The Expression of ERK1/2 in Female Yak (Bos grunniens) Reproductive Organs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Yak’s Reproducitve Organs
2.2. erk1 and erk2 Gene Expression Analysis
2.3. The Quantitative Analysis of ERK1/2 Proteins Expression
2.4. Localizational Analysis of ERK1/2 Proteins Expression
2.5. Data Analysis
3. Results
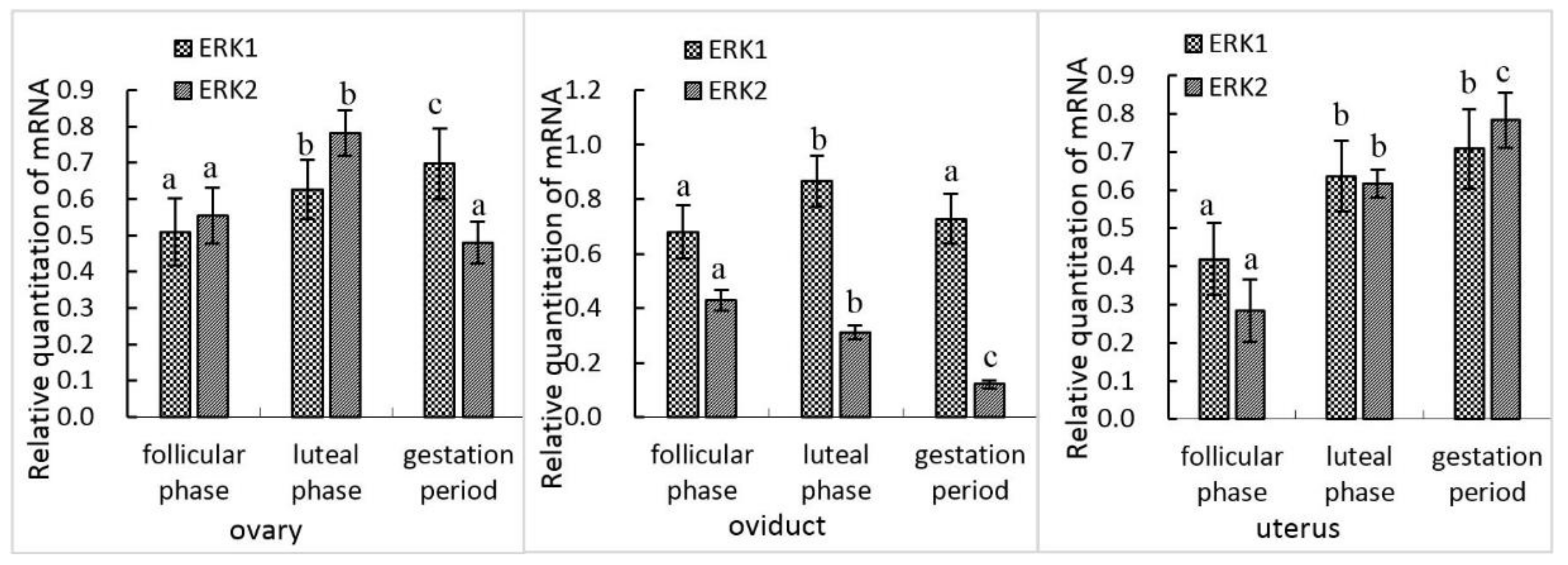
3.1. mRNA Expression of erk1 and erk2 in Female Yak Reproductive Organs
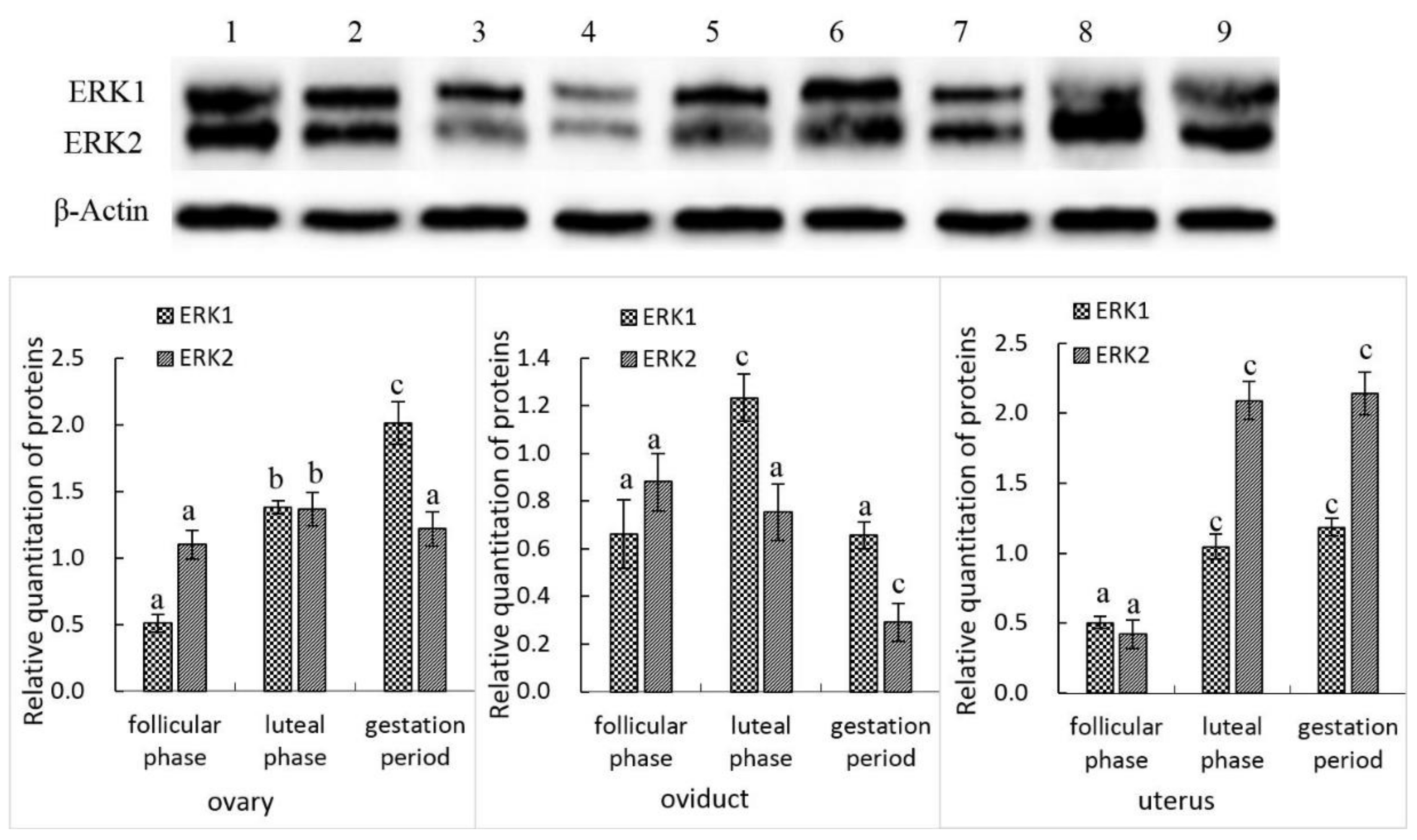
3.2. ERK1 and ERK2 Protein Expression in the Female Yak’s Reproductive Organs
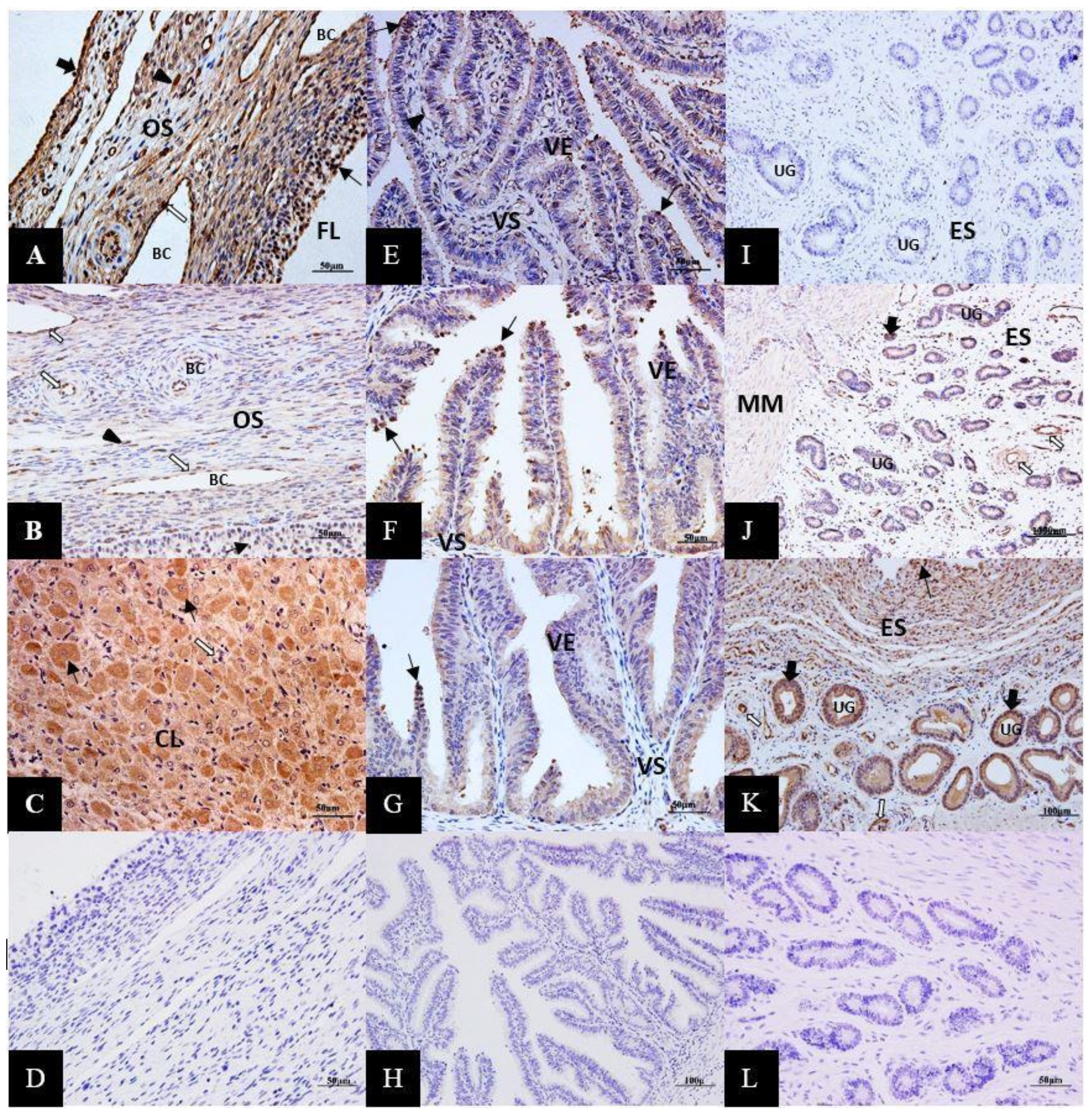
3.3. Immunolocalization of ERK1/2 Proteins in the Female Yak’s Reproductive Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiyokawa, E.; Takai, S.; Tanaka, M.; Iwase, T.; Suzuki, M.; Xiang, Y.; Naito, Y.; Yamada, K.; Sugimura, H.; Kino, I. Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res. 1994, 54, 3645–3650. [Google Scholar]
- Iwase, T.; Tanaka, M.; Suzuki, M.; Naito, Y.; Sugimura, H.; Kino, I. Identification of Protein-Tyrosine Kinase Genes Preferentially Expressed in Embryo Stomach and Gastric Cancer. Biochem. Biophys. Res. Commun. 1993, 194, 700–705. [Google Scholar] [CrossRef]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis . IUBMB Life 2008, 58, 621–631. [Google Scholar]
- Erhardt, P.; Schremser, E.J.; Cooper, G.M. B-Raf inhibits programmed cell death downstream of cytochrome c release from mitochondria by activating the MEK/Erk pathway. Mol. Cell. Biol. 1999, 19, 5308–5315. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Carter, S.; Reardon, D.B.; Schmidt-Ullrich, R.; Dent, P.; Fisher, P.B. Roles for basal and stimulated p21(Cip-1/WAF1/MDA6) expression and mitogen-activated protein kinase signaling in radiation-induced cell cycle checkpoint control in carcinoma cells. Mol. Biol. Cell 1999, 10, 4231–4246. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, D.; Tanemura, S.; Ohata, S.; Shimizu, N.; Seo, J.; Nishitai, G.; Watanabe, T.; Nakagawa, K.; Kishimoto, H.; Wada, T.; et al. Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation. Its implication in an anti-apoptotic function. J. Biol. Chem. 2002, 277, 366–371. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, S.; Joazeiro, C.; Cobb, M.H.; Hunter, T. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol. Cell 2002, 9, 945–956. [Google Scholar] [CrossRef]
- Nagata, Y.; Todokoro, K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood 1999, 94, 853–863. [Google Scholar] [CrossRef]
- Tran, S.E.; Holmstrom, T.H.; Ahonen, M.; Kahari, V.M.; Eriksson, J.E. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J. Biol. Chem. 2001, 276, 16484–16490. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ju, J.W.; Oh, C.D.; Yoon, Y.M.; Song, W.K.; Kim, J.H.; Yoo, Y.J.; Bang, O.S.; Kang, S.S.; Chun, J.S. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J. Biol. Chem. 2002, 277, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The cellular response to oxidative stress: Influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 1998, 333 Pt 2, 291–300. [Google Scholar] [CrossRef]
- Buckley, S.; Driscoll, B.; Barsky, L.; Weinberg, K.; Anderson, K.; Warburton, D. ERK activation protects against DNA damage and apoptosis in hyperoxic rat AEC2. Am. J. Physiol. 1999, 277, L159–L166. [Google Scholar] [CrossRef] [PubMed]
- Karagiota, A.; Kourti, M.; Simos, G.; Mylonis, I. HIF-1α-derived cell-penetrating peptides inhibit ERK-dependent activation of HIF-1 and trigger apoptosis of cancer cells under hypoxia. Cell. Mol. Life Sci. 2018, 76, 809–825. [Google Scholar] [CrossRef]
- Mottet, D.; Michel, G.; Renard, P.; Ninane, N.; Raes, M.; Michiels, C. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J. Cell Physiol. 2003, 194, 30–44. [Google Scholar] [CrossRef]
- Minet, E.; Arnould, T.; Michel, G.; Roland, I.; Mottet, D.; Raes, M.; Remacle, J.; Michiels, C. ERK activation upon hypoxia: Involvement in HIF-1 activation. FEBS Lett. 2000, 468, 53–58. [Google Scholar] [CrossRef]
- Almog, T.; Naor, Z. Mitogen activated protein kinases (MAPKs) as regulators of spermatogenesis and spermatozoa functions. Mol. Cell. Endocrinol. 2008, 282, 39–44. [Google Scholar] [CrossRef]
- Tong, J.S.; Zhang, Q.H.; Huang, X.; Fu, X.Q.; Qi, S.T.; Wang, Y.P.; Hou, Y.; Sheng, J.; Sun, Q.Y. Icaritin causes sustained ERK1/2 activation and induces apoptosis in human endometrial cancer cells. PLoS ONE 2011, 6, e16781. [Google Scholar] [CrossRef]
- Samuels, I.S.; Karlo, J.C.; Faruzzi, A.N.; Pickering, K.; Herrup, K.; Sweatt, J.D.; Saitta, S.C.; Landreth, G.E. Deletion of ERK2 Mitogen-Activated Protein Kinase Identifies Its Key Roles in Cortical Neurogenesis and Cognitive Function. J. Neurosci. 2008, 28, 6983–6995. [Google Scholar] [CrossRef]
- Wang, Y.; Kristensen, G.B.; Helland, A.; Nesland, J.M.; Borresen-Dale, A.L.; Holm, R. Protein expression and prognostic value of genes in the erb-b signaling pathway in advanced ovarian carcinomas. Am. J. Clin. Pathol. 2005, 124, 392–401. [Google Scholar] [CrossRef]
- Wong, C.H.; Cheng, C.Y. Mitogen-activated protein kinases, adherens junction dynamics, and spermatogenesis: A review of recent data. Dev. Biol. 2005, 286, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bao, F.; Zhi, D.; Liu, M.; Yan, Q.; Zheng, X.; Ren, L.; Cong, S.; Li, Y.; Cao, G. Lipopolysaccharide induces SBD-1 expression via the P38 MAPK signaling pathway in ovine oviduct epithelial cells. Lipids Health Dis. 2016, 15, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Cui, H.; Liu, S.; Yu, Q.; Wu, H.; Liang, L.; Guan, Y.; Xin, G.; Long, Z.; Fan, H.Y. Lgr4 Gene Regulates Corpus Luteum Maturation Through Modulation of the WNT-Mediated EGFR-ERK Signaling Pathway. Endocrinology 2014, 155, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, Y.; Rovani, M.T.; Gasperin, B.; Ferreira, R.; Ferst, J.; Madogwe, E.; Gonçalves, P.B.; Bordignon, V.; Duggavathi, R. ERK1/2-dependent gene expression in the bovine ovulating follicle. Sci. Rep. 2018, 8, 16170. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.E.; Casey, S.M.; Canty, M.J.; Crowe, M.A.; Martin, F.; Evans, A.C.O. Akt and Erk signal transduction pathways are early markers of differentiation in dominant and subordinate ovarian follicles in cattle. Reproduction 2007, 133, 617–626. [Google Scholar] [CrossRef]
- Choi, J.; Jo, M.; Lee, E.; Choi, D. ERK1/2 is involved in luteal cell autophagy regulation during corpus luteum regression via an mTOR-independent pathway. Mol. Hum. Reprod. 2014, 20, 972–980. [Google Scholar] [CrossRef]
- Lee, J.H.; Banu, S.K.; McCracken, J.A.; Arosh, J.A. Early pregnancy modulates survival and apoptosis pathways in the corpus luteum in sheep. Reproduction 2016, 151, 187–202. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Zhang, K.; Mao, X.; Ding, X.; Zeng, Q.; Bai, S.; Xuan, Y.; Peng, H. Vanadate oxidative and apoptotic effects are mediated by the MAPK-Nrf2 pathway in layer oviduct magnum epithelial cells. Metallomics 2017, 9, 1562–1575. [Google Scholar] [CrossRef]
- Crepieux, P.; Marion, S.; Martinat, N.; Fafeur, V.; Vern, Y.L.; Kerboeuf, D.; Guillou, F.; Reiter, E. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 2001, 20, 4696–4709. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, Q.Y.; Breitbart, H.; Chen, D.Y. Expression and phosphorylation of mitogen-activated protein kinases during spermatogenesis and epididymal sperm maturation in mice. Arch. Androl. 1999, 43, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sette, C.; Barchi, M.; Bianchini, A.; Conti, M.; Rossi, P.; Geremia, R. Activation of the mitogen-activated protein kinase ERK1 during meiotic progression of mouse pachytene spermatocytes. J. Biol. Chem. 1999, 274, 33571–33579. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.M. Advances in research of yak resources in China. J. Nat. Resour. 2001, 16, 564–569. [Google Scholar]
- Qiu, Q.; Zhang, G.; Ma, T.; Qian, W.; Wang, J.; Ye, Z.; Cao, C.; Hu, Q.; Kim, J.; Larkin, D.M.; et al. The yak genome and adaptation to life at high altitude. Nat. Genet. 2012, 44, 946–949. [Google Scholar] [CrossRef]
- Mann, G.E. Reproduction in the yak. Br. Vet. J. 1993, 149, 513–514. [Google Scholar] [CrossRef]
- Zi, X.D. Reproduction in female yaks (Bos grunniens) and opportunities for improvement. Theriogenology 2003, 59, 1303–1312. [Google Scholar] [CrossRef]
- Lan, D.; Xiong, X.; Huang, C.; Mipam, T.D.; Li, J. Toward Understanding the Genetic Basis of Yak Ovary Reproduction: A Characterization and Comparative Analyses of Estrus Ovary Transcriptiome in Yak and Cattle. PLoS ONE 2016, 11, e0152675. [Google Scholar] [CrossRef]
- Yu, S.J.; Huang, Y.M.; Chen, B.X. Reproductive patterns of the yak. III. Levels of progesterone and oestradiol-17β during pregnancy and the periparturient period. Br. Vet. J. 1993, 149, 595–602. [Google Scholar] [CrossRef]
- Yu, S.J.; Huang, Y.M.; Chen, B.X. Reproductive patterns of the yak. II. Progesterone and oestradiol-17β levels in plasma and milk just before the breeding season; also during normal and short oestrous cycles. Br. Vet. J. 1993, 149, 585–593. [Google Scholar] [CrossRef]
- JiangFeng, F.; Jiu, Y.; Wen, Z.; Ben, L. The expression of Fas/FasL and apoptosis in yak placentomes. Anim. Reprod. Sci. 2011, 128, 107–116. [Google Scholar] [CrossRef]
- Fan, J.; Yu, S.; Cui, Y.; Xu, G.; Wang, L.; Pan, Y.; He, H. Bcl-2/Bax protein and mRNA expression in yak (Bos grunniens) placentomes. Theriogenology 2017, 104, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.; Khan, N. Comparative Expression and Distribution of c-fos, Estrogen Receptorα (ERα), and p38α in the Uterus of Rats, Monkeys, and Humans. Toxicol. Pathol. 2006, 34, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.; Marusak, R.; Morris, D. Species Comparison of the Role of p38 MAP Kinase in the Female Reproductive System. J. Toxicol. Pathol. 2009, 22, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002, 143, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.E.; Glister, C.; Lonergan, P.; Martin, F.; Knight, P.G.; Evans, A.C. Functional significance of the signal transduction pathways Akt and Erk in ovarian follicles: In vitro and in vivo studies in cattle and sheep. J. Ovarian Res. 2008, 1, 2. [Google Scholar] [CrossRef]
- Henriquez, S.; Kohen, P.; Munoz, A.; Godoy, A.; Orge, F.; Strauss, J.F., 3rd; Devoto, L. In-vitro study of gonadotrophin signaling pathways in human granulosa cells in relation to progesterone receptor expression. Reprod. Biomed. Online 2017, 35, 363–371. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chang, H.M.; Taylor, E.L.; Liu, R.Z.; Leung, P.C.K. BMP6 Downregulates GDNF Expression Through SMAD1/5 and ERK1/2 Signaling Pathways in Human Granulosa-Lutein Cells. Endocrinology 2018, 159, 2926–2938. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hishinuma, M.; Shimada, M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J. Ovarian Res. 2009, 2, 20. [Google Scholar] [CrossRef]
- Wayne, C.M.; Fan, H.Y.; Cheng, X.; Richards, J.S. Follicle-stimulating hormone induces multiple signaling cascades: Evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol. Endocrinol. 2007, 21, 1940–1957. [Google Scholar] [CrossRef]
- Siddappa, D.; Beaulieu, E.; Gevry, N.; Roux, P.P.; Bordignon, V.; Duggavathi, R. Effect of the transient pharmacological inhibition of Mapk3/1 pathway on ovulation in mice. PLoS ONE 2015, 10, e0119387. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Q.; Nyegaard, M.; Overgaard, M.T.; Qiao, J.; Giudice, L.C. Participation of mitogen-activated protein kinase in luteinizing hormone-induced differential regulation of steroidogenesis and steroidogenic gene expression in mural and cumulus granulosa cells of mouse preovulatory follicles. Biol. Reprod. 2006, 75, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Cooke, I.D. The corpus luteum. Hum. Reprod. 1988, 3, 153–156. [Google Scholar] [CrossRef]
- Maekawa, R.; Lee, L.; Okada, M.; Asada, H.; Shinagawa, M.; Tamura, I.; Sato, S.; Tamura, H.; Sugino, N. Changes in gene expression of histone modification enzymes in rat granulosa cells undergoing luteinization during ovulation. J. Ovarian Res. 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Jiang, J.; Jin, P.; Kuang, M.; Wei, Q.; Shi, F.; Mao, D. Expression patterns of claudin-5 and its related signals during luteal regression in pseudopregnant rats: The enhanced effect of additional PGF treatment. Acta Histochem. 2018, 120, 221–227. [Google Scholar] [CrossRef]
- Chen, D.B.; Davis, J.S. Epidermal growth factor induces c-fos and c-jun mRNA via Raf-1/MEK1/ERK-dependent and -independent pathways in bovine luteal cells. Mol. Cell. Endocrinol. 2003, 200, 141–154. [Google Scholar] [CrossRef]
- Rueda, B.R.; Hendry, I.R.; Ndjountche, L.; Suter, J.; Davis, J.S. Stress-induced mitogen-activated protein kinase signaling in the corpus luteum. Mol. Cell. Endocrinol. 2000, 164, 59–67. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Wang, D.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; DuBois, R.N.; Dey, S.K. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nat. Med. 2004, 10, 1074–1080. [Google Scholar] [CrossRef]
- Lopez-Cardona, A.P.; Perez-Cerezales, S.; Fernandez-Gonzalez, R.; Laguna-Barraza, R.; Pericuesta, E.; Agirregoitia, N.; Gutierrez-Adan, A.; Agirregoitia, E. CB1 cannabinoid receptor drives oocyte maturation and embryo development via PI3K/Akt and MAPK pathways. FASEB J. 2017, 31, 3372–3382. [Google Scholar] [CrossRef]
- Leese, H.J.; Hugentobler, S.A.; Gray, S.M.; Morris, D.G.; Sturmey, R.G.; Whitear, S.-L.; Sreenan, J.M. Female reproductive tract fluids: Composition, mechanism of formation and potential role in the developmental origins of health and disease. Reprod. Fertil. Dev. 2007, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buhi, W.C.; Alvarez, I.M.; Kouba, A.J. Secreted proteins of the oviduct. Cells Tissues Organs 2000, 166, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, S.; Rehfeld, S.; Ulbrich, S.E.; Mallok, S.; Prelle, K.; Wenigerkind, H.; Einspanier, R.; Blum, H.; Wolf, E. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J. Mol. Endocrinol. 2004, 32, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Cerny, K.L.; Garrett, E.; Walton, A.J.; Anderson, L.H.; Bridges, P.J. A transcriptomal analysis of bovine oviductal epithelial cells collected during the follicular phase versus the luteal phase of the estrous cycle. Reprod. Biol. Endocrinol. 2015, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Soleilhavoup, C.; Riou, C.; Tsikis, G.; Labas, V.; Harichaux, G.; Kohnke, P.; Reynaud, K.; de Graaf, S.P.; Gerard, N.; Druart, X. Proteomes of the Female Genital Tract During the Oestrous Cycle. Mol. Cell. Proteom. 2016, 15, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef]
- Acuña, O.; Avilés, M.; López-Úbeda, R.; Guillen-Martinez, A.; Soriano-Úbeda, C.; Torrecillas, A.; Coy, P.; Izquierdo Rico, M.J. Differential gene expression in porcine oviduct during the oestrous cycle. Reprod. Fertil. Dev. 2017, 29, 2387–2399. [Google Scholar] [CrossRef]
- Seytanoglu, A.; Georgiou, A.S.; Sostaric, E.; Watson, P.F.; Holt, W.V.; Fazeli, A. Oviductal cell proteome alterations during the reproductive cycle in pigs. J. Proteome Res. 2008, 7, 2825–2833. [Google Scholar] [CrossRef]
- Tone, A.A.; Begley, H.; Sharma, M.; Murphy, J.; Rosen, B.; Brown, T.J.; Shaw, P.A. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin. Cancer Res. 2008, 14, 4067–4078. [Google Scholar] [CrossRef]
- George, S.H.; Greenaway, J.; Milea, A.; Clary, V.; Shaw, S.; Sharma, M.; Virtanen, C.; Shaw, P.A. Identification of abrogated pathways in fallopian tube epithelium from BRCA1 mutation carriers. J. Pathol. 2011, 225, 106–117. [Google Scholar] [CrossRef]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Handbook of Toxicologic Pathology; Academic Press: San Diego, CA, USA, 2002; pp. 47–894. [Google Scholar]
- Marusak, R.A.; Radi, Z.A.; Obert, L. Expression of Ki-67 in the uterus during various stages of the estrous cycle in rats. Exp. Toxicol. Pathol. 2007, 59, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Pan, H.; Zhu, L.; Deng, Y.; Pollard, J.W. Progesterone inhibits the estrogen-induced phosphoinositide 3-kinase-->AKT-->GSK-3beta-->cyclin D1-->pRB pathway to block uterine epithelial cell proliferation. Mol. Endocrinol. 2005, 19, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Lessey, B. Two pathways of progesterone action in the human endometrium: Implications for implantation and contraception. Steroids 2003, 68, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Kokatam, S.; Blesson, C.; Fatima, I.; Kitchlu, S.; Jain, S.; Mehrotra, P.; Dwivedi, A. Expression of αVβ3 integrin in rat endometrial epithelial cells and its functional role during implantation. Gen. Comp. Endocrinol. 2008, 160, 124–133. [Google Scholar]
- Okulicz, W.C.; Ace, C.I.; Longcope, C.; Tast, J. Analysis of differential gene regulation in adequate versus inadequate secretory-phase endometrial complementary deoxyribonucleic acid populations from the rhesus monkey. Endocrinology 1996, 137, 4844–4850. [Google Scholar] [CrossRef]
- Thienel, T.; Chwalisz, K.; Winterhager, E. Expression of MAPkinases (Erk1/2) during decidualization in the rat: Regulation by progesterone and nitric oxide. Mol. Hum. Reprod. 2002, 8, 465–474. [Google Scholar] [CrossRef]
- Casals, G.; Ordi, J.; Creus, M.; Fabregues, F.; Casamitjana, R.; Quinto, L.; Campo, E.; Balasch, J. Osteopontin and alphavbeta3 integrin expression in the endometrium of infertile and fertile women. Reprod. Biomed. Online 2008, 16, 808–816. [Google Scholar] [CrossRef]
- Sayem, A.S.M.; Giribabu, N.; Muniandy, S.; Salleh, N. Effects of thyroxine on expression of proteins related to thyroid hormone functions (TR-alpha, TR-beta, RXR and ERK1/2) in uterus during peri-implantation period. Biomed. Pharm. 2017, 96, 1016–1021. [Google Scholar] [CrossRef]
- Welsh, T.; Johnson, M.; Yi, L.; Tan, H.; Rahman, R.; Merlino, A.; Zakar, T.; Mesiano, S. Estrogen receptor (ER) expression and function in the pregnant human myometrium: Estradiol via ERalpha activates ERK1/2 signaling in term myometrium. J. Endocrinol. 2012, 212, 227–238. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Han, X.; He, H.; Luo, Y.; Yu, S.; Cui, Y.; Xu, G.; Wang, L.; Pan, Y. The Expression of ERK1/2 in Female Yak (Bos grunniens) Reproductive Organs. Animals 2020, 10, 334. https://doi.org/10.3390/ani10020334
Fan J, Han X, He H, Luo Y, Yu S, Cui Y, Xu G, Wang L, Pan Y. The Expression of ERK1/2 in Female Yak (Bos grunniens) Reproductive Organs. Animals. 2020; 10(2):334. https://doi.org/10.3390/ani10020334
Chicago/Turabian StyleFan, Jiangfeng, Xiaohong Han, Honghong He, Yuzhu Luo, Sijiu Yu, Yan Cui, Gengquan Xu, Libin Wang, and Yangyang Pan. 2020. "The Expression of ERK1/2 in Female Yak (Bos grunniens) Reproductive Organs" Animals 10, no. 2: 334. https://doi.org/10.3390/ani10020334
APA StyleFan, J., Han, X., He, H., Luo, Y., Yu, S., Cui, Y., Xu, G., Wang, L., & Pan, Y. (2020). The Expression of ERK1/2 in Female Yak (Bos grunniens) Reproductive Organs. Animals, 10(2), 334. https://doi.org/10.3390/ani10020334




