Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Feeds, and Experimental Design
2.2. Tissue Collection and RNA Isolation
2.3. Reverse Transcription–Quantitative PCR (RT-qPCR)
2.4. Gene Selection
2.4.1. Reference Genes
2.4.2. Target Genes
2.5. Relative Quantification of Gene Expression and Statistical Analysis
3. Results
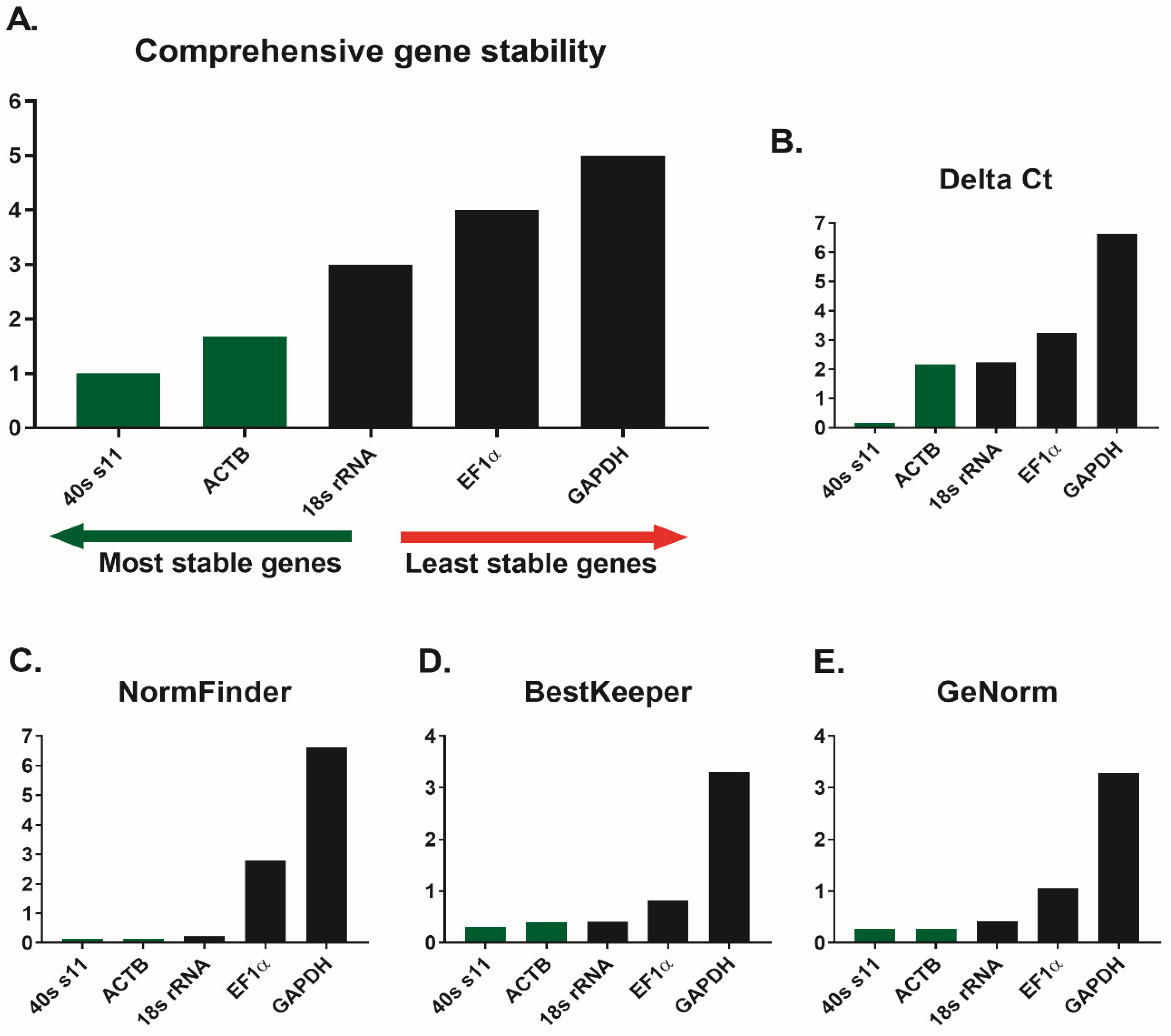
3.1. Reference Genes
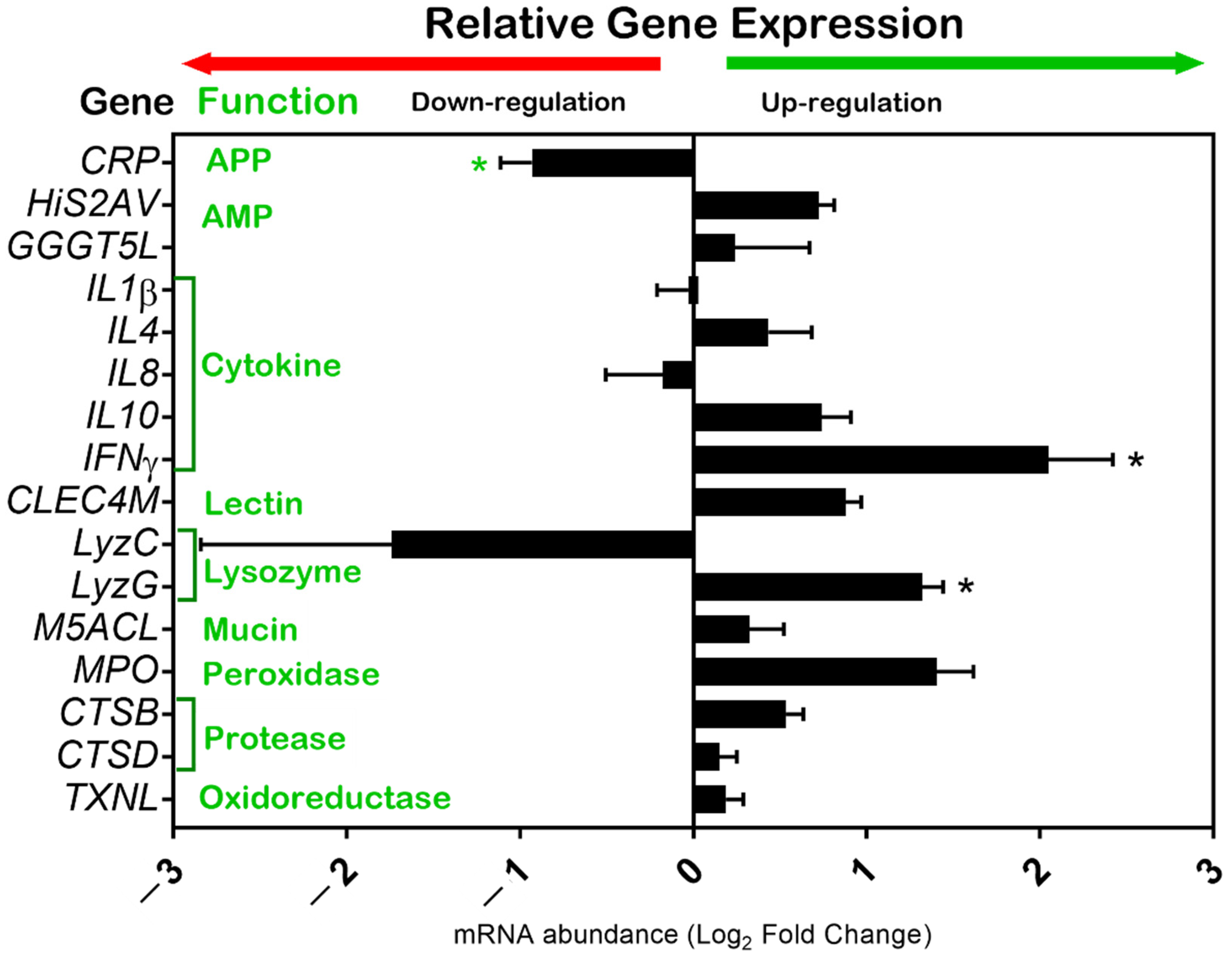
3.2. Immune-Related Gene Expression
4. Discussion
4.1. mRNA Expression Stability of the Reference Genes
4.2. Immune-Related Gene Expression in Skin Mucosa
4.3. Effects of GOS in Fish
4.4. Immunomodulatory Role of GOS
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Wang, Y.; Ma, J.; Ding, Y.; Zhang, S. Phosvitin plays a critical role in the immunity of zebrafish embryos via acting as a pattern recognition receptor and an antimicrobial effector. J. Biol. Chem. 2011, 286, 22653–22664. [Google Scholar] [CrossRef]
- Hawkes, J.W. The structure of fish skin—I. General organization. Cell Tissue Res. 1974, 149, 147–158. [Google Scholar] [CrossRef]
- Fast, M.; Sims, D.; Burka, J.; Mustafa, A.; Ross, N. Skin morphology and humoral non-specific defence parameters of mucus and plasma in rainbow trout, coho and Atlantic salmon. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002, 132, 645–657. [Google Scholar] [CrossRef]
- Arasu, A.; Kumaresan, V.; Sathyamoorthi, A.; Palanisamy, R.; Prabha, N.; Bhatt, P.; Roy, A.; Thirumalai, M.K.; Gnanam, A.J.; Pasupuleti, M.; et al. Fish lily type lectin-1 contains β-prism architecture: Immunological characterization. Mol. Immunol. 2013, 56, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.; Dash, S.; Sahoo, P.; Routray, P.; Sahoo, S.; Gupta, S.; Meher, P.; Sarangi, N. Non-specific immune parameters of brood Indian major carp Labeo rohita and their seasonal variations. Fish Shellfish Immunol. 2007, 22, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ángeles Esteban, M. An Overview of the Immunological Defenses in Fish Skin. ISRN Immunol. 2012, 2012, 1–29. [Google Scholar] [CrossRef]
- Lazado, C.C.; Caipang, C.M.A. Bacterial viability differentially influences the immunomodulatory capabilities of potential host-derived probiotics in the intestinal epithelial cells of Atlantic cod Gadus morhua. J. Appl. Microbiol. 2014, 116, 990–998. [Google Scholar] [CrossRef]
- Streilein, J.W. Skin-associated lymphoid tissues (SALT): Origins and functions. J. Invest. Dermatol. 1983, 80 Suppl, 12s–16s. [Google Scholar] [CrossRef]
- Xu, Z.; Parra, D.; Gomez, D.; Salinas, I.; Zhang, Y.-A.; von Gersdorff Jorgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Sunyer, J.O. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef]
- Austin, B. The bacterial microflora of fish, revised. Sci. World J. 2006, 6, 931–945. [Google Scholar] [CrossRef]
- Musharrafieh, R.; Tacchi, L.; Trujeque, J.; LaPatra, S.; Salinas, I. Staphylococcus warneri, a resident skin commensal of rainbow trout (Oncorhynchus mykiss) with pathobiont characteristics. Vet. Microbiol. 2014, 169, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Boutin, S.; Bernatchez, L.; Audet, C.; Derôme, N. Antagonistic effect of indigenous skin bacteria of brook charr (Salvelinus fontinalis) against Flavobacterium columnare and F. psychrophilum. Vet. Microbiol. 2012, 155, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-González, M.; Fregeneda-Grandes, J.; Suárez-Ramos, S.; Rodríguez Cadenas, F.; Aller-Gancedo, J. Bacterial skin flora variation and in vitro inhibitory activity against Saprolegnia parasitica in brown and rainbow trout. Dis. Aquat. Organ. 2011, 96, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Horsley, R.W. The bacterial flora of the Atlantic salmon (Salmo salar L.) in relation to its environment. J. Appl. Bacteriol. 1973, 36, 377–386. [Google Scholar] [CrossRef]
- Cahill, M.M. Bacterial flora of fishes: A review. Microb. Ecol. 1990, 19, 21–41. [Google Scholar] [CrossRef]
- Sullam, K.E.; Essinger, S.D.; Lozupone, C.A.; O’Connor, M.P.; Rosen, G.L.; Knight, R.; Kilham, S.S.; Russell, J.A. Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis. Mol. Ecol. 2012, 21, 3363–3378. [Google Scholar] [CrossRef]
- van Kessel, M.A.H.J.; Dutilh, B.E.; Neveling, K.; Kwint, M.P.; Veltman, J.A.; Flik, G.; Jetten, M.S.M.; Klaren, P.H.M.; Op den Camp, H.J.M. Pyrosequencing of 16s rRNA gene amplicons to study the microbiota in the gastrointestinal tract of carp (Cyprinus carpio L.). AMB Express 2011, 1, 1–9. [Google Scholar] [CrossRef]
- Kazuñ, B.; Kazuñ, K.; Siwicki, A.K. Probiotyki, prebiotyki i synbiotyki w ochronie zdrowia ryb. Komunikaty Rybackie 2016, 4, 14–17. [Google Scholar]
- Hoseinifar, S.H.; Khalili, M.; Khoshbavar Rostami, H.; Esteban, M.Á. Dietary galactooligosaccharide affects intestinal microbiota, stress resistance, and performance of Caspian roach (Rutilus rutilus) fry. Fish Shellfish Immunol. 2013, 35, 1416–1420. [Google Scholar] [CrossRef]
- Do Huu, H.; Jones, C.M. Effects of dietary mannan oligosaccharide supplementation on juvenile spiny lobster Panulirus homarus (Palinuridae). Aquaculture 2014, 432, 258–264. [Google Scholar] [CrossRef]
- Guerreiro, I.; Enes, P.; Rodiles, A.; Merrifield, D.; Oliva-Teles, A. Effects of rearing temperature and dietary short-chain fructooligosaccharides supplementation on allochthonous gut microbiota, digestive enzymes activities and intestine health of turbot ( Scophthalmus maximus L.) juveniles. Aquac. Nutr. 2016, 22, 631–642. [Google Scholar] [CrossRef]
- Luna-González, A.; Almaraz-Salas, J.C.; Fierro-Coronado, J.A.; Flores-Miranda, M. del C.; González-Ocampo, H.A.; Peraza-Gómez, V. The prebiotic inulin increases the phenoloxidase activity and reduces the prevalence of WSSV in whiteleg shrimp (Litopenaeus vannamei) cultured under laboratory conditions. Aquaculture 2012, 362–363, 28–32. [Google Scholar]
- Hoseinifar, S.H.; Mirvaghefi, A.; Amoozegar, M.A.; Merrifield, D.L.; Ringø, E. In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Nutr. 2015, 23, 111–118. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringø, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Benítez-Santana, T.; Caballero, M.J.; Montero, D.; Sweetman, J.; Izquierdo, M. Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Fish Shellfish Immunol. 2011, 30, 674–681. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Collado, M.C.; Vesterlund, S.; Mazurkiewicz, J.; Salminen, S. Adhesion abilities of commensal fish bacteria by use of mucus model system: Quantitative analysis. Aquaculture 2011, 318, 33–36. [Google Scholar] [CrossRef]
- Rungrassamee, W.; Kingcha, Y.; Srimarut, Y.; Maibunkaew, S.; Karoonuthaisiri, N.; Visessanguan, W. Mannooligosaccharides from copra meal improves survival of the Pacific white shrimp (Litopenaeus vannamei) after exposure to Vibrio harveyi. Aquaculture 2014, 434, 403–410. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ringø, E.; Shenavar Masouleh, A.; Esteban, M.Á. Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Rev. Aquac. 2016, 8, 89–102. [Google Scholar] [CrossRef]
- Khalil, S.R.; Reda, R.M.; Awad, A. Efficacy of Spirulina platensis diet supplements on disease resistance and immune-related gene expression in Cyprinus carpio L. exposed to herbicide atrazine. Fish Shellfish Immunol. 2017, 67, 119–128. [Google Scholar] [CrossRef]
- Ringø, E.; Song, S.K. Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac. Nutr. 2016, 22, 4–24. [Google Scholar] [CrossRef]
- Subramanian, S.; MacKinnon, S.L.; Ross, N.W. A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, H. Carp. Yoshoku 1997, 34, 108–111. [Google Scholar]
- Metz, J.R.; Huising, M.O.; Leon, K.; Verburg-van Kemenade, B.M.L.; Flik, G. Central and peripheral interleukin-1 and interleukin-1 receptor I expression and their role in the acute stress response of common carp, Cyprinus carpio L. J. Endocrinol. 2006, 191, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, J.; Rašković, B.; Blust, R.; De Boeck, G. Exercise improves growth, alters physiological performance and gene expression in common carp (Cyprinus carpio). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 226, 38–48. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, Y.; Ji, X.; Zhang, R.; Liang, T.; Du, Q.; Chang, Z. Optimal reference genes in different tissues, gender, and gonad of Yellow River carp (Cyprinus carpio var) at various developmental periods. Pak. J. Zool. 2016, 48, 1615–1622. [Google Scholar]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Silver, N.; Best, S.; Jiang, J.; Thein, S. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar] [PubMed]
- Dawar, F.U.; Tu, J.; Xiong, Y.; Lan, J.; Dong, X.X.; Liu, X.; Khattak, M.N.K.; Mei, J.; Lin, L. Chemotactic Activity of Cyclophilin A in the Skin Mucus of Yellow Catfish (Pelteobagrus fulvidraco) and Its Active Site for Chemotaxis. Int. J. Mol. Sci. 2016, 17, 1422. [Google Scholar] [CrossRef] [PubMed]
- Byadgi, O.; Chen, Y.-C.; Maekawa, S.; Wang, P.-C.; Chen, S.-C. Immune-Related Functional Differential Gene Expression in Koi Carp (Cyprinus carpio) after Challenge with Aeromonas sobria. Int. J. Mol. Sci. 2018, 19, 2107. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Watanuki, H.; Ota, K.; Tassakka, A.C.M.A.R.; Kato, T.; Sakai, M. Immunostimulant effects of dietary Spirulina platensis on carp, Cyprinus carpio. Aquaculture 2006, 258, 157–163. [Google Scholar] [CrossRef]
- Pietsch, C. Zearalenone (ZEN) and Its Influence on Regulation of Gene Expression in Carp (Cyprinus carpio L.) Liver Tissue. Toxins 2017, 9, 283. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Song, S.K.; Beck, B.R.; Kim, D.; Park, J.; Kim, J.; Kim, H.D.; Ringø, E. Prebiotics as immunostimulants in aquaculture: A review. Fish Shellfish Immunol. 2014, 40, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cerezuela, R.; Meseguer, J.; Esteban, A. Current Knowledge in Synbiotic Use for Fish Aquaculture: A Review. J. Aquac. Res. Dev. 2011. [Google Scholar] [CrossRef]
- Huynh, T.-G.; Shiu, Y.-L.; Nguyen, T.-P.; Truong, Q.-P.; Chen, J.-C.; Liu, C.-H. Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: A review. Fish Shellfish Immunol. 2017, 64, 367–382. [Google Scholar] [CrossRef]
- Mo, F.; Zhao, J.; Liu, N.; Cao, L.-H.; Jiang, S.-X. Validation of reference genes for RT-qPCR analysis of CYP4T expression in crucian carp. Genet. Mol. Biol. 2014, 37, 500–507. [Google Scholar] [CrossRef]
- McCurley, A.T.; Callard, G.V. Characterization of housekeeping genes in zebrafish: Male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef]
- Filby, A.L.; Tyler, C.R. Appropriate “housekeeping” genes for use in expression profiling the effects of environmental estrogens in fish. BMC Mol. Biol. 2007, 8, 10. [Google Scholar] [CrossRef]
- Julin, K.; Johansen, L.-H.; Sommer, A.-I. Reference genes evaluated for use in infectious pancreatic necrosis virus real-time RT-qPCR assay applied during different stages of an infection. J. Virol. Methods 2009, 162, 30–39. [Google Scholar] [CrossRef]
- Jorgensen, S.M.; Kleveland, E.J.; Grimholt, U.; Gjoen, T. Validation of Reference Genes for Real-Time Polymerase Chain Reaction Studies in Atlantic Salmon. Mar. Biotechnol. 2006, 8, 398–408. [Google Scholar] [CrossRef]
- Small, B.C.; Murdock, C.A.; Bilodeau-Bourgeois, A.L.; Peterson, B.C.; Waldbieser, G.C. Stability of reference genes for real-time PCR analyses in channel catfish (Ictalurus punctatus) tissues under varying physiological conditions. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 296–304. [Google Scholar] [CrossRef]
- Yabu, T.; Toda, H.; Shibasaki, Y.; Araki, K.; Yamashita, M.; Anzai, H.; Mano, N.; Masuhiro, Y.; Hanazawa, S.; Shiba, H.; et al. Antiviral protection mechanisms mediated by ginbuna crucian carp interferon gamma isoforms 1 and 2 through two distinct interferon gamma-receptors. J. Biochem. 2011, 150, 635–648. [Google Scholar] [CrossRef]
- Savan, R.; Aman, A.; Sakai, M. Molecular cloning of G type lysozyme cDNA in common carp (Cyprinus carpio L.). Fish Shellfish Immunol. 2003, 15, 263–268. [Google Scholar] [CrossRef]
- Ellis, A.E. Immunity to bacteria in fish. Fish Shellfish Immunol. 1999, 9, 291–308. [Google Scholar] [CrossRef]
- Hicks, P.S.; Saunero-Nava, L.; Du Clos, T.W.; Mold, C. Serum amyloid P component binds to histones and activates the classical complement pathway. J. Immunol. 1992, 149, 3689–3694. [Google Scholar] [PubMed]
- Falco, A.; Cartwright, J.R.; Wiegertjes, G.F.; Hoole, D. Molecular characterization and expression analysis of two new C-reactive protein genes from common carp (Cyprinus carpio). Dev. Comp. Immunol. 2012, 37, 127–138. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ahmadi, A.; Raeisi, M.; Hoseini, S.M.; Khalili, M.; Behnampour, N. Comparative study on immunomodulatory and growth enhancing effects of three prebiotics (galactooligosaccharide, fructooligosaccharide and inulin) in common carp (Cyprinus carpio). Aquac. Res. 2017, 48, 3298–3307. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Zoheiri, F.; Dadar, M.; Rufchaei, R.; Ringø, E. Dietary galactooligosaccharide elicits positive effects on non-specific immune parameters and growth performance in Caspian white fish ( Rutilus frisii kutum ) fry. Fish Shellfish Immunol. 2016, 56, 467–472. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sharifian, M.; Vesaghi, M.J.; Khalili, M.; Esteban, M.Á. The effects of dietary xylooligosaccharide on mucosal parameters, intestinal microbiota and morphology and growth performance of Caspian white fish (Rutilus frisii kutum) fry. Fish Shellfish Immunol. 2014, 39, 231–236. [Google Scholar] [CrossRef]
- Miandare, H.K.; Farvardin, S.; Shabani, A.; Hoseinifar, S.H.; Ramezanpour, S.S. The effects of galactooligosaccharide on systemic and mucosal immune response, growth performance and appetite related gene transcript in goldfish (Carassius auratus gibelio). Fish Shellfish Immunol. 2016, 55, 479–483. [Google Scholar] [CrossRef]
- Nawaz, A.; Bakhsh javaid, A.; Irshad, S.; Hoseinifar, S.H.; Xiong, H. The functionality of prebiotics as immunostimulant: Evidences from trials on terrestrial and aquatic animals. Fish Shellfish Immunol. 2018, 76, 272–278. [Google Scholar] [CrossRef]
- Tzortzis, G.; Goulas, A.K.; Gibson, G.R. Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl. Microbiol. Biotechnol. 2005, 68, 412–416. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.R. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [PubMed]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’Anson, K.; Gibson, G.R. Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: A randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Drakoularakou, A.; Tzortzis, G.; Rastall, R.A.; Gibson, G.R. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur. J. Clin. Nutr. 2010, 64, 146–152. [Google Scholar] [CrossRef]
- Vulevic, J.; Tzortzis, G.; Juric, A.; Gibson, G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 2018, 30, e13440. [Google Scholar] [CrossRef]
- Slawinska, A.; Dunislawska, A.; Plowiec, A.; Radomska, M.; Lachmanska, J.; Siwek, M.; Tavaniello, S.; Maiorano, G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery In Ovo. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Slawinska, A.; Plowiec, A.; Siwek, M.; Jaroszewski, M.; Bednarczyk, M. Long-Term Transcriptomic Effects of Prebiotics and Synbiotics Delivered In Ovo in Broiler Chickens. PLoS ONE 2016, 11, e0168899. [Google Scholar] [CrossRef]
- Siwek, M.; Slawinska, A.; Stadnicka, K.; Bogucka, J.; Dunislawska, A.; Bednarczyk, M. Prebiotics and synbiotics—In ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018, 14, 1–17. [Google Scholar] [CrossRef]
- Slawinska, A.; Mendes, S.; Dunislawska, A.; Siwek, M.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Tavaniello, S.; Maiorano, G. Avian model to mitigate gut-derived immune response and oxidative stress during heat. Biosystems. 2019, 178, 10–15. [Google Scholar] [CrossRef]
- Slawinska, A.; Zampiga, M.; Sirri, F.; Meluzzi, A.; Bertocchi, M.; Tavaniello, S.; Maiorano, G. Impact of galactooligosaccharides delivered in ovo on mitigating negative effects of heat stress on performance and welfare of broilers. Poult. Sci. 2020, 99, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Composition (%) | |
|---|---|---|
| CON 11 | GOS 12 | |
| Wheat meal | 32.8 | 30.8 |
| Fish meal 1 | 12.3 | 12.3 |
| Blood meal 2 | 10.0 | 10.0 |
| DDGS 3 | 11.0 | 11.0 |
| Soybean meal 4 | 15.0 | 15.0 |
| Rapeseed meal 5 | 10.0 | 10.0 |
| Fish oil 6 | 4.6 | 4.6 |
| Soybean lecithin 7 | 1.0 | 1.0 |
| Vitamin-mineral premix 8 | 1.5 | 1.5 |
| Vitamin premix 9 | 0.1 | 0.1 |
| Choline chloride | 0.2 | 0.2 |
| Fodder chalk | 1.5 | 1.5 |
| Prebiotic 10 | 0 | 2 |
| Proximate composition (% dry matter) | ||
| Crude protein | 35.06 | |
| Essential amino acids (g 100 g −1 of crude protein) Arginine | 4.53 | |
| Histidine | 2.80 | |
| Lysine | 3.50 | |
| Tryptophan | 1.04 | |
| Phenylalanine + Tyrosine | 4.96 | |
| Methionine + Cysteine | 1.75 | |
| Threonine | 3.13 | |
| Leucine | 6.72 | |
| Isoleucine | 3.90 | |
| Valine | 4.97 | |
| Total lipid | 9.08 | |
| Crude fiber | 3.93 | |
| Total phosphorus | 0.83 | |
| Calcium | 1.36 | |
| Ash | 7.17 | |
| Gross energy (MJ·kg −1) | 18.51 | |
| Name | Gene | NCBI Gene ID | Primer Sequences (5’→3’) | Ref |
|---|---|---|---|---|
| Beta-actin | ACTB | 109073280 | F:ATCCGTAAAGACCTGTATGCCA R:GGGGAGCAATGATCTTGATCTTCA | [24] |
| Elongation factor 1-alpha | EF-1α | 109111735 | F:TGGAGATGCTGCCATTGT R:TGCAGACTTCGTGACCTT | [34] |
| Glyceraldehyde-3-phosphate dehydrogenase-like | GAPDH | 109106399 | F:ATCTGACGGTCCGTCT R:CCAGCACCGGCATCAAA | [34] |
| 18S ribosomal RNA | 18s rRNA | FJ710826.1 | F:GAGTATGGTTGCAAAGCTGAAAC R:AATCTGTCAATCCTTTCCGTGTCC | [35] |
| 40S ribosomal protein S11 | 40s s11 | 109061205 | F:CCGTGGGTGACATCGTTACA R:TCAGGACATTGAACCTCACTGTCT | [33] |
| Name | Gene | Gene ID | Function 1 | Primer Sequences (5’→3’) | Ref. 2 |
|---|---|---|---|---|---|
| Acute-phase protein | |||||
| C-reactive protein | CRP | 109083752 | Host defense: it promotes agglutination, bacterial capsular swelling, phagocytosis, and complement fixation through its calcium-dependent binding to phosphorylcholine. | F:AGCTTTGGAAAATTCGGTTCACC R:ACTCACCCTCGTGTCACTGC | This study |
| Antimicrobial peptides (AMP) | |||||
| Histone H2A.V-like | His2Av | 109068402 | Main role in transcription regulation, DNA repair, DNA replication, and chromosomal stability | F:CTGGTGGAGGTGTGATTCCT R:AGCGGGAACTACACGGTCTT | This study |
| Protein-glutamine gamma-glutamyltransferase 5-like | GGGT5L | 109112827 | Key role in the gamma-glutamyl cycle and maintains normal redox status | F:AGCTGCATATCATGGACGAGTT R:CTCCGCAGAACCAGAGTGCT | This study |
| Cytokines | |||||
| Interleukin 1 beta-like | IL1β | 109097442 | Mediator of the inflammatory response, and is involved in a variety of cellular activities, including cell proliferation, differentiation, and apoptosis | F:AAGGAGGCCAGTGGCTCTGT R:CCTGAAGAAGAGGAGGCTGTCA | [46] |
| Interleukin 4 | IL4 | 109064937 | Participates in at least several B-cell activation processes as well as other cell types. It is a costimulator of DNA-synthesis. It induces the expression of class II MHC molecules on resting B-cells | F:TTTCTGGGCTGTCTGGTGCCAA R:TTTCTTGTCAGTACGGAAATGCTCA | [47] |
| Interleukin 8-like | IL8 | 109085034 | Chemotactic factor that attracts neutrophils, basophils, and T-cells, but not monocytes. It is also involved in neutrophil activation. It is released from several cell types in response to an inflammatory stimulus | F:GATGCAAATGCCCTCAAATACA R:GGCTCTTGACGTTCCTTTTG | [43] |
| Interleukin 10-like | IL10 | 109076801 | Major immune-regulatory cytokine that acts on many cells of the immune system where it has profound anti-inflammatory functions, limiting excessive tissue disruption caused by inflammation | F:CGCCAGCATAAAGAACTCGT R:TGCCAAATACTGCTCGATGT | [46] |
| Interferon gamma | IFNγ | 109053615 | Produced by lymphocytes activated by specific antigens or mitogens | F:TGAGCTTAAAGAATGTGTGGCCCAA R:ACTCCATATGTGACGGCTTTTGGT | [47] |
| Lectins | |||||
| C-type lectin 4 | CLEC4M | 109066444 | Binds carbohydrates mannose and fucose | F:TCAACTGGTCAGAGGCACGA R:GAAAGGCCCACTCTTCATCGTC | This study |
| Lyzosymes | |||||
| Lyzosyme C | LyzC | 109090952 | Protection against pathogens | F:ATGAAGGTGACTATTGCTGTCTTG R:AGTAGGCCGTGCACACATAGTT | This study |
| Lyzosyme G | LyzG | 109087581 | Protection against pathogens | F:GGCCTTCAGACGATACTTACCA R:TGGAAGCCTCGACACCCTTT | This study |
| Mucins | |||||
| Mucin-5AC-like | M5ACL3 (LOC109110796) | 109110796 | Forming protective mucous barriers on epithelial surfaces | F:CGATCAGTGCTATGTCCTGTCA R:ACAGTTGGGCTCACGTTTGT | This study |
| Peroxidases | |||||
| Myeloperoxidase-like | MPO | 109052003 | Produces hypochlorous acid from hydrogen peroxide and chloride anion during the neutrophil’s respiratory burst, oxidizes tyrosine to the tyrosyl radical using hydrogen peroxide as an oxidizing agent | F:CAACCTGGTCCACAAGGTGTAGC R:GGCAGACTGTTGTCCTGTGG | This study |
| Proteases | |||||
| Cathepsin B | CTSB | 109064698 | Bacteriolytic activity against fish pathogen | F:CACTGACTGGGGTGATAATGGATA R:GGTGCTCATTTCAGCCCTCCT | This study |
| Cathepsin D | CTSD | 109105685 | Regulates production of parasin I | F:CGACGGCTCGCCAAAATGAG R:AGAGGAATCCGTACAATTGCGT | This study |
| Oxidoreductase | |||||
| Thioredoxin-like | TXNL3 (LOC109108046) | 109108046 | Cell redox homeostasis | F:GCGGGCTGCTGCTTTGACTG R:GTCGAAGGCAGGCTTATCCTCA | This study |
| Reference genes | |||||
| Beta-actin | ACTB | 109073280 | Actins are highly conserved proteins that are involved in cell motility, structure, integrity, and intercellular signaling | F:ATCCGTAAAGACCTGTATGCCA R:GGGGAGCAATGATCTTGATCTTCA | [24] |
| 40S ribosomal protein S11 | 40s s11 | 109061205 | Relation with viral mRNA translation and activation of the mRNA pathways upon binding of the cap-binding complex and eIFs, and subsequent binding to 43S | F:CCGTGGGTGACATCGTTACA R:TCAGGACATTGAACCTCACTGTCT | [33] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, E.; Mazurkiewicz, J.; Slawinska, A. Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides. Animals 2020, 10, 438. https://doi.org/10.3390/ani10030438
Pietrzak E, Mazurkiewicz J, Slawinska A. Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides. Animals. 2020; 10(3):438. https://doi.org/10.3390/ani10030438
Chicago/Turabian StylePietrzak, Elzbieta, Jan Mazurkiewicz, and Anna Slawinska. 2020. "Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides" Animals 10, no. 3: 438. https://doi.org/10.3390/ani10030438
APA StylePietrzak, E., Mazurkiewicz, J., & Slawinska, A. (2020). Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides. Animals, 10(3), 438. https://doi.org/10.3390/ani10030438







