Simple Summary
Hydrogen sulfide (H2S) is an important second messenger, which has been implicated in regulating placental angiogenesis. Our findings revealed that gestational dietary methionine could affect maternal and placental H2S concentrations. With the increase of dietary methionine, maternal plasma and placental H2S concentrations changed quadratically, which was consistent with placental vascular density and reproductive performance. The decrease in H2S production caused by an increase in dietary methionine was likely to be the cause for the increase in the rate of low birth weight piglets and needs further study.
Abstract
The placenta is a unique bond between the mother and the fetus during pregnancy, and a proper placental angiogenesis is vital for fetal development. H2S is an endogenous stimulator of angiogenesis that is mainly produced by the methionine transsulfurationpathway. The goal of this study was to evaluate the effect of gestational dietary methionine on maternal and placental H2S production in sows. Multiparous sows (Large×White; third parity; n = 65) were randomly allocated into five groups, with feed diets comprisingstandardized ileal digestible methionine/lysine (Met/Lys) ratios of 0.27 (nutrient requirements of swine (NRC); 2012 level), 0.32, 0.37, 0.42, and 0.47, respectively. The litter size and weight at birth were measured and recorded. Maternal blood samples were obtained at embryonic day (E) E40 d, E90 d, and E114 d of gestation. The placental samples were collected at parturition. The results showed that maternal plasma H2S concentration was not affected at E40 d. However, the maternal plasma H2S concentration changed quadratically with the dietary Met/Lys ratio at E90 d (p < 0.01) and E114 d (p = 0.03). The maximum maternal plasma H2S concentration was at the dietary Met/Lys ratio of 0.37. Meanwhile, maternal plasma H2S concentration was positively correlated with piglets born alive (p < 0.01) and litter weight (p < 0.01). Consistent with the maternal plasma, the placental H2S concentration also changed quadratically with the dietary Met/Lys ratio (p = 0.03); the Met/Lys ratio of 0.37 showed the maximum H2S concentration. In conclusion, our findings revealed that the gestational dietary Met/Lys ratio could affect maternal and placental H2S concentrations, which may be an important molecular mechanism affecting placental angiogenesis and piglet development.
1. Introduction
Methionine (Met) is an essential amino acid in the diets of pigs [1,2]. In addition to being a substrate for protein synthesis, Met is also a methyldonor in DNA, RNA, and protein methylation. This methylation reaction results in epigenetic changes in gene expression and protein function during embryonic development [3,4]. Functional amino acids are important for the development of the pig placenta [5]. In our previous work, we demonstrated that an appropriate increase in the methionine/lysine (Met/Lys) ratio in a sow’s diet had a positive effect on the sow’splacental vascular density and on the birth weight of the piglets [6]. However, the underlying mechanism by which Met promotes placental angiogenesis and fetal development is unclear.
Hydrogen sulfide (H2S) is a ubiquitous signaling molecule with important functions in many mammalian cells and tissues and is now considered to be the third gas-phase signaling molecule, in addition to carbon monoxide (CO) and nitric oxide (NO) [7,8,9]. Endogenous H2S is mainly produced by Met and the cysteine transsulfuration pathway (TSP), via the direct enzymatic action of cystathionine-γ-lyase (CGL) and cystathionine-β-synthase (CBS) [10,11]. Studies in humans have found that preeclampsia and intrauterine growth retardation (IUGR) correspond to a significant reduction in placental H2S production, placental angiogenesis, and blood flow velocity [12,13]. However, exogenous H2S donors significantly attenuated placental vascular dysplasia and IUGR, caused by CGL inhibition [14]. In vitro studies have also shown that H2S produced by trophoblast cells can promote angiogenesis in placental artery endothelial cells [15]. However, there were no reports of the regulation of H2S production in sow plasma and placenta. The goal of the current study was to investigate the role of the gestational methionine/lysine ratio on maternal and placental H2S production, both in vivo and in vitro.
2. Materials and Methods
2.1. Animals, Diets and Management
The experimental design and the dietary formula were described previously [6]. In this study, multiparous sows (Large×White; third parity; n = 65) were randomly allocated into five groups and fed diets with standardized ileal digestible methionine/lysine (Met/Lys) ratios of 0.27 (nutrient requirements of swine (NRC); 2012 level), 0.32, 0.37, 0.42, and 0.47 respectively. The diet was maintained at constant levels of Lys (average SID Lys 0.71%); the ingredients and nutrient compositions of the experimentaldiets are listed in Table S1. Sows were fed a gestational dietary 2.0 kg/day (from embryonic day E0 d to E3 d), 2.8 kg/day (E4 d to E30 d), 2.3 kg/day (E31 d to E90 d), and 2.9 kg/day (E91 d to farrowing). All sows were moved to individual farrowing crates with stalls at E90 d. The experimental protocol was approved by the Institutional Animal Ethics Committee of Huazhong Agricultural University(Ethics Code: HZAU SW-2016-014).
2.2. Recording and Sampling
The sample collection of blood and placenta was described previously [6]. Twelve sows by group were selected for blood sampling, and eight sows of each group were selected for placenta sampling. The litter size, the piglets born alive, the litter weight, and the average pig birth weight of each litter were recorded within 24 h after farrowing.
2.3. Preparation of Placenta Homogenate
The placenta was selected based on the average pig birth weight ± one standard deviation of each group. A total of 100 mg of placental tissue was separated and ground to a fine powder using a porcelain mortar and pestle, chilled with liquid nitrogen. Next, 10% of the tissue homogenate was made with 100 mM of ice cold KH2PO4 buffer (pH 7.4) and was centrifuged for 5 min at 13,000 rpm. The supernatant wasused for H2S content and production assay. The protein concentration was determined by the bicinchoninic acid (BCA) protein assay kit (Beyotime, Shanghai, China).
2.4. Cell Culture and Treatment
Porcine iliac artery endothelial cells (PIEC) were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China), cultured in Roswell Park memorial institute (RPMI) 1640 medium (Gibco, San Diego, CA, USA), and supplemented with 10% fetal bovine serum (FBS, Gibco, San Diego, CA, USA) and 1% penicillin/streptomycin solution (Sigma, St. Louis, MO, USA) at 37 °C in a 5% CO2 incubator. Before the Met treatment, the PIEC cells were seeded in a 12-well plate (2 × 105 cells/well); 80% confluent PIEC cells were placed in serum-free basic medium for 18 h, followed by Met-free RPMI 1640 medium for another 6 h, then treated with different concentrations of Met (0, 50, 200, 500, and 1000 μM) for 24 h (n = 4). The supernatant culture medium was collected for H2S content assay.
2.5. H2S Measurement
In this study, the H2S levels were measured through the formation of methylene blue [16,17]. For the plasma, 100 μL samples were mixed with 400 μL of 100 mM KH2PO4 buffer (pH 7.4) and 250 μL of 1% (w/v) zinc acetate; for the culture medium and placental tissue homogenate, 500 μL samples were mixed with 250 μL of 1% (w/v) zinc acetate. The reaction mixture was incubated at 37 °C for 2.5 h. The protein content in the samples was removed by adding 10% trichloroacetic acid (250 μL) to the reaction mixture, which was pelleted by centrifugation. The supernatant was then mixed with 100 μL of N-dimethyl-p-phenylenediamine sulfate (20 mM in 7.2 M HCl) and 100 μL of FeCl3 (30 mM in 1.2 M HCl) in a test tube. The mixture was incubated at room temperature for 15 min. The absorbance of the resulting solution was measured at 670 nm in a 96-well plate with a microplate reader. The H2S concentration was calculated using a standard (NaHS) calibration curve (0.04, 0.2, 0.4, 2, 4, 20, and 40 μM) (Aladdin, Shanghai, China).
2.6. Statistical Analyses
Statistical analysis was performed using the SAS statistical package (v 8.2; SAS Inst. Inc., Cary, NC, USA). A one-way analysis of variance (ANOVA) followed by a least-significant-difference (LSD) post-hoc test was conducted to explore the impact of the gestational dietary Met/lysine ratio on maternal plasma and placenta H2S concentrations, as shown in Figure 1, Figure 2 and Figure 3, where p < 0.05 was considered to indicate statistical significance. Regression analyses were performed to evaluate the quadratic effects of dietary Met/Lys ratio and H2S concentration, as shown in Figure 1 and Figure 2; the linear effects of dietary Met/Lys ratio and H2S concentration are shown in Figure 4. Spearman correlations were used to determine the association between the dietary Met/Lys ratio and the piglets born alive, the litter weight of those born alive, and the average pig birth weight; see Figure 4. Data in all the Figures are expressed as mean ± standard errors of the mean (SEM).
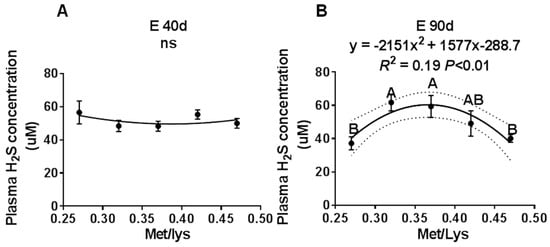
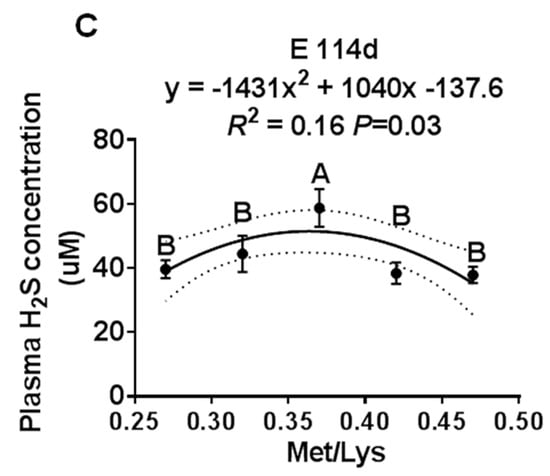
Figure 1.
The effect of the gestational dietary Met/lysine ratio on maternal plasma hydrogen sulfide (H2S) concentration at E40, E90, and E114: (A) maternal plasma H2S concentration at E40 d, (B) maternal plasma H2S concentration at E90 d, and (C) maternal plasma H2S concentration at E114 d, n = 8–12/group.
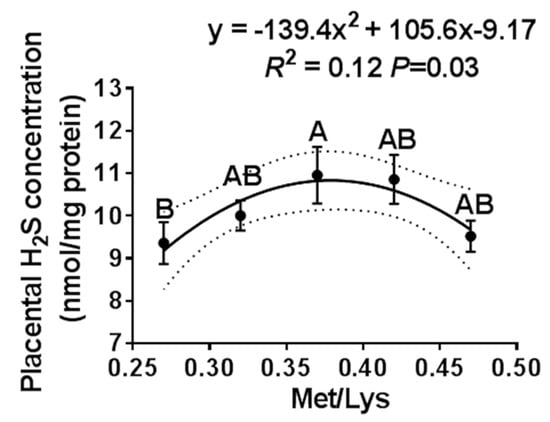
Figure 2.
The effect of the gestational dietary Met/lysine ratio on placental H2S concentration. Met/Lys ratios 0.27, n = 12; Met/Lys ratios 0.32, n = 14; Met/Lys ratios 0.37, n = 11; Met/Lys ratios 0.42, n = 11; and Met/Lys ratios 0.47, n = 11.
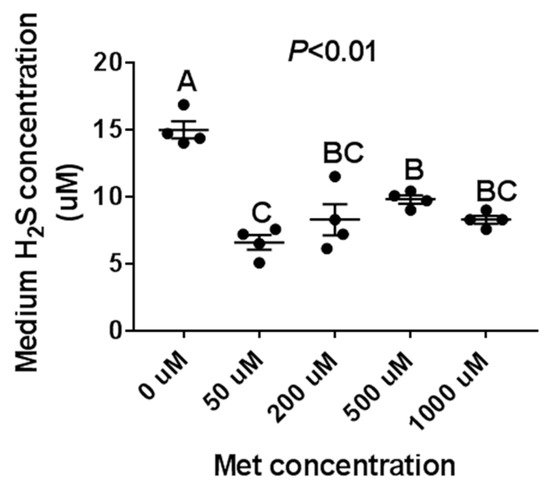
Figure 3.
The effect of the Met treatment on porcine iliac artery endothelial cells (PIEC) H2S production. The PIEC cells were seeded in a 12-well cell plate. After 80% confluence, the PIEC cells were placed in a serum-free medium for 18 h, followed by a Met-free RPMI 1640 medium for another 6 h, then treated with Met for 24 h. The supernatant culture medium was collected for H2S assay, n = 4/group.
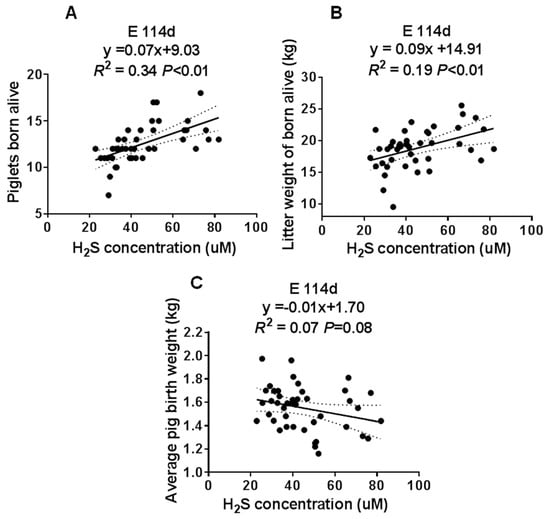
Figure 4.
The correlation analysis of the maternal plasma H2S concentration at E114d and the sow’s reproductive performance. (A) Piglets born alive. (B) Litter weight of piglets born alive. (C) Average pig birth weight. Met/Lys ratios 0.27, n = 8; Met/Lys ratios 0.32, n = 9; Met/Lys ratios 0.37, n = 10; Met/Lys ratios 0.42, n = 7; Met/Lys ratios 0.47, n = 7.
3. Results
3.1. The Gestational Dietary Met/Lys Ratio Affected Maternal Plasma H2S Concentration
To study the effect of the gestation dietary Met/Lys ratio on maternal plasma H2S concentration, we collected maternal plasma at different stages of pregnancy. We found that by E40 d, increasing the Met/Lys ratio in the diet had no effect on the maternal plasma H2S concentration, as shown in Figure 1A. However, at E90 d and E114 d, the maternal plasma H2S concentration changed quadratically, with an increase in the dietary Met/Lys ratio, as shown in Figure 1B,C. According to the quadratic polynomial regression equation, at E90 d (y = −2151x2 + 1577x −288.7, R2 = 0.19, p < 0.01) and at E114 d (y = −1431x2 + 1040x −137.6, R2 = 0.16, p = 0.03), the maximum maternal plasma H2S concentration was at the dietary Met/Lys ratio of 0.37. These results indicated that maternal plasma H2S concentration could be affected by the dietary Met/Lys ratio.
3.2. Maternal Plasma H2S Concentration Was Positively Correlated with the Sows’ reproductive performance
To determine the involvement of H2S in the reproductive performance of the sows, we analyzed the correlation between the maternal plasma H2S concentration and the number of piglets born alive, the litter weight, and average pig birth weight at E114 d. We found that the maternal plasma H2S concentration had a positive correlation with the number of piglets born alive and the litter weight, as shown in Figure 4A,B. However, there was no correlation with the average pig birth weight, as shown in Figure 4C. These data suggested that maternal plasma H2S may have played an important role in the sows’ reproductive performance.
3.3. The Gestational Dietary Met/Lys Ratio Affected Placental H2S Concentration
We further examined the effect of the gestation dietary Met/Lys ratio on the placental H2S concentration. The placenta was selected based on the average pig birth weight ± one standard deviation of each group. Similar to the maternal plasma, the placental H2S concentration also changed quadratically with the dietary Met/Lys ratio, as shown in Figure 2. According to the quadratic polynomial regression equation (y = −139.4x2 + 105.6x −9.17, R2 = 0.12, p = 0.03), the maximum placental H2S concentration was at the dietary Met/Lys ratio of 0.37.
3.4. The H2S Productionof Met Treatment Affected PIEC Cells
To further confirm the effect of Met on H2S production in vitro, PIEC cells were treated with variable mediums which were (1) Met-free (0 μM), (2) had a physiological concentration of Met (50 μM), and (3) had a high concentration of Met (200, 500, and 1000 μM), respectively. We found that the H2S concentration was highest in the Met-free medium condition and lowest in a physiological concentration of Met (50 μM), as shown in Figure 3. Subsequently, with an increase in Met concentration, the H2S production increased initially (from 50 to 500 μM) and then maintained relative stability (from 500 to 1000 μM).
4. Discussion
H2S is an endogenous stimulator of angiogenesis that promotes vascular endothelial cell proliferation, migration, and angiogenesis by activating the NO/cGMP pathway, opening KATP channels, and promoting the phosphorylation of PI3K/AKT and MAPK pathways [7,18,19]. In the present study, we found that the maternal plasma H2S concentration was positively correlated with the sows’ reproductive performance. With an increase in Met/Lys ratio in the sows’ diet, the maternal plasma and the placental H2S concentrations changed quadratically. These results indicated that there was a dose-effect of Met concentration on H2S production.
Although Met is a precursor for H2S production, recent studies have found that dietary restriction (50% restriction) or Met restriction is the most effective way to increase H2S production. Studies have found that limiting the feed intake can increase liver CGL expression, increase endogenous H2S production, and alleviate liver ischemia-reperfusion injury [11]. Additionally, Met restriction also promotes vascular regeneration and recovery after rodent femoral artery ligation [20] and maintains capillary density in skeletal muscle [21]. Recently, two important studies have further confirmed that by restricting the sulfur amino acid, H2S production can increase in the skeletal muscles by up-regulating the GCN2/ATF4 pathway and activating NAD+/SIRT1 signaling, thus triggering skeletal muscle angiogenesis and delaying muscle aging [22,23]. We found similar results in our study, where medium H2S concentrations were highest under Met-free conditions (0 μM), whereas relative to physiological concentrations, high concentrations of Met did not significantly inhibit H2S production in PIEC cells, which suggested that there were some differences between in vitro and in vivo experiments.
In modern animal production, gestational restriction feeding is an effective and widely used practice to improve the reproductive performance of sows. Our previous study found that under the restriction feeding conditions, increasing the Met/Lys ratio from 0.27 to 0.37 can significantly increase the average pig birth weight and the litter weight in the pigs that are born alive [6]. However, when Met/Lys ratio was further increased to 0.47, the rate of piglets with a weight <0.9kg increased and the placental vascular density decreased significantly [6]. In this study, the maternal plasma at E114 d and the placental H2S concentrations were consistent with the sows’ reproductive performance. The decrease in H2S production caused by an increase in dietary Met was likely to be the cause for the increase in the rate of low birth weight, along with the failure to further improve the reproductive performance of the sows. Studies of rodents have found that a high concentration of Met can significantly inhibit CGL activity in peritoneal macrophages, reducing serum H2S content and increasing inflammatory reactions [16]. A recent study showed that exogenous H2S could promote mammary epithelial cell proliferation by activating the PI3K/Akt-mTOR pathway in porcine [24]. Therefore, determination of the appropriate dietary Met content is important for improving the reproductive performance of sows. Meanwhile, increasing the activity of maternal and placenta H2S producing enzymes, or increasing the H2S content by using H2S donors, may be an effective way to alleviate the adverse effects of high Met production. Our current results found that H2S donors diallyl trisufide(DATs) could significantly promote PIEC angiogenesis (unpublished data), which suggests that H2S is a potential target for improving the reproductive performance of sows.
5. Conclusions
In conclusion, our findings revealed that gestational dietary Met could affect maternal and placental H2S concentration, which may be an important molecular mechanism affecting placental angiogenesis and piglet development.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/10/2/251/s1, Table S1: Ingredients and nutrient compositions of the experimental diets.
Author Contributions
J.P. (Jie Peng) designed and carried out experimental work, wrote the manuscript and revised the manuscript. M.X.—acquisition and analysis of data and carried out experimental work. Y.Z. designed the work and analysis of data. J.X.; C.C. and N.H. carried out experimental work. H.W. designed the work and analysis of data. J.P. (Jian Peng) designed the work and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the National key Research and Development project of China (2017YFD0502004); Hubei Provincial Creative Team Project of Agricultural Science and Technology (2007-620); China Agriculture Research System (CARS-36).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Gaines, A.M.; Yi, G.F.; Ratliff, B.W.; Srichana, P.; Kendall, D.C.; Allee, G.L.; Knight, C.D.; Perryman, K.R. Estimation of the ideal ratio of true ileal digestible sulfur amino acids:lysine in 8- to 26-kg nursery pigs. J. Anim. Sci. 2005, 83, 2527–2534. [Google Scholar] [CrossRef]
- Rees, W.D.; Wilson, F.A.; Maloney, C.A. Sulfur amino acid metabolism in pregnancy: The impact of methionine in the maternal diet. J. Nutr. 2006, 136, 1701S–1705S. [Google Scholar] [CrossRef] [PubMed]
- Kalhan, S.C.; Marczewski, S.E. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 2012, 13, 109–119. [Google Scholar] [CrossRef]
- Kalhan, S.C. One Carbon Metabolism, Fetal Growth and Long Term Consequences; Nestlé Nutr Inst Workshop Ser.; Nestec Ltd.: Basel, Switzerland, 2013; pp. 127–138. [Google Scholar]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Herring, C.; Seo, H.; Dai, Z.; Wang, J.; Wu, Z.; Wang, X. Functional amino acids in the development of the pig placenta. Reprod. Dev. 2017, 84, 870–882. [Google Scholar] [CrossRef]
- Xia, M.; Pan, Y.; Guo, L.L.; Wei, X.X.; Xiong, J.; Wang, L.; Peng, J.; Wang, C.; Peng, J.; Wei, H.K. Effect of gestation dietary methionine/lysine ratio on placental angiogenesis and reproductive performance of sows. J. Anim. Sci. 2019, 97, 3487–3497. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [PubMed]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.E.; Longchamp, A.; Trevinovillarreal, J.H.; Mejia, P.; Ozaki, C.K. Endogenous Hydrogen Sulfide Production Is Essential for Dietary Restriction Benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Kingdom, J.; Burton, G.J.; Cindrovadavies, T. Placental Stem Villus Arterial Remodeling Associated with Reduced Hydrogen Sulfide Synthesis Contributes to Human Fetal Growth Restriction. Am. J. Pathol. 2017, 187, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, K.M.; Bos, E.M.; Rajakumar, A.; Risstalpers, C.; Van Pampus, M.G.; Timmer, A.; Erwich, J.; Faas, M.M.; Goor, V.H.; Lely, A. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta 2012, 33, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.J.; Hadoke, P.W.F. Dysregulation of Hydrogen Sulfide Producing Enzyme Cystathionine γ-lyase Contributes to Maternal Hypertension and Placental Abnormalities in Preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-B.; Feng, L.; Hodges, J.K.; Lechuga, T.J.; Zhang, H. Human trophoblast-derived hydrogen sulfide stimulates placental artery endothelial cell angiogenesis. Biol. Reprod. 2017, 97, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Li, Q.; Du, H.P.; Wang, Y.L.; You, S.J.; Wang, F.; Xu, X.S.; Cheng, J.; Cao, Y.J.; Liu, C.F.; et al. Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter. Int. J. Mol. Sci. 2015, 16, 12560–12577. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Gungor, N.; McVie, R.; Jain, S.K. Decreased cystathionine-gamma-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients. J. Biol. Chem. 2014, 289, 11767–11778. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular Biology of Hydrogen Sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A Review of Hydrogen Sulfide (H2S) Donors: Chemistry and Potential Therapeutic Applications. Biochem. Pharmacol. 2017, 149, 110–123. [Google Scholar] [CrossRef]
- Kondo, M.; Shibata, R.; Miura, R.; Shimano, M.; Kondo, K.; Li, P.; Ohashi, T.; Kihara, S.; Maeda, N.; Walsh, K. Caloric Restriction Stimulates Revascularization in Response to Ischemia via Adiponectin-mediated Activation of Endothelial Nitric-oxide Synthase. J. Biol. Chem. 2009, 284, 1718–1724. [Google Scholar] [CrossRef]
- Omodei, D.; Fontana, L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011, 585, 1537–1542. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; Macarthur, M.R.; Das, A.; Trevinovillarreal, J.H.; Hine, C.; Bensahra, I.; Knudsen, N.H.; Brace, L.E. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD(+)-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, J.; Yuan, C.; Fu, Q.; Zhang, F.; Zhu, X.; Wang, L.; Gao, P.; Shu, G.; Jiang, Q. Exogenous H 2 S exerts biphasic effects on porcine mammary epithelial cells proliferation through PI3K/Akt-mTOR signaling pathway. J. Cell. Physiol. 2018, 233, 7071–7081. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).