In Vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Peptides
2.3. In Vitro Susceptibility Testing
2.4. Statistical Analysis
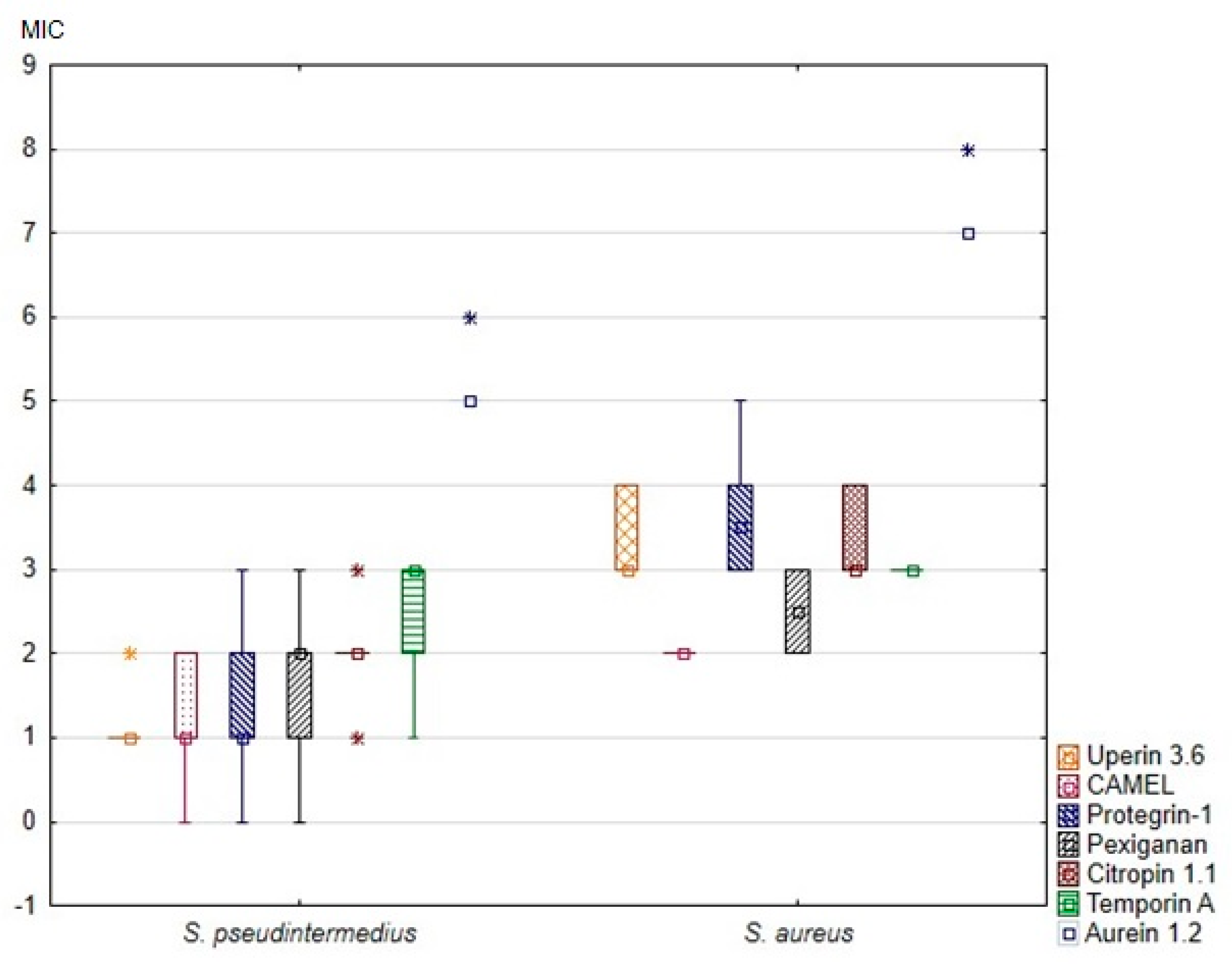
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, P.B.; Lo, A.; Eden, C.A.; Huntley, S.; Morey, V.; Ramsey, S.; Richardson, C.; Smith, D.J.; Sutton, C.; Taylor, M.D.; et al. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. Vet. Rec. 2006, 158, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Maldonado, A.; Ginel, P.J.; Tarradas, C.; Gomez-Gascon, L.; Astorga, R.J.; Luque, I. Risk factors associated with the antimicrobial resistance of staphylococci in canine pyoderma. Vet. Microbiol. 2011, 150, 302–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeffler, A.; Lloyd, D.H. What has changed in canine pyoderma? A narrative review. Vet. J. 2018, 235, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbacz, K.; Żarnowska, S.; Piechowicz, L.; Haras, K. Staphylococci isolated from carriage sites and infected sites of dogs as a reservoir of multidrug resistance and methicillin resistance. Curr. Microbiol. 2013, 66, 169–173. [Google Scholar] [CrossRef]
- Bloom, P. Canine superficial bacterial folliculitis: Current understanding of its etiology, diagnosis and treatment. Vet. J. 2014, 199, 217–222. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of methicillin resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Hryniewicz, M.M.; Garbacz, K. Borderline oxacillin-resistant Staphylococcus aureus (BORSA)–a more common problem than expected? J. Med. Microbiol. 2017, 66, 1367–1373. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in development of antimicrobial peptidomimetics as potential drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef] [Green Version]
- Greco, I.; Hummel, B.D.; Vasir, J.; Watts, J.L.; Koch, J.; Johannes, E.; Hansen, J.E.; Nielsen, H.M.; Damborg, P.; Hansen, P.R. In vitro adme properties of two novel antimicrobial peptoid-based compounds as potential agents against canine pyoderma. Molecules 2018, 23, 630. [Google Scholar] [CrossRef] [Green Version]
- McPhee, J.B.; Hancock, R.E. Function and therapeutic potential of host defence peptides. J. Pept. Sci. 2005, 11, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, T.R.; Rhomberg, P.R.; Sader, H.S.; Jones, R.N. Antimicrobial activity of omiganan pentahydrochloride tested against contemporary bacterial pathogens commonly responsible for catheter-associated infections. J. Antimicrob. Chemother. 2008, 61, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Sarno, O.; Zauli, S.; Borghi, A.; Minghetti, S.; Ricci, M.; Mantovani, L.; Toni, G.; Virgili, A. What’s new in acne? New therapeutic approaches. Ann. Dermatol. Venereol. 2010, 137, 70033–70038. [Google Scholar] [CrossRef]
- Elad, S.; Epstein, J.B.; Raber-Durlacher, J.; Donnelly, P.; Strahilevitz, J. The antimicrobial effect of Iseganan HCl oral solution in patients receiving stomatotoxic chemotherapy: Analysis from a multicenter, double-blind, placebo-controlled, randomized, phase III clinical trial. J. Oral Pathol. Med. 2012, 41, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dumville, J.C.; Lipsky, B.A.; Hoey, C.; Cruciani, M.; Fiscon, M.; Xia, J. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2017, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Ahrens, K.; Vesny, R.; Navarro, C.; Gatto, H.; Marsella, R. Evaluation of the in vitro effect of Boldo and Meadowsweet plant extracts on the expression of antimicrobial peptides and inflammatory markers in canine keratinocytes. Res. Vet. Sci. 2017, 115, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Cabassi, C.S.; Taddei, S.; Cavirani, S.; Baroni, M.C.; Sansoni, P.; Romani, A.A. Broad-spectrum activity of a novel antibiotic peptide against multidrug-resistant veterinary isolates. Vet. J. 2013, 198, 534–537. [Google Scholar] [CrossRef]
- Cabassi, C.S.; Sala, A.; Santospirito, D.; Alborali, G.; Carretto, E. Activity of AMP2041 against human and animal multidrug resistant Pseudomonas aeruginosa clinical isolates. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Morroni, G.; Simonetti, O.; Brenciani, A.; Brescini, L.; Kamysz, W.; Kamysz, E.; Neubauer, D. In vitro activity of Protegrin-1, alone and in combination with clinically useful antibiotics, against Acinetobacter baumannii strains isolated from surgical wounds. Med. Microbiol. Immunol. 2019, 208, 877–883. [Google Scholar] [CrossRef]
- Garbacz, K.; Żarnowska, S.; Piechowicz, L.; Haras, K. Pathogenicity potential of Staphylococcus pseudintermedius strains isolated from canine carriers and from dogs with infection signs. Virulence 2013, 4, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Bannoehr, J.; Franco, A.; Iurescia, M.; Battisti, A.; Fitzgerald, J.R. Molecular diagnostic identification of Staphylococcus pseudintermedius. J. Clin. Microbiol. 2009, 47, 469–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement; Document VET01-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; Document M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Milheirico, C.; Oliveira, D.C.; de Lencastre, H. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3374–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbacz, K.; Kamysz, W.; Piechowicz, L. Activity of antimicrobial peptides, alone or combined with conventional antibiotics, against Staphylococcus aureus isolated from the airways of cystic fibrosis patients. Virulence 2017, 8, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Hillier, A.; Lloyd, D.H.; Weese, J.S.; Blondeau, J.M.; Boothe, D.; Breitschwerdt, E.; Guardabassi, L.; Papich, M.G.; Rankin, S.; Turnidge, J.D.; et al. Guidelines for the diagnosis and antimicrobial therapy of canine superficial bacterial folliculitis (Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases). Vet. Dermatol. 2014, 25, 163-e43. [Google Scholar] [CrossRef]
- Chia, B.C.; Carver, J.A.; Mulhern, T.D.; Bowie, J.H. The solution structure of uperin 3.6, an antibiotic peptide from the granular dorsal glands of the Australian toadlet, Uperoleia mjobergii. J. Pept. Res. 1999, 54, 137–145. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Kamysz, W.; Silvestri, C.; Licci, A.; D’Amato, G.; Nadolski, P.; Riva, A.; Lukasiak, J.; Scalise, G. In vitro activity and killing effect of uperin 3.6 against gram-positive cocci isolated from immunocompromised patients. Antimicrob. Agents Chemother. 2005, 49, 3933–3936. [Google Scholar] [CrossRef] [Green Version]
- Barańska-Rybak, W.; Cirioni, O.; Dawgul, M.; Sokolowska-Wojdylo, M.; Naumiuk, L.; Szczerkowska-Dobosz, A.; Nowicki, R.; Roszkiewicz, J.; Kamysz, W. Activity of antimicrobial peptides and conventional antibiotics against superantigen positive Staphylococcus aureus isolated from the patients with neoplastic and inflammatory erythrodermia. Chemother. Res. Pract. 2011, 2011, 270932. [Google Scholar] [CrossRef]
| Peptide | Amino Acid Sequence |
|---|---|
| aurein 1.2 | GLFDIIKKIAESF-NH2 |
| CAMEL | KWKLFKKIGAVLKVL-NH2 |
| citropin 1.1 | GLFDVIKKVASVIGGL-NH2 |
| pexiganan | GIGKFLKKAKKFGKAFVKILKK-NH2 |
| * protegrin-1 | RGGLCYCRGRFCVCVGR-NH2 |
| temporin A | FLPLIGRVLSGIL-NH2 |
| uperin 3.6. | GVIDAAKKVVNVLKNLF-NH2 |
| Parameter | Uperin 3.6 | CAMEL | Protegrin-1 | Pexiganan | Citropin 1.1 | Temporin A | Aurein 1.2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | I | II | I | II | I | II | I | II | I | II | I | II | ||
| M I C | Median (IQR) | 2 (2–2) | 8 (8–14) | 2 (2–4) | 4 (4–4) | 2 (2–4) | 12 (8–16) | 4 (2–4) | 6 (4–8) | 4 (4–4) | 8 (8–14) | 8 (4–8) | 8 (8–8) | 32 (32–32) | 128 (128–128) |
| MIC50 | 2 | 8 | 2 | 4 | 2 | 8 | 4 | 4 | 4 | 8 | 8 | 8 | 32 | 128 | |
| MIC90 | 2 | 16 | 4 | 4 | 4 | 32 | 4 | 8 | 4 | 16 | 8 | 8 | 32 | 256 | |
| p-value * | <0.001 | 0.014 | <0.001 | 0.006 | <0.001 | 0.041 | <0.001 | ||||||||
| M B C | Median (IQR) | 2 (2–4) | 16 (16–16) | 4 (2–4) | 4 (4–4) | 4 (2–4) | 12 (8–16) | 4 (2–4) | 8 (8–8) | 4 (4–4) | 12 (8–16) | 8 (8–8) | 8 (8–14) | 64 (64–64) | 128 (128–224) |
| MBC50 | 2 | 16 | 4 | 4 | 4 | 8 | 4 | 8 | 4 | 8 | 8 | 8 | 64 | 128 | |
| MBC90 | 4 | 16 | 4 | 4 | 8 | 32 | 8 | 8 | 8 | 16 | 8 | 16 | 64 | 256 | |
| p-value * | <0.001 | 0.055 | <0.001 | <0.001 | <0.001 | 0.045 | <0.001 | ||||||||
| Peptide | Species | MIC [µg/mL] | Range | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ≥256 | [µg/mL] | ||
| Uperin 3.6 | S. pseudintermedius (MSSP) | 50 | 3 | 0.5–64 | |||||||
| S. pseudintermedius (MRSP) | 6 | 1 | 0.5–64 | ||||||||
| S. aureus | 4 | 2 | 0.5–64 | ||||||||
| S. aureus ATCC 6538 | 1 | 0.5–64 | |||||||||
| S. aureus ATCC 43300 | 1 | 0.5–64 | |||||||||
| S. intermedius PCM 2405 | 1 | 0.5–64 | |||||||||
| Protegrin-1 | S. pseudintermedius(MSSP) | 1 | 28 | 21 | 3 | 0.5–64 | |||||
| S. pseudintermedius (MRSP) | 4 | 2 | 1 | 0.5–64 | |||||||
| S. aureus | 3 | 2 | 1 | 0.5–64 | |||||||
| S. aureus ATCC 6538 | 1 | 0.5–64 | |||||||||
| S. aureus ATCC 43300 | 1 | 0.5–64 | |||||||||
| S. intermedius PCM 2405 | 1 | 0.5–64 | |||||||||
| CAMEL | S. pseudintermedius (MSSP) | 2 | 25 | 26 | 0.5–32 | ||||||
| S. pseudintermedius (MRSP) | 6 | 1 | 0.5–32 | ||||||||
| S. aureus | 6 | 0.5–32 | |||||||||
| S. aureus ATCC 6538 | 1 | 0.5–32 | |||||||||
| S. aureus ATCC 43300 | 1 | 0.5–32 | |||||||||
| S. intermedius PCM 2405 | 1 | 0.5–32 | |||||||||
| Pexiganan | S. pseudintermedius(MSSP) | 1 | 23 | 26 | 3 | 0.5–32 | |||||
| S. pseudintermedius (MRSP) | 6 | 1 | 0.5–32 | ||||||||
| S. aureus | 3 | 3 | 0.5–32 | ||||||||
| S. aureus ATCC 6538 | 1 | 0.5–32 | |||||||||
| S. aureus ATCC 43300 | 1 | 0.5–32 | |||||||||
| S. intermedius PCM 2405 | 1 | 0.5–32 | |||||||||
| Citropin 1.1 | S. pseudintermedius (MSSP) | 1 | 49 | 3 | 0.5–32 | ||||||
| S. pseudintermedius (MRSP) | 3 | 4 | 0.5–32 | ||||||||
| S. aureus | 4 | 2 | 0.5–32 | ||||||||
| S. aureus ATCC 6538 | 1 | 0.5–32 | |||||||||
| S. aureus ATCC 43300 | 1 | 0.5–32 | |||||||||
| S. intermedius PCM 2405 | 1 | 0.5–32 | |||||||||
| Temporin A | S. pseudintermedius (MSSP) | 24 | 29 | 0.5–32 | |||||||
| S. pseudintermedius (MRSP) | 1 | 6 | 0.5–32 | ||||||||
| S. aureus | 6 | 0.5–32 | |||||||||
| S. aureus ATCC 6538 | 1 | 0.5–32 | |||||||||
| S. aureus ATCC 43300 | 1 | 0.5–32 | |||||||||
| S. intermedius PCM 2405 | 1 | 0.5–32 | |||||||||
| Aurein 1.2 | S. pseudintermedius (MSSP) | 49 | 4 | 8–256 | |||||||
| S. pseudintermedius (MRSP) | 7 | 8–256 | |||||||||
| S. aureus | 5 | 1 | 8–256 | ||||||||
| S. aureus ATCC 6538 | 1 | 8–256 | |||||||||
| S. aureus ATCC 43300 | 1 | 8–256 | |||||||||
| S. intermedius PCM 2405 | 1 | 8–256 | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarosiewicz, M.; Garbacz, K.; Neubauer, D.; Kamysz, W. In Vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma. Animals 2020, 10, 470. https://doi.org/10.3390/ani10030470
Jarosiewicz M, Garbacz K, Neubauer D, Kamysz W. In Vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma. Animals. 2020; 10(3):470. https://doi.org/10.3390/ani10030470
Chicago/Turabian StyleJarosiewicz, Małgorzata, Katarzyna Garbacz, Damian Neubauer, and Wojciech Kamysz. 2020. "In Vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma" Animals 10, no. 3: 470. https://doi.org/10.3390/ani10030470
APA StyleJarosiewicz, M., Garbacz, K., Neubauer, D., & Kamysz, W. (2020). In Vitro Efficiency of Antimicrobial Peptides against Staphylococcal Pathogens Associated with Canine Pyoderma. Animals, 10(3), 470. https://doi.org/10.3390/ani10030470






