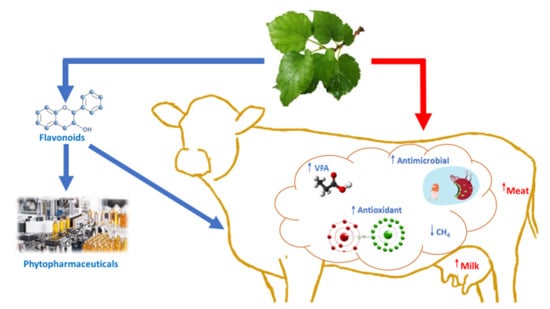
Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects
Simple Summary
Abstract
1. Introduction
2. Plant Secondary Metabolites of Mulberry Leaf Biomass
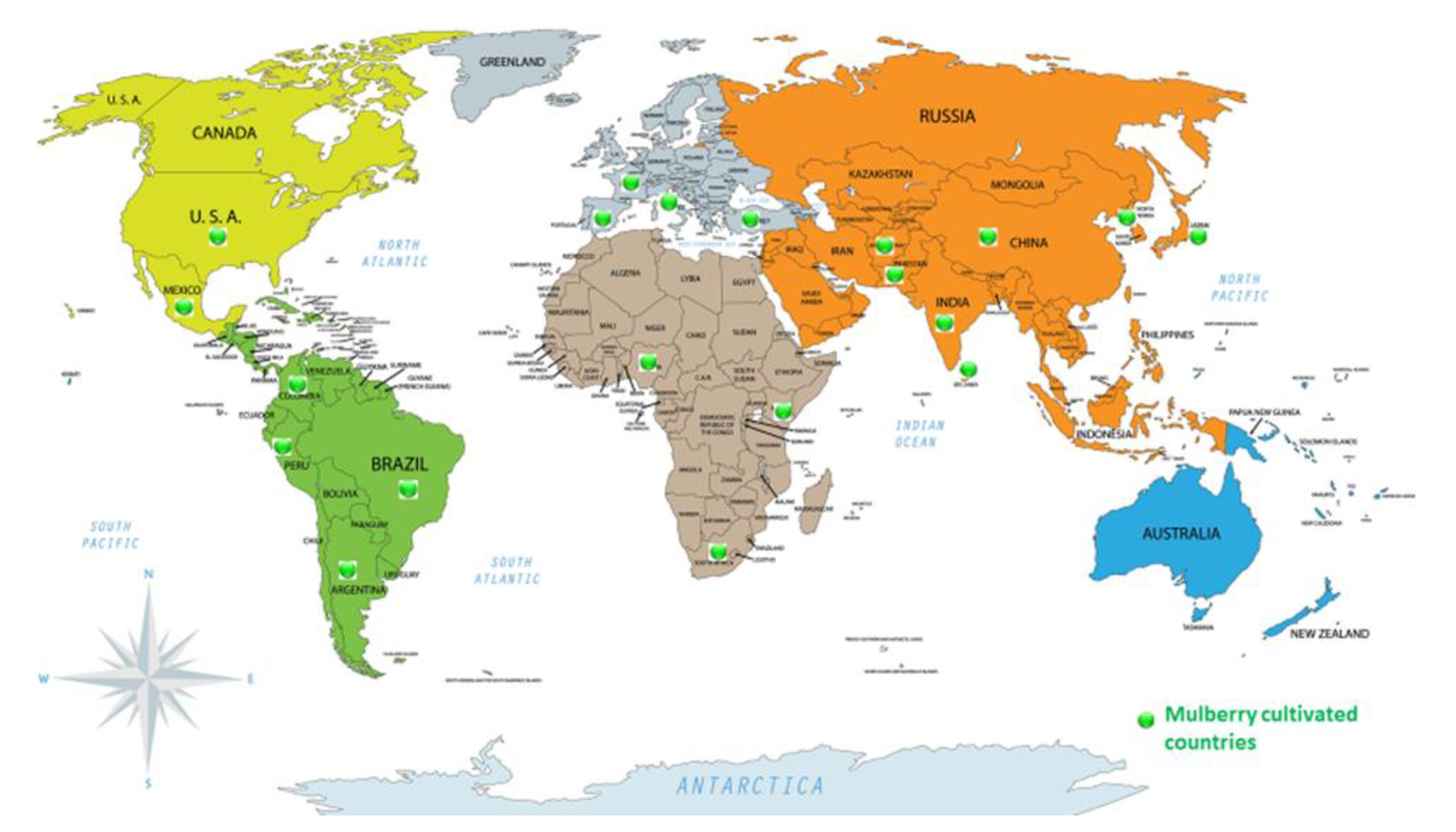
3. Mulberry Tree Cultivation, Global Distribution, and Leaf Biomass Yield
4. Nutritional Profile of Mulberry Leaves
5. Anti-Nutritional Factors in Mulberry Leaves
6. Structure, Bioavailability, and Absorption of Mulberry Leaf Flavonoids
6.1. Structure of Mulberry Leaf Flavonoids
6.2. Flavonoid Contents of Mulberry Leaf and Their Bioactivities
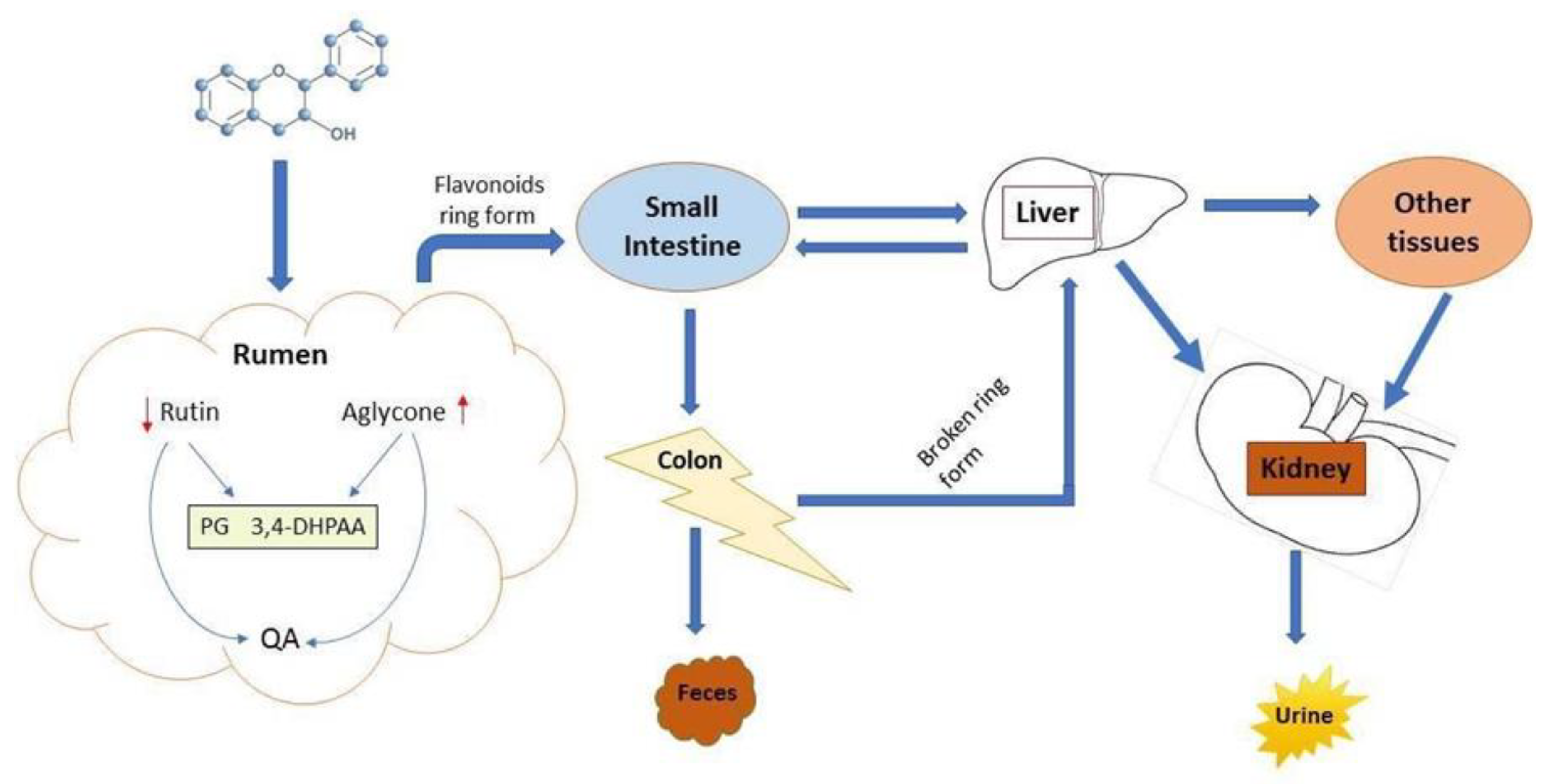
6.3. Ruminal Degradation, Absorption, and Bioavailability of Mulberry Flavonoids
7. Feeding of Mulberry Leaves and Its Flavonoids in Ruminants
7.1. Effects of Mulberry Leaf and Its Flavonoids on Ruminal Development and Calf Health
7.2. Effects of Mulberry Leaf and Its Flavonoids on Animal Health and Performance
7.3. Effects of Mulberry Leaf and Its Flavonoids on Rumen Microbiota and Methanogenesis
7.4. Effects of Mulberry Leaf and Its Flavonoids on Feed Digestibility and Ruminal Fermentation Parameters
7.5. Effects of Mulberry Leaf Flavonoids on Oxidative Stress and Antioxidant Parameters
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ash, A. The effect of supplementation with leaves from the leguminous trees Sesbania grandiflora, Albizia chinensis and Gliricidia sepium on the intake and digestibility of guinea grass hay by goats. Anim. Feed Sci. Technol. 1990, 28, 225–232. [Google Scholar] [CrossRef]
- Emile, J.-C. Nutritive value and degradability of leaves from temperate woody resources for feeding ruminants in summer. In Proceedings of the 3rd European Agroforestry Conference Montpellier, Montpellier, France, 23–25 May 2016. [Google Scholar]
- Ku-Vera, J.C.; Castelán-Ortega, O.; Galindo-Maldonado, F.; Arango, J.; Chirinda, N.; Jiménez-Ocampo, R.; Valencia-Salazar, S.; Flores-Santiago, E.; Montoya-Flores, M.D.; Molina-Botero, I.C. Strategies for enteric methane mitigation in cattle fed tropical forages. Animal 2020, 14, s453–s463. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Khan, N.; Sultan, A.; Ali, M. Nutritive value of common tree leaves for livestock in the semi-arid and arid rangelands of Northern Pakistan. Livest. Sci. 2016, 184, 64–70. [Google Scholar] [CrossRef]
- Omar, S.; Shayo, C.; Uden, P. Voluntary intake and digestibility of mulberry (Morus alba) diets by growing goats. Trop. Grassl. 1999, 33, 177–181. [Google Scholar]
- Yao, J.; Yan, B.; Wang, X.; Liu, J. Nutritional evaluation of mulberry leaves as feeds for ruminants. Livest. Res. Rural Dev. 2000, 12, 9–16. [Google Scholar]
- Chen, D.; Chen, X.; Tu, Y.; Wang, B.; Lou, C.; Ma, T.; Diao, Q. Effects of mulberry leaf flavonoid and resveratrol on methane emission and nutrient digestion in sheep. Anim. Nutr. 2015, 1, 362–367. [Google Scholar] [CrossRef]
- Huo, Y. Mulberry cultivation and utilization in China. FAO Anim. Prod. Health Pap. 2000, 1, 11–44. [Google Scholar]
- Sánchez, M.D. World distribution and utilization of mulberry and its potential for animal. In Proceedings of the Mulberry for Animal Production: Proceedings of an Electronic Conference Carried out between May and August 2000; Food and Agriculture Organization: Rome, Italy, 2002; p. 1. [Google Scholar]
- Hao, Y.; Huang, S.; Si, J.; Zhang, J.; Gaowa, N.; Sun, X.; Lv, J.; Liu, G.; He, Y.; Wang, W. Effects of Paper Mulberry Silage on the Milk Production, Apparent Digestibility, Antioxidant Capacity, and Fecal Bacteria Composition in Holstein Dairy Cows. Animal 2020, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Ba, N.X.; Giang, V.D.; Ngoan, L.D. Ensiling of mulberry foliage (Morus alba) and the nutritive value of mulberry foliage silage for goats in central Vietnam. Livest. Res. Rural Dev. 2005, 17, 23–25. [Google Scholar]
- Simbaya, J.; Chibinga, O.; Salem, A.Z. Nutritional evaluation of selected fodder trees: Mulberry (Molus alba Lam.), Leucaena (Leucaena luecocephala Lam de Wit.) and Moringa (Moringa oleifera Lam.) as dry season protein supplements for grazing animals. Agrofor. Syst. 2020, 94, 1189–1197. [Google Scholar] [CrossRef]
- Marchii, H. Varietal differences of nitrogen and amino acid contents in mulberry leaves. Acta Sericologica Entomol. 1989, 1, 51–61. [Google Scholar]
- Tesfay, G.; Tamir, B.; Berhane, G. Substitution of mulberry leaf meal on feed intake, body weight and carcass characteristics of Tigray highland lambs. Indones. J. Anim. Vet. Sci. 2018, 23, 28–37. [Google Scholar] [CrossRef]
- Phiny, C.; Preston, T.; Ly, J. Mulberry (Morus alba) leaves as protein source for young pigs fed rice-based diets: Digestibility studies. Livest. Res. Rural Dev. 2003, 15, 1. [Google Scholar]
- Liu, J.; Yao, J.; Yan, B.; Yu, J.; Shi, Z. Effects of mulberry leaves to replace rapeseed meal on performance of sheep feeding on ammoniated rice straw diet. Small Rumin. Res. 2001, 39, 131–136. [Google Scholar] [CrossRef]
- Cheong, S.; Kim, K.; Jeon, B.; Park, P.; Hwang, I.; Choi, N.; Kim, E.; Hong, S.; Park, J.; Sung, S. Effect of mulberry silage supplementation during late fattening stage of Hanwoo (Bos taurus coreanae) steer on antioxidative enzyme activity within the longissimus muscle. Anim. Prod. Sci. 2012, 52, 240–247. [Google Scholar] [CrossRef]
- Wang, B.; Yang, C.; Diao, Q.; Tu, Y. The influence of mulberry leaf flavonoids and Candida tropicalis on antioxidant function and gastrointestinal development of preweaning calves challenged with Escherichia coli O141: K99. J. Dairy Sci. 2018, 101, 6098–6108. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Peng, Y.; He, J.; Xiao, D.; Chen, C.; Li, F.; Huang, R.; Yin, Y. Dietary mulberry leaf powder affects growth performance, carcass traits and meat quality in finishing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1934–1945. [Google Scholar] [CrossRef]
- Zhou, B.; Meng, Q.; Ren, L.; Shi, F.; Wei, Z.; Zhou, Z. Evaluation of chemical composition, in situ degradability and in vitro gas production of ensiled and sun-dried mulberry pomace. J. Anim. Feed Sci. 2012, 21, 188–197. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Ren, L.; Meng, Q. Effect of ensiled mulberry leaves and sun-dried mulberry fruit pomace on finishing steer growth performance, blood biochemical parameters, and carcass characteristics. PLoS ONE 2014, 9, e85406. [Google Scholar] [CrossRef]
- Benavides, J. Utilisation of mulberry in animal production systems (part 1/3). In FAO Electronic Conference on Mulberry for Animal Production; FAO: Rome, Italy, 2000. [Google Scholar]
- Salem, A.Z.; Kunst, C.R.; Jose, S. Alternative animal feeds from agroforestry plants. Agrofor. Syst. 2020, 94, 1133–1138. [Google Scholar] [CrossRef]
- Ebrahim, H.; Negussie, F. Effect of secondary compounds on nutrients utilization and productivity of ruminant animals: A review. J. Agric. Sci. Prac. 2020, 5, 60–73. [Google Scholar]
- Naboulsi, I.; Aboulmouhajir, A.; Kouisni, L.; Bekkaoui, F.; Yasri, A. Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review. Acad. J. Med. Plants 2018, 6, 223–240. [Google Scholar]
- Balcells, J.; Aris, A.; Serrano, A.; Seradj, A.; Crespo, J.; Devant, M. Effects of an extract of plant flavonoids (Bioflavex) on rumen fermentation and performance in heifers fed high-concentrate diets. J. Anim. Sci. 2012, 90, 4975–4984. [Google Scholar] [CrossRef]
- Polumackanycz, M.; Sledzinski, T.; Goyke, E.; Wesolowski, M.; Viapiana, A. A comparative study on the phenolic composition and biological activities of Morus alba L. commercial samples. Molecules 2019, 24, 3082. [Google Scholar] [CrossRef]
- Cirne, L.; Sobrinho, A.; Oliveira, E.; Carvalho, G.; Moreno, G.; Valença, R.; Almeida, F.; Endo, V.; Zeola, N. Nutritional characteristics of meat from lambs fed diets containing mulberry hay. S. Afr. J. Anim. Sci. 2019, 49, 20–28. [Google Scholar] [CrossRef]
- Peng, C.-H.; Lin, H.-T.; Chung, D.-J.; Huang, C.-N.; Wang, C.-J. Mulberry Leaf Extracts prevent obesity-induced NAFLD with regulating adipocytokines, inflammation and oxidative stress. J. Food Drug Anal. 2018, 26, 778–787. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Numhom, S.; Aramwit, P. Mulberry leaves and their potential effects against cardiometabolic risks: A review of chemical compositions, biological properties and clinical efficacy. Pharm. Biol. 2018, 56, 109–118. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Besle, J.-M.; Viala, D.; Martin, B.; Pradel, P.; Meunier, B.; Berdagué, J.-L.; Fraisse, D.; Lamaison, J.; Coulon, J.B. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J. Dairy Sci. 2010, 93, 2846–2856. [Google Scholar] [CrossRef]
- Stoldt, A.-K.; Derno, M.; Das, G.; Weitzel, J.M.; Wolffram, S.; Metges, C.C. Effects of rutin and buckwheat seeds on energy metabolism and methane production in dairy cows. J. Dairy Sci. 2016, 99, 2161–2168. [Google Scholar] [CrossRef]
- Seradj, A.R.; Abecia, L.; Crespo, J.; Villalba, D.; Fondevila, M.; Balcells, J. The effect of Bioflavex® and its pure flavonoid components on in vitro fermentation parameters and methane production in rumen fluid from steers given high concentrate diets. Anim. Feed Sci. Technol. 2014, 197, 85–91. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, R.; Bi, Y.; Bilal, M.; Kuang, Z.; Iqbal, H.; Luo, Q. Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs. Animal 2020, 10, 35. [Google Scholar] [CrossRef]
- Mohamaden, W.I.; Hegab, I.M.; Shang-li, S. In situ ruminal degradation kinetics and blood metabolites as affected by feeding different sources of tannin and flavonoids to small-tailed Han rams. Livest. Sci. 2020, 239, 104029. [Google Scholar] [CrossRef]
- Ouyang, J.; Hou, Q.; Wang, M.; Zhao, W.; Feng, D.; Pi, Y.; Sun, X.-z. Effects of dietary mulberry leaf powder on growth performance, blood metabolites, meat quality and antioxidant enzyme related gene expression of fattening Hu lambs. Can. J. Anim. Sci. 2020, 100, 510–521. [Google Scholar] [CrossRef]
- Dong, T.; Liu, Z.; Xuan, Q.; Wang, Z.; Ma, W.; Zhang, Q. Tumor LDH-A expression and serum LDH status are two metabolic predictors for triple negative breast cancer brain metastasis. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Radi, Z.A.; Koza-Taylor, P.H.; Bell, R.R.; Obert, L.A.; Runnels, H.A.; Beebe, J.S.; Lawton, M.P.; Sadis, S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am. J. Pathol. 2011, 179, 240–247. [Google Scholar] [CrossRef]
- Nguyen-Lefebvre, A.T.; Horuzsko, A. Kupffer cell metabolism and function. J. Enzymol. Metab. 2015, 1, 101. [Google Scholar]
- Khan, M.A.; Rahman, A.A.; Islam, S.; Khandokhar, P.; Parvin, S.; Islam, M.B.; Hossain, M.; Rashid, M.; Sadik, G.; Nasrin, S. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L.(Moraceae). BMC Res. Notes 2013, 6, 24. [Google Scholar] [CrossRef]
- Rohela, G.K.; Muttanna, P.S.; Kumar, R.; Chowdhury, S.R. Mulberry (Morus spp.): An ideal plant for sustainable development. Trees For. People 2020, 2, 2666–7193. [Google Scholar] [CrossRef]
- Alfrey, P. Mo’ Mulberry—A guide to probably everything you need to know about growing Mulberry. Noteworthy J. Blog 2017. Available online: https://blog.usejournal.com/mo-mulberry-the-essential-guide-to-all-you-need-to-know-about-mulberry-28a0c11b611 (accessed on 10 October 2020).
- Sánchez-Salcedo, E.M.; Amorós, A.; Hernández, F.; Martínez, J.J. Physicochemical properties of white (Morus alba) and black (Morus nigra) mulberry leaves, a new food supplement. J. Food Nutr. Res. 2017, 5, 253–261. [Google Scholar]
- Yang, X.; Yang, L.; Zheng, H. Hypolipidemic and antioxidant effects of mulberry (Morus alba L.) fruit in hyperlipidaemia rats. Food Chem. Toxicol. 2010, 48, 2374–2379. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F.; Luo, X.; Li, X. Effects of mulberry fruit (Morus alba L.) consumption on health outcomes: A mini-review. Antioxidants 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Fallon, E.; Zhong, L.; Furne, J.K.; Levitt, M.D. A mixture of extracts of black and green teas and mulberry leaf did not reduce weight gain in rats fed a high-fat diet. Altern. Med. Rev. 2008, 13, 43. [Google Scholar] [PubMed]
- Oh, K.-S.; Ryu, S.Y.; Lee, S.; Seo, H.W.; Oh, B.K.; Kim, Y.S.; Lee, B.H. Melanin-concentrating hormone-1 receptor antagonism and anti-obesity effects of ethanolic extract from Morus alba leaves in diet-induced obese mice. J. Ethnopharmacol. 2009, 122, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Lakshmi, A.J.; Manjunath, M.; Prakash, J. Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT-Food Sci. Technol. 2005, 38, 339–345. [Google Scholar] [CrossRef]
- Kandylis, K.; Hadjigeorgiou, I.; Harizanis, P. The nutritive value of mulberry leaves (Morus alba) as a feed supplement for sheep. Trop. Anim. Health Prod. 2009, 41, 17–24. [Google Scholar] [CrossRef]
- Vu, C.C.; Verstegen, M.; Hendriks, W.; Pham, K. The nutritive value of mulberry leaves (Morus alba) and partial replacement of cotton seed in rations on the performance of growing Vietnamese cattle. Asian-Australas J. Anim. Sci. 2011, 24, 1233–1242. [Google Scholar] [CrossRef]
- Wanapat, M.; Kang, S.; Polyorach, S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J. Anim. Sci. Biotechnol. 2013, 4, 32. [Google Scholar] [CrossRef]
- Chandang, P.; Thongprajukaew, K.; Chotimanothum, B.; Kovitvadhi, A.; Kovitvadhi, U.; Pakkong, P. The effects on in vitro digestibility from different developmental stages of silkworm larvae, Bombyx mori (Lepidoptera: Bombycidae) and position of mulberry leaves, Morus alba (Rosales: Moraceae). J. Asia-Pac. Entomol. 2017, 20, 1134–1139. [Google Scholar] [CrossRef]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical characterization and antioxidative properties of Polish variety of Morus alba L. leaf aqueous extracts from the laboratory and pilot-scale processes. Agric. Sci. 2013, 4, 141. [Google Scholar] [CrossRef]
- Srivastava, S.; Kapoor, R.; Thathola, A.; Srivastava, R.P. Nutritional quality of leaves of some genotypes of mulberry (Morus alba). Int. J. Food Sci. Nutr. 2006, 57, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Devi, B.; Sharma, N.; Kumar, D.; Jeet, K. Morus alba Linn: A phytopharmacological review. Int. J. Pharm. Pharm. Sci. 2013, 5, 14–18. [Google Scholar]
- Jeszka-Skowron, M.; Flaczyk, E.; Jeszka, J.; Krejpcio, Z.; Król, E.; Buchowski, M.S. Mulberry leaf extract intake reduces hyperglycaemia in streptozotocin (STZ)-induced diabetic rats fed high-fat diet. J. Funct. Foods 2014, 8, 9–17. [Google Scholar] [CrossRef]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Yang, J.; Zhang, Y.; Deng, Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef]
- Radojković, M.; Zeković, Z.; Mašković, P.; Vidović, S.; Mandić, A.; Mišan, A.; Đurović, S. Biological activities and chemical composition of Morus leaves extracts obtained by maceration and supercritical fluid extraction. J. Supercrit. Fluid 2016, 117, 50–58. [Google Scholar] [CrossRef]
- Adeduntan, S.; Oyerinde, A. Evaluation of chemical and antinutritional characteristics of obeche (Triplochition scleroxylon) and some mulberry (Morus alba) leaves. Int. J. Biol Chem Sci. 2009, 3, 681–687. [Google Scholar] [CrossRef]
- Bamikole, M.; Ikhatua, M.; Ikhatua, U.; Ezenwa, I. Nutritive value of mulberry (Morus spp.) leaves in the growing rabbits in Nigeria. Pak. J. Nutr. 2005, 4, 231–236. [Google Scholar] [CrossRef]
- Cai, M.; Mu, L.; Wang, Z.-l.; Liu, J.-y.; Liu, T.-l.; Wanapat, M.; Huang, B.-z. Assessment of mulberry leaf as a potential feed supplement for animal feeding in PR China. Asian-Australas J. Anim. Sci. 2019, 32, 1145. [Google Scholar] [CrossRef]
- Guven, I. Effect of species on Nutr.itive value of mulberry leaves. Kafkas Univ. Vet. Fak. Derg. 2012, 18, 865–869. [Google Scholar]
- Todaro, M.; Bonanno, A.; Tornambè, G.; Di Grigoli, A.; Luisa Scatassa, M.; Giaccone, P. Utilization of mulberry leaves (Morus latifolia cv. Kokusou 21) in diets for dairy ewes. Ital. J. Anim. Sci. 2009, 8, 438–440. [Google Scholar] [CrossRef]
- Wang, W.; Yang, H.; Bo, Y.; Ding, S.; Cao, B. Nutr.ient composition, polyphenolic contents, and in situ protein degradation kinetics of leaves from three mulberry species. Livest. Sci. 2012, 146, 203–206. [Google Scholar] [CrossRef]
- Al-Kirshi, R.; Alimon, A.; Zulkifli, I.; Zahari, M.; Sazili, A. The chemical composition and Nutritive value of mulberry leaf as a protein source in poultry diets. In Proceedings of the International Seminar on Animal Industry, Jakarta, Indonesia, 5–6 July 2012. [Google Scholar]
- Doliș, M.; Donose, R.; Simeanu, C.; Usturoi, A.; Rațu, R. Research regarding chemical composition of the mulberry leaves from Kokuso 21 variety. An. Univ. Oradea Fasc. Ecotoxicol. Zooteh. Tehnol. Ind. Aliment. 2016, 15, 207–212. [Google Scholar]
- Ganai, A.; Mattoo, F.; Singh, P.; Ahmad, H.; Samoon, M. Chemical composition of some feeds, fodders and plane of Nutr.ition of livestock of Kashmir valley. SKUAST J. Res. 2006, 8, 145–151. [Google Scholar]
- Iqbal, S.; Younas, U.; Chan, K.W.; Sarfraz, R.A.; Uddin, M. Proximate composition and antioxidant potential of leaves from three varieties of Mulberry (Morus sp.): A comparative study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef]
- Kang, J.; Wang, R.; Tang, S.; Wang, M.; Tan, Z.; Bernard, L. Chemical composition and in vitro ruminal fermentation of pigeonpea and mulberry leaves. Agrofor. Syst. 2019, 94, 1521–1528. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, M.; Hou, Q.; Feng, D.; Pi, Y.; Zhao, W. Effects of Dietary Mulberry Leaf Powder in Concentrate on the Rumen Fermentation and Ruminal Epithelium in Fattening Hu Sheep. Animal 2019, 9, 218. [Google Scholar] [CrossRef]
- Sahoo, A.; Singh, B.; Sharma, O. Evaluation of feeding value of Eupatorium adenophorum in combination with mulberry leaves. Livest. Sci. 2011, 136, 175–183. [Google Scholar] [CrossRef]
- Sun, C.; Wu, W.; Ma, Y.; Min, T.; Lai, F.; Wu, H. Physicochemical, functional properties, and antioxidant activities of protein fractions obtained from mulberry (morus atropurpurea roxb.) leaf. Int. J. Food Prop. 2017, 20, S3311–S3325. [Google Scholar] [CrossRef]
- Kamalak, A.; Canbolat, O.; Gurbuz, Y.; Ozay, O.; Ozkan, C.; Sakarya, M. Chemical composition and in vitro gas production characteristics of several tannin containing tree leaves. Livest. Res. Rural Dev. 2004, 16, 2004. [Google Scholar]
- St-Pierre, N.R. Meta-analyses of experimental data in the animal Sciences. Rev. Bras. Zootec. 2007, 36, 343–358. [Google Scholar] [CrossRef]
- Ustundag, A.O.; Ozdogan, M. Usage possibilities of mulberry leaves in poultry Nutrition. Sci. Pap. Ser. D Anim. Sci. Int. Sess. Sci. Commun. Fac. Anim. Sci. 2015, 58, 170–178. [Google Scholar]
- Hassan, F.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic additives can modulate rumen microbiome to mediate fermentation kinetics and methanogenesis through exploiting diet- microbe interaction. Front. Vet. Sci. 2020, 7, 892. [Google Scholar] [CrossRef]
- McSweeney, C.; Palmer, B.; McNeill, D.; Krause, D. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative determination of 1-deoxynojirimycin in mulberry leaves from 132 varieties. Ind. Crops Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Kwon, H.J.; Chung, J.Y.; Kim, J.Y.; Kwon, O. Comparison of 1-deoxynojirimycin and aqueous mulberry leaf extract with emphasis on postprandial hypoglycemic effects: In vivo and in vitro studies. J. Agric. Food Chem. 2011, 59, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, H.-J.; Bucheli, P.; Zhang, P.-F.; Wei, D.-Z.; Lu, Y.-H. Phytochemical profiles of different mulberry (Morus sp.) species from China. J. Agric. Food Chem. 2009, 57, 9133–9140. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahman, M.R.; Niimi, M.; Khadijah, W.E.W.; Akashi, R.; Abdullah, R. Effects of different levels of oxalic acid administration on feed intake and Nutrient digestibility in goats. Sains Malays. 2017, 46, 515–519. [Google Scholar] [CrossRef]
- Rahman, M.; Nakagawa, T.; Niimi, M.; Fukuyama, K.; Kawamura, O. Effects of feeding oxalate containing grass on intake and the concentrations of some minerals and parathyroid hormone in blood of sheep. Asian-Australas J. Anim. Sci. 2011, 24, 940–945. [Google Scholar] [CrossRef]
- Rahman, M.; Abdullah, R.; Wan Khadijah, W. A review of oxalate poisoning in domestic animals: Tolerance and performance aspects. J. Anim. Physiol. Anim. Nutr. 2013, 97, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Soto-Blanco, B.; Stegelmeier, B.; Pfister, J.; Gardner, D.; Panter, K. Comparative effects of prolonged administration of cyanide, thiocyanate and chokecherry (Prunus virginiana) to goats. J. Appl. Toxicol. An. Int. J. 2008, 28, 356–363. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant. Physiol 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.-T.; Kwon, O.-C.; Kim, H.-B.; Sung, G.-B.; Kim, H.-W.; Kim, Y.-S. Qualitative and quantitative analysis of flavonoids from 12 species of Korean mulberry leaves. J. Food Sci. Technol 2018, 55, 1789–1796. [Google Scholar] [CrossRef]
- Sugiyama, M.; Katsube, T.; Koyama, A.; Itamura, H. Varietal differences in the flavonol content of mulberry (Morus spp.) leaves and genetic analysis of quercetin 3-(6-malonylglucoside) for component breeding. J. Agric. Food Chem. 2013, 61, 9140–9147. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco. 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Rogerio, A.; Kanashiro, A.; Fontanari, C.; Da Silva, E.; Lucisano-Valim, Y.; Soares, E.; Faccioli, L. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.; Krishna, D. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, M.N.C.A., Ashihara, H., Eds.; Blackwell: Oxford, UK, 2006; pp. 1–24. [Google Scholar]
- Middleton, E., Jr.; Drzewiecki, G. Flavonoid inhibition of human basophil histamine release stimulated by various agents. Biochem. Pharmacol. 1984, 33, 3333–3338. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; Van Gameren, Y.; Cnossen, E.P.; De Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Gohlke, A.; Ingelmann, C.; Nürnberg, G.; Starke, A.; Wolffram, S.; Metges, C. Bioavailability of quercetin from its aglycone and its glucorhamnoside rutin in lactating dairy cows after intraduodenal administration. J. Dairy Sci. 2013, 96, 2303–2313. [Google Scholar] [CrossRef]
- Matsuoka, T.; Kimura, T.; Muraoka, N. Research of the available constituents from mulberry tree. Tohoku Agric. Res. 1994, 47, 361–362. [Google Scholar]
- Onogi, A.; Osawa, K.; Yasuda, H.; Sakai, A.; Morita, H.; Itokawa, H. Flavonol Glycosides from the Leaves of Morus alba L. J. Pharm. Sci. 1993, 47, 423–425. [Google Scholar]
- He, X.; Chen, X.; Ou, X.; Ma, L.; Xu, W.; Huang, K. Evaluation of flavonoid and polyphenol constituents in mulberry leaves using HPLC fingerprint analysis. Int. J. Food Sci. Technol. 2020, 55, 526–533. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Katsube, T.; Yamasaki, M.; Shiwaku, K.; Ishijima, T.; Matsumoto, I.; Abe, K.; Yamasaki, Y. Effect of flavonol glycoside in mulberry (Morus alba L.) leaf on glucose metabolism and oxidative stress in liver in diet-induced obese mice. J. Sci. Food Agric. 2010, 90, 2386–2392. [Google Scholar] [CrossRef]
- Ren, M.Q.; Kuhn, G.; Wegner, J.; Chen, J. Isoflavones, substances with multi-biological and clinical properties. Eur. J. Nutr. 2001, 40, 135–146. [Google Scholar] [CrossRef]
- Cushnie, T.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Li, D.; Chen, G.; Ma, B.; Zhong, C.; He, N. Metabolic Profiling and Transcriptome Analysis of Mulberry Leaves Provide Insights into Flavonoid Biosynthesis. J. Agric. Food Chem. 2020, 68, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; Yang, Z.; Zeng, Q.; Luo, Y.; He, N. Flavones Produced by Mulberry Flavone Synthase Type I Constitute a Defense Line against the Ultraviolet-B Stress. Plants 2020, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Chao, P.-Y.; Lin, S.-Y.; Lin, K.-H.; Liu, Y.-F.; Hsu, J.-I.; Yang, C.-M.; Lai, J.-Y. Antioxidant activity in extracts of 27 indigenous Taiwanese vegetables. Nutrtion 2014, 6, 2115–2130. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Jiménez-Aliaga, K.; Bermejo-Bescós, P.; Benedí, J.; Martín-Aragón, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-K.; Gao, J.; Zhu, D.-N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef]
- Lin, M.-J.; Chang, S.-C.; Jea, Y.-S.; Liao, J.-W.; Fan, Y.-K.; Lee, T.-T. In vitro antioxidant capability and performance assessment of White Roman goose supplemented with dried Toona sinensis. J. Appl. Anim. Res. 2016, 44, 395–402. [Google Scholar] [CrossRef]
- Hassan, W.; Rongyin, G.; Daoud, A.; Ding, L.; Wang, L.; Liu, J.; Shang, J. Reduced oxidative stress contributes to the lipid lowering effects of isoquercitrin in free fatty acids induced hepatocytes. Oxid. Med. Cell Longev. 2014, 2014. [Google Scholar] [CrossRef]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Tsalkidou, E.G.; Tsaroucha, A.K.; Chatzaki, E.; Lambropoulou, M.; Papachristou, F.; Trypsianis, G.; Pitiakoudis, M.; Vaos, G.; Simopoulos, C. The effects of apigenin on the expression of Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Papapetropoulos, A.; Mauromatis, A.; Economou, M.; Fotsis, T.; Roussos, C. Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J. Pharmacol. Exp. Ther. 2001, 296, 181–187. [Google Scholar] [PubMed]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Lopes, M.C.; García-Rodríguez, C.; Geraldes, C.F.; Cruz, M.T.; Batista, M.T. Chemical characterization and anti-inflammatory activity of luteolin glycosides isolated from lemongrass. J. Funct. Foods 2014, 10, 436–443. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018. [Google Scholar] [CrossRef]
- Qu, D.; Han, J.; Ren, H.; Yang, W.; Zhang, X.; Zheng, Q.; Wang, D. Cardioprotective effects of astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. Oxid. Med. Cell Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Olagaray, K.; Bradford, B. Plant flavonoids to improve productivity of ruminants–A review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Berger, L.; Wein, S.; Blank, R.; Metges, C.; Wolffram, S. Bioavailability of the flavonol quercetin in cows after intraruminal application of quercetin aglycone and rutin. J. Dairy Sci. 2012, 95, 5047–5055. [Google Scholar] [CrossRef]
- Wein, S.; Beyer, B.; Zimmermann, B.F.; Blank, R.H.; Wolffram, S. Bioavailability of quercetin from onion extracts after intraruminal application in cows. J. Agric. Food Chem. 2018, 66, 10188–10192. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef]
- Wein, S.; Wolffram, S. Oral bioavailability of quercetin in horses. J. Equine Vet. Sci. 2013, 33, 441–445. [Google Scholar] [CrossRef]
- Cheng, K.-J.; Jones, G.; Simpson, F.; Bryant, M. Isolation and identification of rumen bacteria capable of anaerobic rutin degradation. Can. J. Microbiol. 1969, 15, 1365–1371. [Google Scholar] [CrossRef]
- Krishnamurty, H.; Cheng, K.-J.; Jones, G.; Simpson, F.; Watkin, J. Identification of products produced by the anaerobic degradation of rutin and related flavonoids by Butyrivibrio sp. C3. Can. J. Microbiol. 1970, 16, 759–767. [Google Scholar] [CrossRef]
- Labib, S.; Erb, A.; Kraus, M.; Wickert, T.; Richling, E. The pig caecum model: A suitable tool to study the intestinal metabolism of flavonoids. Mol. Nutr. Food Res. 2004, 48, 326–332. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Hsiu, S.-L.; Hou, Y.-C.; Chen, H.-Y.; Chao, P.-D.L. Degradation of flavonoid aglycones by rabbit, rat and human fecal flora. Biol. Pharm. Bull. 2003, 26, 747–751. [Google Scholar] [CrossRef]
- Aura, A.-M.; O’leary, K.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.-M.; Oksman-Caldentey, K.-M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Maciej, J. Oral Bioavailability of Flavonoids and Their Effects on the Metabolic and Antioxidative Status in Neonatal Calves. Ph.D. Thesis, Christian-Albrechts Universität Kiel, Kiel, Germany, 2015. [Google Scholar]
- Drackley, J.K. Calf Nutr.ition from birth to breeding. Vet. Clin. North. Am. Small Anim. Prac. 2008, 24, 55–86. [Google Scholar] [CrossRef]
- Guilloteau, P.; Zabielski, R.; Blum, J. Gastrointestinal tract and digestion in the young ruminant: Ontogenesis, adaptations, consequences and manipulations. J. Physiol. Pharmacol. 2009, 60, 37–46. [Google Scholar] [PubMed]
- Heinrichs, A.; Jones, C. Feeding the newborn calf. In College of Agricultural Sciences, Agricultural Research, and Cooperative Extension; Pennsylvania State University: University Park, PA, USA, 2003. [Google Scholar]
- Salinas-Chavira, J.; Castillo-Martínez, O.; Ramirez-Bribiesca, J.E.; Mellado, M. Effect of increasing levels of white mulberry leaves (Morus alba) on ruminal dry matter degradability in lambs. Trop. Anim. Health Prod. 2011, 43, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Yulistiani, D.; Jelan, Z.; Liang, J.; Yaakub, H.; Abdullah, N. Effects of supplementation of mulberry (Morus alba) foliage and urea-rice bran as fermentable energy and protein sources in sheep fed urea-treated rice straw based diet. Asian-Australas J. Anim. Sci. 2015, 28, 494. [Google Scholar] [CrossRef]
- Rieu, F.; Fonty, G.; Gaillard, B.; Gouet, P. Electron microscopy study of the bacteria adherent to the rumen wall in young conventional lambs. Can. J. Microbiol. 1990, 36, 140–144. [Google Scholar] [CrossRef]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef]
- Yang, C.; Diao, Q.; Qu, P.; Si, B.; Ma, J.; Zhou, Y.; Tu, Y. Effects of Candida tropicalis and mulberry leaf flavonoids on Nutr.ient metabolism and rumen fermentation in calves. J. Anim. Nutr. 2016, 28, 224–234. [Google Scholar]
- Zhang, L.; PB, Q.; CT, Y. Effects of flavonoids from mulberry leaves and candida tropicalis on performance and Nutr.ient digestibility in calves. Kafkas Üniversitesi Vet. Fakültesi Derg. 2017, 23, 473–479. [Google Scholar]
- Kong, L.; Yang, C.; Dong, L.; Diao, Q.; Si, B.; Ma, J.; Tu, Y. Rumen Fermentation Characteristics in Pre-and Post-Weaning Calves upon Feeding with Mulberry Leaf Flavonoids and Candida tropicalis Individually or in Combination as a Supplement. Animal 2019, 9, 990. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Van Camp, J.; Smagghe, G.; Raes, K.; Mackie, A. Flavonoid–gastrointestinal mucus interaction and its potential role in regulating flavonoid bioavailability and mucosal biophysical properties. Food Res. Int. 2016, 88, 342–347. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, C.; Diao, Q.; Tu, Y. Effects of dietary supplementation with two alternatives to antibiotics on intestinal microbiota of preweaned calves challenged with Escherichia coli K99. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Q.; Zhong, S.; Chen, Y.; Yang, Y. Comparison of Rumen Microbiota and Serum Biochemical Indices in White Cashmere Goats Fed Ensiled or Sun-Dried Mulberry Leaves. Microorganisms 2020, 8, 981. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Arjmandi, B.; Khalil, D.; Hollis, B. Ipriflavone, a synthetic phytoestrogen, enhances intestinal calcium transport in vitro. Calcif. Tissue Int. 2000, 67, 225–229. [Google Scholar] [CrossRef]
- Zafar, T.A.; Weaver, C.M.; Jones, K.; Moore, D.R.; Barnes, S. Inulin effects on bioavailability of soy isoflavones and their calcium absorption enhancing ability. J. Agric. Food Chem. 2004, 52, 2827–2831. [Google Scholar] [CrossRef]
- Li, M.; Hassan, F.; Tang, Z.; Peng, L.; Liang, X.; Li, L.; Peng, K.; Xie, F.; Yang, C. Mulberry leaf flavonoids improve milk production, antioxidant and metabolic status in buffaloes during summer season. Front. Vet. Sci. 2020, 7, 599. [Google Scholar] [CrossRef]
- Miksicek, R.J. Commonly occurring plant flavonoids have estrogenic activity. Mol. Pharmacol. 1993, 44, 37–43. [Google Scholar]
- Zand, R.S.R.; Jenkins, D.J.; Diamandis, E.P. Steroid hormone activity of flavonoids and related compounds. Breast Cancer Res. Treat. 2000, 62, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R. Role of plant polyphenols in genomic stability. Mutat Res.-Fund Mol. M 2001, 475, 89–111. [Google Scholar] [CrossRef]
- Stoldt, A.-K.; Derno, M.; Nürnberg, G.; Weitzel, J.M.; Otten, W.; Starke, A.; Wolffram, S.; Metges, C.C. Effects of a 6-wk intraduodenal supplementation with quercetin on energy metabolism and indicators of liver damage in periparturient dairy cows. J. Dairy Sci. 2015, 98, 4509–4520. [Google Scholar] [CrossRef] [PubMed]
- Kehrli, M.; Neill, J.; Burvenich, C.; Goff, J.; Lippolis, J.; Reinhardt, T.; Nonnecke, B. Energy and protein effects on the immune system. In Ruminant Physiology. Digestion, Metabolism and Impact of Nutrition on Gene Expression, Immunology and Stress; Wageningen Academic Publishers: Wageningen, The Netherlands, 2006; pp. 455–471. [Google Scholar]
- Tan, N.; Wanapat, M.; Uriyapongson, S.; Cherdthong, A.; Pilajun, R. Enhancing mulberry leaf meal with urea by pelleting to improve rumen fermentation in cattle. Asian-Australas J. Anim. Sci. 2012, 25, 452. [Google Scholar] [CrossRef]
- Bakshi, M.; Wadhwa, M. Tree leaves as complete feed for goat bucks. Small Rumin. Res. 2007, 69, 74–78. [Google Scholar] [CrossRef]
- Anbarasu, C.; Dutta, N.; Sharma, K.; Rawat, M. Response of goats to partial replacement of dietary protein by a leaf meal mixture containing Leucaena leucocephala, Morus alba and Tectona grandis. Small Rumin. Res. 2004, 51, 47–56. [Google Scholar] [CrossRef]
- Ma, T.; Chen, D.D.; Tu, Y.; Zhang, N.F.; Si, B.W.; Diao, Q.Y. Dietary supplementation with mulberry leaf flavonoids inhibits methanogenesis in sheep. Anim. Sci. J. 2017, 88, 72–78. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Zhou, B.; Zhou, Z. Effect of ensiled mulberry leaves and sun-dried mulberry fruit pomace on the fecal bacterial community composition in finishing steers. BMC Microbiol. 2017, 17, 97. [Google Scholar] [CrossRef]
- Niu, Y.; Meng, Q.; Li, S.; Ren, L.; Zhou, B.; Schonewille, T.; Zhou, Z. Effects of diets supplemented with ensiled mulberry leaves and sun-dried mulberry fruit Pomace on the Ruminal bacterial and Archaeal community composition of finishing steers. PLoS ONE 2016, 11, e0156836. [Google Scholar] [CrossRef]
- Huyen, N.; Wanapat, M.; Navanukraw, C. Effect of mulberry leaf pellet (MUP) supplementation on rumen fermentation and Nutr.ient digestibility in beef cattle fed on rice straw-based diets. Anim. Feed Sci. Technol. 2012, 175, 8–15. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Gh, G.; Satari, R. Antimicrobial activity of Iranian propolis and its chemical composition. DARU J. Pharml. Sci. 2007, 15, 45–48. [Google Scholar]
- Santas, J.; Almajano, M.P.; Carbó, R. Antimicrobial and antioxidant activity of crude onion (Allium cepa, L.) extracts. Int. J. Food Sci. Technol. 2010, 45, 403–409. [Google Scholar] [CrossRef]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In Vitro bioactivity of various pure flavonoids in ruminal fermentation, with special reference to methane formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar]
- Cui, H.; Lu, T.; Wang, M.; Zou, X.; Zhang, Y.; Yang, X.; Dong, Y.; Zhou, H. Flavonoids from Morus alba L. leaves: Optimization of extraction by response surface methodology and comprehensive evaluation of their antioxidant, antimicrobial, and inhibition of α-amylase activities through analytical hierarchy process. Molecules 2019, 24, 2398. [Google Scholar] [CrossRef]
- Mirzoeva, O.; Grishanin, R.; Calder, P. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Oskoueian, E.; Abdullah, N.; Oskoueian, A. Effects of flavonoids on rumen fermentation activity, methane production, and microbial population. BioMed Res. Int. 2013, 152, 239–246. [Google Scholar] [CrossRef]
- AL-Bayati, M.; Hassan, A. Effects of in vitro supplementation of mulberry leaf flavonoids on microbial flora, methanogenesis and fermentative products in rumen fluid of sheep. J. Res. Ecol. 2018, 6, 2067–2077. [Google Scholar]
- Winter, J.; Moore, L.; Dowell, V.; Bokkenheuser, V. C-ring cleavage of flavonoids by human intestinal bacteria. Appl. Environ. Microbiol. 1989, 55, 1203–1208. [Google Scholar] [CrossRef]
- Schneider, H.; Schwiertz, A.; Collins, M.D.; Blaut, M. Anaerobic transformation of quercetin-3-glucoside by bacteria from the human intestinal tract. Arch. Microbiol. 1999, 171, 81–91. [Google Scholar] [CrossRef]
- Stack, R.J.; Hungate, R.E.; Opsahl, W.P. Phenylacetic acid stimulation of cellulose digestion by Ruminococcus albus 8. Appl. Environ. Microbiol. 1983, 46, 539–544. [Google Scholar] [CrossRef]
- Morrison, M.; Mackie, R.I.; Kistner, A. 3-Phenylpropanoic acid improves the affinity of Ruminococcus albus for cellulose in continuous culture. Appl. Environ. Microbiol. 1990, 56, 3220–3222. [Google Scholar] [CrossRef] [PubMed]
- Doran, M.; Laca, E.; Sainz, R. Total tract and rumen digestibility of mulberry foliage (Morus alba), alfalfa hay and oat hay in sheep. Anim. Feed Sci. Technol. 2007, 138, 239–253. [Google Scholar] [CrossRef]
- Venkatesh Kumar, R.; Gautam, C.; Shobha, N.; Lingappa, R.S. Use of mulberry leaves as supplementary food in cow and goat to improve milk production. Int. J. Appl. Res. 2015, 1, 81–84. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant. Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Fandy, T.E.; Jiemjit, A.; Thakar, M.; Rhoden, P.; Suarez, L.; Gore, S.D. Decitabine induces delayed reactive oxygen species (ROS) accumulation in leukemia cells and induces the expression of ROS generating enzymes. Clin. Cancer Res. 2014, 20, 1249–1258. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamiya, Y.; Kamiya, M.; Nakai, Y. Effect of high environmental temperatures on ascorbic acid, sulfhydryl residue and oxidized lipid concentrations in plasma of dairy cows. Anim. Sci. J. 2007, 78, 301–306. [Google Scholar] [CrossRef]
- Enríquez, D.; Hötzel, M.J.; Ungerfeld, R. Minimising the stress of weaning of beef calves: A review. Acta Vet. Scand. 2011, 53, 1–8. [Google Scholar] [CrossRef]
- Antunes, F.; Han, D.; Cadenas, E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H2O2 detoxification in in vivo conditions. Free Radic. Biol. Med. 2002, 33, 1260–1267. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Kaya, E.; Ozgok, Y.; Zor, M.; Eken, A.; Bedir, S.; Erdem, O.; Ebiloglu, T.; Ergin, G. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: A prospective controlled study. Adv. Clin. Exp. Med. 2017, 26, 1095–1099. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Piao, H.; Li, G. Study on antioxidant activity of constituents from mulberry leaf. J. Chin. Med. Mater. 2008, 31, 519. [Google Scholar]
- Kim, G.N.; Jang, H.D. Flavonol content in the water extract of the mulberry (Morus alba L.) leaf and their antioxidant capacities. J. Food Sci. 2011, 76, C869–C873. [Google Scholar] [CrossRef] [PubMed]
- Xiaofeng, Y.; Shuang, Z.; Li, Z.; Dan, W.; Xiaoman, F.; Zhen, O. Effect of frost on flavonol glycosides accumulation and antioxidant activities of mulberry (Morus alba L.) leaves. Pharmacogn. Mag. 2019, 15, 466. [Google Scholar]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993, 57, 715S–725S. [Google Scholar] [CrossRef]
- Nerland, D.E. The antioxidant/electrophile response element motif. Drug Metab. Rev. 2007, 39, 235–248. [Google Scholar] [CrossRef]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Arojojoye, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O. Quercetin attenuates hypertension induced by sodium fluoride via reduction in oxidative stress and modulation of HSP 70/ERK/PPARγ signaling pathways. Biofactors 2018, 44, 465–479. [Google Scholar] [CrossRef]
- Liao, B.-Y.; Zhu, D.-Y.; Thakur, K.; Li, L.; Zhang, J.-G.; Wei, Z.-J. Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (Morus alba L.). Molecules 2017, 22, 2271. [Google Scholar] [CrossRef]

| Nutrient | Range | Average † | SEM * | References |
|---|---|---|---|---|
| Dry matter | 18 to 30.5 | 27.3 | 1.61 | [6,21,51,55,60,61,62,63,64,65] |
| Crude protein | 14 to 34.2 | 21.4 | 0.88 | [6,21,50,51,55,58,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] |
| Organic matter | 86.4 to 89.8 | 87.9 | 0.72 | [21,51,61,70] |
| Fat | 3.5 to 8.1 | 5.1 | 0.46 | [50,51,55,60,61,64,65,67,68,69,70,71,72,73,74] |
| Fiber | 5.4 to 38.4 | 16.4 | 2.83 | [50,51,55,58,60,61,66,67,68,70] |
| NFE | 25 to 47.9 | 40.1 | 5.11 | [50,61,67,70] |
| NDF | 19.4 to 49.7 | 32.6 | 1.72 | [6,21,50,51,55,58,63,64,65,68,69,70,71,72,73,74] |
| ADF | 10.2 to 31.8 | 40.1 | 1.46 | [6,21,51,58,62,64,65,66,72,73] |
| Ash | 7.6 to 22.4 | 13.1 | 0.85 | [50,55,60,61,62,63,64,66,67,71,72,73,74] |
| Total Flavonoids | Rutin | CHA | IQT | QMG | AG | KMG | References |
|---|---|---|---|---|---|---|---|
| 21.36–56.41 | 0.42–4.31 | 2.45–10.24 | 0.70–4.83 | 0.68–3.05 | 0.30–1.32 | 0.46–1.19 | [58] |
| ND | 0.90 | ND | ND | 0.47 | ND | 0.19 | [54] |
| 24.34–58.42 | 1.09–8.35 | 4.10–9.67 | ND | 0.36–13.92 | ND | 0.07–3.21 | [99] |
| 9.84–26.6 | ND | ND | ND | ND | ND | ND | [100] |
| 22.5–33.3 | 2.1 | 0.13–0.27 | 3.70–4.01 | ND | ND | ND | [59] |
| Flavonoid | Mechanism | Major Activities | Reference |
|---|---|---|---|
| Quercetin | Inhibition of xanthine oxidase and lipoxygenase, potential ROS scavenger, DPPH scavenging activity, radical oxygen absorption activity | Antioxidant | [108,109,110] |
| Rutin | DPPH radical scavenging activity, Reducing ROS generation in H2O2-treated APPswe cells | Inhibition of lipid peroxidation and act as an antioxidant, revert the β-amyloid toxicity | [111,112] |
| Kaempferol | Improve glucose uptake of 3T3-L1 adipocytes acting as partial agonists of PPARγ, superoxide anion radical scavenging activity | Ameliorate hyperglycemia, antioxidant effects | [113,114] |
| Isoquercitrin | Lipid-lowering effect and reduced ROS within the Hepatocytes | Reduce oxidative stress | [115,116] |
| Apigenin | Scavenging ROS and regulation of Fas/FasL pathway | Protects from toxicity and hepatic necrosis | [117,118] |
| Luteolin | Scavenging reactive oxygen and nitrogen species, inhibiting nuclear factor-kappa B activity and Activator protein 1 | Antioxidant and anti-inflammatory activity | [119,120,121] |
| Astragalin | Suppression of 6-hydroxydopamine-stimulated neurotoxicity, decreased expression of MDA, TNF-α, IL-6, ROS | Alleviation of oxidative stress, cardioprotective Activity | [122,123] |
| Animal | Dose Rate | Major Findings | References |
|---|---|---|---|
| Fattening Hu sheep | Inclusion of MLP at 15, 30, 45, or 60% in concentrate diet | DM intake and average daily gain was optimized up to 30% MLP | [37] |
| Calves | MLFs at 2 and 4 g/d during pre and poet-weaning respectively | Improved growth performance and feed digestibility | [144] |
| Ewes | 2 g of MLFs in forage diet (6 weeks) | Reduction in CH4 emission by 12% | [161] |
| Simmental crossbred steers | Ensiled MLs (16 weeks) | Higher abundance of Ruminococcus albus and Ruminococcus albus in the fecal sample | [162] |
| Simmental crossbred steers | Corn grain and cottonseed meal diet replaced by 8% ensiled MLs group, and 6.3% sun-dried mulberry fruit pomace (16 weeks) | The concentration of total VFA improved with ensiled MLs compared to sun-dried mulberry fruit pomace | [21] |
| Simmental crossbred steers | Corn grain and cottonseed meal diet replaced by 8% ensiled MLs group, and 6.3% sun-dried mulberry fruit pomace (16 weeks) | Bacterial community composition was similar among the three groups | [163] |
| Beef cattle | Mulberry leaf pellet supplementation at 200, 400, and 600 g/d with rice straw (21 d) | Improved DM intake, ruminal NH3-N, and cellulolytic bacteria | [158] |
| Beef cattle | Mulberry leaf pellet supplementation at 200, 400, and 600 g/d with rice straw (21 d) | Improved apparent metabolizable energy of DM, CP, organic matter, NDF and ADF | [164] |
| Sheep | Basel diet supplemented with 2 g of MLFs | Reduced energy losses of CH4 emission | [7] |
| Sheep | Mulberry foliage 1.2% of body weight | Improved total VFA concentration and digestibility of ADF and NDF | [140] |
| Goats | Feeding of different tree leaves (Azadirachta indica, Melia azedarach, Morus alba, and Leucaena) | Morus alba show higher DM intake and digestibility coefficients | [159] |
| Growing lambs | Replacement of rapeseed meal with MLs in ammoniated rice straw diet (75 days) | Improved feed intake and growth rate | [16] |
| Goats | 50% replacement of conventional supplements with a mixture of leaf meal of (Leucaena leucocephala, M. alba, and Tectona grandis) | Improved DM intake and comparable nitrogen balance with soybean meal group | [160] |
| Cattle | Compare different grasses (Bermuda grass, elephant grass, and buffalo grass) | Improved digestibility of DM and OM and ME and NE value of the ML compare to other | [51] |
| Holstein calves challenged with E. coli | 5% mulberry flavonoids at 3 g/d (36 days) | Improved feed efficiency and gut beneficial bacterial Count | [147] |
| Calves | MLFs at 3 g/d during pre- and post-weaning period (21–80 d of age) | The ADG was improved post-weaning and overall period with similar feed Intake | [145] |
| Calves challenged with E. coli | MLFs at 3 g/d | Improved ADG and feed efficiency and reduce oxidative stress | [18] |
| Buffalo | MLFs at 15, 30, and 45 g/d | Dose-dependent increase in milk yield; while a higher level of MLFs also increased milk fat (%) and protein (%) | [152] |
| Dairy cows | Paper mulberry silage at 13.5% and 18.0% | Increased milk urea nitrogen and decreased somatic cell count with similar milk yield, DM digestibility | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, F.-u.; Arshad, M.A.; Li, M.; Rehman, M.S.-u.; Loor, J.J.; Huang, J. Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects. Animals 2020, 10, 2076. https://doi.org/10.3390/ani10112076
Hassan F-u, Arshad MA, Li M, Rehman MS-u, Loor JJ, Huang J. Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects. Animals. 2020; 10(11):2076. https://doi.org/10.3390/ani10112076
Chicago/Turabian StyleHassan, Faiz-ul, Muhammad Adeel Arshad, Mengwei Li, Muhammad Saif-ur Rehman, Juan J. Loor, and Jiaxiang Huang. 2020. "Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects" Animals 10, no. 11: 2076. https://doi.org/10.3390/ani10112076
APA StyleHassan, F.-u., Arshad, M. A., Li, M., Rehman, M. S.-u., Loor, J. J., & Huang, J. (2020). Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects. Animals, 10(11), 2076. https://doi.org/10.3390/ani10112076