, S.; Kwon, Y.; Ricke, S. Applications of In Ovo technique for the optimal development of the gastrointestinal tract and the potential influence on the establishment of its microbiome in poultr. Front. Vet. Sci. 2016, 3, 63. [Google Scholar] [CrossRef]
| Group | No.Chick/ House at Day 1 | No.Chick/ m2 at Day 1 | Final No.Chick/ House at Day 42 | Hatchability (%) | Mortality at Day 7 (%) | Total Mortality (%) |
|---|---|---|---|---|---|---|
| RFO | 24500 | 22 | 22769 | 84.56 | 1.78 | 2.9 |
| Control | 24500 | 22 | 22705 | 85.35 | 2.08 | 3.3 |
| SE | n/a | n/a | 32 | 0.3950 | 0.15 | 0.2 |
| p | n/a | n/a | 0.2952 | 0.2958 | 0.2952 | 0.2926 |
| Group | Average BW (kg/Bird) | FI (g/day/Bird) | FCR (kg/kg) | EBI |
|---|---|---|---|---|
| RFO | 2.24 | 94.20 | 1.72 | 322.70 |
| Control | 2.30 | 95.20 | 1.69 | 335.50 |
| SE | 0.03 | 0.49 | 0.01 | 6.40 |
| p | 0.2804 | 0.2952 | 0.2804 | 0.2951 |
| Day of Rearing | Day 21 | ANOVA | Day 42 | ANOVA | ||
|---|---|---|---|---|---|---|
| RFO | Control | p | RFO | Control | p | |
| Body weight (g) | 750.9 a ± 22.9 | 744.7 a ± 9.2 | 0.338 | 2249.6 a ± 535.9 | 2168.6 a ± 103.2 | 0.570 |
| (mm) | ||||||
| Length of D | 24.9 a ± 2.5 | 25.2 a ± 1.4 | 0.687 | 28.7 b ± 2.6 | 31.9 a ± 1.7 | <0.001 |
| Length of J | 61.9 a ± 3.7 | 60.1 a ± 3.4 | 0.194 | 65.7 b ± 4.5 | 75.8 a ± 5.0 | <0.001 |
| Length of I | 61.3 a ± 5.2 | 57.8 a ± 7.9 | 0.162 | 67.1 b ± 3.4 | 74.6 a ± 5.0 | <0.001 |
| Length of cecum | 26.1 a ± 2.6 | 25.7 a ± 2.4 | 0.649 | 35.8 b ± 3.0 | 38.8 a ± 2.9 | 0.009 |
| Length of colon | 8.3 a ± 1.0 | 8.0 a ± 0.5 | 0.275 | 8.7 b ± 0,7 | 10.4 a ± 0.9 | <0.001 |
| Weight of D (g) | 6.8 a ± 1.0 | 7.3 a ± 0.6 | 0.121 | 12.4 b ± 1.7 | 13.9 a ± 1.4 | 0.011 |
| Weight of J (g) | 12.6 a ± 1.7 | 13.1 a ± 1.3 | 0.390 | 23.3 b ± 3.3 | 27.5 a ± 5.0 | 0.010 |
| Weight of I (g) | 10.5 a ± 1.5 | 10.5 a ± 1.1 | 0.938 | 19.3 a ± 2.5 | 21.2 a ± 3.2 | 0.073 |
| Weight of cecum (g) | 3.8 b ± 0.8 | 4,5 a ± 1,0 | 0.029 | 11.6 a ± 1.9 | 11.9 a ± 2.5 | 0.743 |
| Weight of colon (g) | 2.0 a ± 0.7 | 1.8 a ± 0.4 | 0.545 | 3.2 b ± 0.4 | 4.0 a ± 0.5 | <0.001 |
| Villus height (µm) | 1281.0 a ± 160.4 | 1333.9 a ± 114.6 | 0.308 | 1586.8 a ± 199.7 | 1437.2 b ± 94.4 | 0.014 |
| Villus width(µm) | 137.6 a ± 16.3 | 106.4 b ± 7.9 | <0.001 | 111.2 a ± 7.5 | 118.8 a ± 19.8 | 0.175 |
| Villus surface area (µm) | 549,263.3 a ± 63431.3 | 442,127.3 b ± 46013.5 | <0.001 | 549,100.6 a ± 78401.0 | 538,983.8 a ± 107540.2 | 0.771 |
| Crypt depth (µm) | 140.4 a ± 8.2 | 124.0 b ± 11.5 | <0.001 | 166.1 a ± 28.8 | 178.4 a ± 11.2 | 0.135 |
| Days of Rearing | Day 21 | Day 35 | Day 42 |
|---|---|---|---|
| Gut structure | |||
| Lenght of D, J, I, C, colon | + | ||
| Weight of D, J, colon | + | ||
| Weight of cecum | + | ||
| Height of the villi | + | ||
| With and surface of the villi | + | ||
| Biochemical indices | |||
| HDL | + | ||
| ALT | + | ||
| AST | + | ||
| Ca | + | ||
| Bacteriological counts | |||
| Clostridium perfringens | + | + | |
| Oocysts counts | + | + |
| Day | Group | Protein (g/dL) | Albumins (g/dL) | Globulins (g/dL) | Bilirubin (mg/dL) | Triglycerides (mg/dL) | Cholesterol (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | ALT (u/L) | AST (u/L) | Ca (mg/dL) | Fe (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | RFO | 2.5 a ± 0.2 | 1.1 a ± 0.1 | 1.3 a ± 0.1 | 0.2 a ± 0.0 * | 68.8 a ± 10.9 | 474.5 a ± 21.9 | 228.0 a ± 16.6 | 232.7 a ± 12.2 | 17.8 a ± 1.3 | 227.8 a ± 17.5 | 9.6 a ± 0.4 | 103.8 a ± 59.8 |
| Control | 2.4 a ± 0.4 | 1.0 b ± 0.1 | 1.3 a ± 0.2 | 0.2 b ± 0.0 * | 79.3 a ± 11.5 | 484.5 a ± 50.0 | 187.7 a ± 137.0 | 152.3 a ± 130.9 | 20.9 a ± 5.3 | 325.6 a ± 253.1 | 10.0 a ± 0.4 | 90.4 a ± 54.4 | |
| p | 0.794 | 0.033 | 0.674 | 0.018 | 0.112 | 0.657 | 0.492 | 0.163 | 0.197 | 0.368 | 0.087 | 0.668 | |
| 21 | RFO | 2.6 a ± 0.7 | 1.3 a ± 0.5 | 1.2a ± 0.2 | 0.2 a ± 0.0 | 78.9 a ± 22.6 | 121.6 a ± 15.0 | 66.7 a ± 10.3 | 39.2 a ± 6.1 | 14.4 a ± 1.9 | 237.5 a ± 24.8 | 10.5 a ± 0.7 | 92.8 a ± 60.5 |
| Control | 2.9 a ± 0.4 | 1.6 a ± 0.2 | 1.3 a ± 0.2 | 0.2 a ± 0.0 | 87.3 a ± 24.5 | 137.9 a ± 26.3 | 79.8 a ± 16.1 | 40.7 a ± 9.3 | 15.5 a ± 2.5 | 233.4 a ± 35.3 | 10.2 a ± 0.8 | 123.3 a ± 62.8 | |
| p | 0.152 | 0.120 | 0.341 | 0.689 | 0.450 | 0.131 | 0.058 | 0.688 | 0.292 | 0.780 | 0.369 | 0.293 | |
| 42 | RFO | 3.1 b ± 0.2 | 1.6 b ± 0.0 | 1.5 b ± 0.2 | 0.1 b ± 0.0 | 67.4 a ± 13.5 | 119.4 a ± 14.0 | 67.7 a ± 10.8 | 38.2 a ± 8.0 | 10.9 b ± 1.4 | 245.1 b ± 24.1 | 11.0 a ± 0.8 | 166.1 a ± 84.3 |
| Control | 3.6 a ± 0.3 | 1.8 a ± 0.1 | 1.8 a ± 0.2 | 0.2 a ± 0.0 | 79.4 a ± 34.2 | 115.3 a ± 20.6 | 48.3 b ± 22.8 | 39.6 a ± 31.8 | 15.1 a ± 2.4 | 353.3 a ± 75.9 | 10.2 b ± 0.5 | 124.8 a ± 85.7 | |
| p | 0.002 | <0.001 | 0.026 | 0.006 | 0.372 | 0.647 | 0.047 | 0.909 | 0.001 | 0.002 | 0.032 | 0.347 |
| Day | Bacteria Groups | RFO (cfu/g) | Control (cfu/g) | p-Value |
|---|---|---|---|---|
| 21 | Lactobacillus | 4.6 × 108 ± 2.2 × 108 | 6.4 × 108± 5.4 × 108 | 0.733 |
| Bifidobacterium | 2.6 × 108 ± 2.8 × 108 | 2.9 × 108 ± 2.2 × 108 | 0.385 | |
| Enterobacterium | 8.9 × 107 ± 7.1 × 107 | 4.5 × 108 ± 4.0 × 108 | 0.252 | |
| Clostridium | <1.0 × 101,b ± <1.0 × 101 | 4.7 × 102,a ± 4.8 × 102 | 0.026 | |
| Campylobacter | <1.0 × 101 ± <1.0 × 101 | <1.0 × 101 ± <1.0 × 101 | − | |
| 42 | Lactobacillus | 1.9 × 108 ± 7.1 × 107 | 1.6 × 108 ± 1.0 × 108 | 0.434 |
| Bifidobacterium | <1.0 × 101 ± <1.0 × 101 | 2.0 × 108 ± 4.5 × 107 | 0.347 | |
| Enterobacterium | 3.0 × 107 ± 3.3 × 107 | 4.4 × 108 ± 4.1 × 107 | 0.767 | |
| Clostridium | <1.0 × 101,b ± <1.0 × 101 | 2.2 × 103,a ± 2.9 × 103 | 0.046 | |
| Campylobacter | 5.2 × 106 ± 2.5 × 106 | 2.0 × 106 ± 1.8 × 106 | 0.125 |
| Day | Group | Oocysts Number | p-Value |
|---|---|---|---|
| 21 | RFO | 169,600.0 a ± 7372.9 | 0.002 |
| Control | 131,333.3 b ± 4772.1 | ||
| 35 | RFO | 416.7 B± 28.9 | <0.001 |
| Control | 716.7 A± 28.9 | ||
| 38 | RFO | 516.7 b ± 28.9 | 0.013 |
| Control | 616.7 a ± 28.9 | ||
| 42 | RFO | 50.0 B ± 0.0 | <0.001 |
| Control | 450.0 A ± 50.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stadnicka, K.; Bogucka, J.; Stanek, M.; Graczyk, R.; Krajewski, K.; Maiorano, G.; Bednarczyk, M. Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens. Animals 2020, 10, 592. https://doi.org/10.3390/ani10040592
Stadnicka K, Bogucka J, Stanek M, Graczyk R, Krajewski K, Maiorano G, Bednarczyk M. Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens. Animals. 2020; 10(4):592. https://doi.org/10.3390/ani10040592
Chicago/Turabian StyleStadnicka, Katarzyna, Joanna Bogucka, Magdalena Stanek, Radomir Graczyk, Krzysztof Krajewski, Giuseppe Maiorano, and Marek Bednarczyk. 2020. "Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens" Animals 10, no. 4: 592. https://doi.org/10.3390/ani10040592
APA StyleStadnicka, K., Bogucka, J., Stanek, M., Graczyk, R., Krajewski, K., Maiorano, G., & Bednarczyk, M. (2020). Injection of Raffinose Family Oligosaccharides at 12 Days of Egg Incubation Modulates the Gut Development and Resistance to Opportunistic Pathogens in Broiler Chickens. Animals, 10(4), 592. https://doi.org/10.3390/ani10040592





