Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Light Lambs Given Different Maternal Feedings and Fattening Concentrates
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Management and Experimental Design
2.2. Measurements and Sampling Procedures
2.3. Secondary Compound Analyses
2.3.1. Extraction of Secondary Compounds in the Feedstuffs
2.3.2. Analytical Procedures
2.4. Calculations and Statistical Analyses
3. Results
3.1. Secondary Compounds in Feedstuffs of Ewes and Concentrates of Lambs
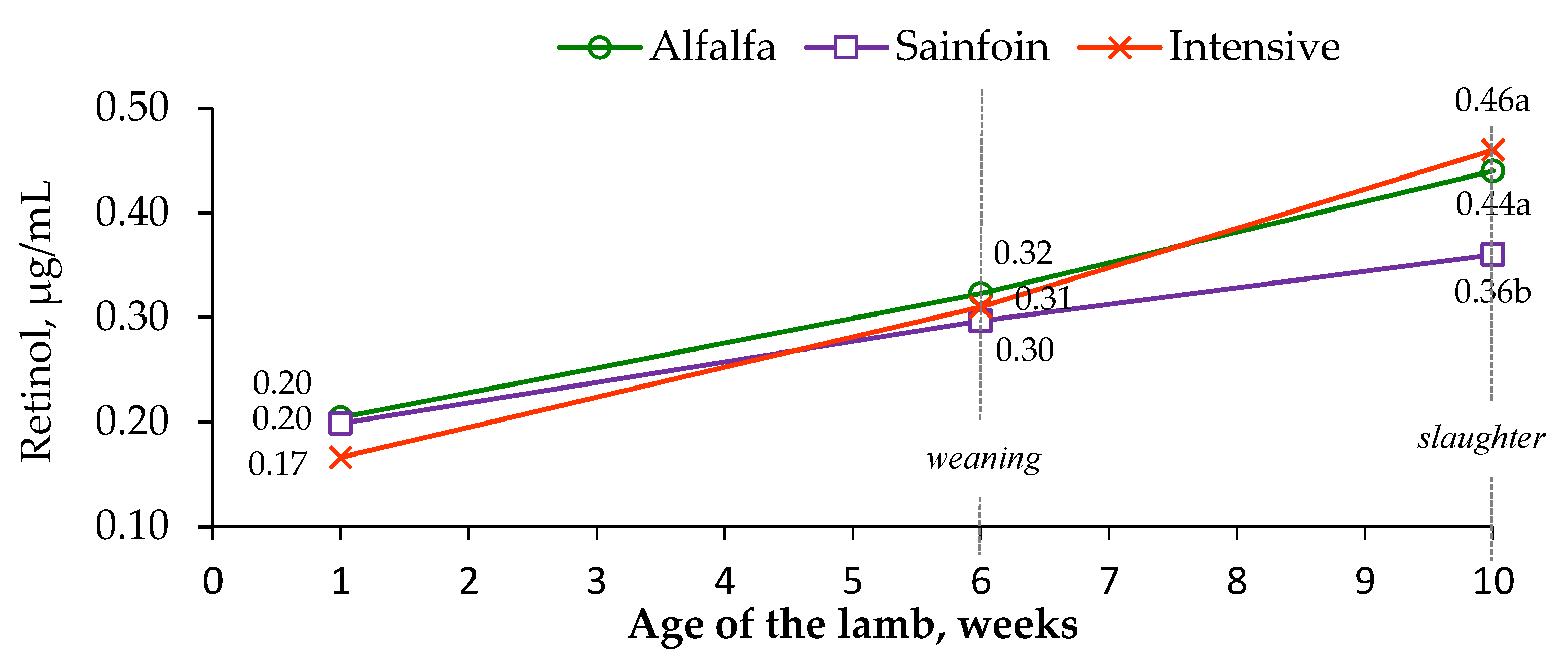
3.2. Concentration of Carotenoids and Liposoluble Vitamins in the Plasma of Lambs
3.3. Carotenoid and Liposoluble Vitamin Content in the Lamb Tissues
3.4. Relationship between the Concentration of the Analytes in the Plasma and the Content in the Lamb Tissues
3.5. Discriminant Analysis Based on Carotenoids and Liposoluble Vitamins
4. Discussion
4.1. Carotenoids and Tocopherols in the Feedstuffs
4.2. Effect of Maternal Feeding on Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Lambs
4.3. Effect of the Inclusion of Quebracho on the Contents of Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Lambs
4.4. Discrimination Analysis Based on Carotenoids and Tocopherols
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ripoll Bosch, R.; Joy Torrens, M.; Bernués Jal, A. Role of self-sufficiency, productivity and diversification on the economic sustainability of farming systems with autochthonous sheep breeds in less favoured areas in Southern Europe. Animal 2014, 8, 1229–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prache, S. Diet authentication in sheep from the composition of animal tissues and products. Rev. Bras. De Zootec. 2009, 38, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Lobón, S.; Blanco, M.; Sanz, A.; Ripoll, G.; Bertolín, J.; Joy, M. Meat quality of light lambs is more affected by the dam’s feeding system during lactation than by the inclusion of quebracho in the fattening concentrate. J. Anim. Sci. 2017, 95, 4998–5011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasta, V.; Priolo, A.; Scerra, M.; Hallett, K.G.; Wood, J.D.; Doran, O. Δ9 desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lambs fed green herbage or concentrate with or without added tannins. Meat Sci. 2009, 82, 357–364. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; McAllan, A. Tannins: Their biochemistry and nutritional properties. Adv. Plant Cell Biochem. Biotechnol. 1992, 1, 151–217. [Google Scholar]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Dian, P.; Andueza, D.; Barbosa, C.; Amoureux, S.; Jestin, M.; Carvalho, P.; Prado, I.; Prache, S. Methodological developments in the use of visible reflectance spectroscopy for discriminating pasture-fed from concentrate-fed lamb carcasses. Animal 2007, 1, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, R.; Meléndez-Martínez, A.; Vicario, I.; Alcalde, M. Effect of pasture and concentrate diets on concentrations of carotenoids, vitamin A and vitamin E in plasma and adipose tissue of lambs. J. Food Compos. Anal. 2014, 36, 59–65. [Google Scholar] [CrossRef]
- Nozière, P.; Graulet, B.; Lucas, A.; Martin, B.; Grolier, P.; Doreau, M. Carotenoids for ruminants: From forages to dairy products. Anim. Feed Sci. Technol. 2006, 131, 418–450. [Google Scholar] [CrossRef]
- Blanco, M.; Lobón, S.; Bertolín, J.R.; Joy, M. Effect of the maternal feeding on the carotenoid and tocopherol content of suckling lamb tissues. Arch. Anim. Nutr. 2019, 73, 472–484. [Google Scholar] [CrossRef]
- Gallardo, B.; Manca, M.G.; Mantecon, A.R.; Nudda, A.; Manso, T. Effects of linseed oil and natural or synthetic vitamin E supplementation in lactating ewes’ diets on meat fatty acid profile and lipid oxidation from their milk fed lambs. Meat Sci. 2015, 102, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.T.; Zumalacarregui, J.M.; Cabeza, E.A.; Figueira, A.; Mateo, J. Effect of rearing system on some meat quality traits and volatile compounds of suckling lamb meat. Small Rumin. Res. 2008, 78, 1–12. [Google Scholar] [CrossRef]
- Capper, J.L.; Wilkinson, R.G.; Kasapidou, E.; Pattinson, S.E.; Mackenzie, A.M.; Sinclair, L.A. The effect of dietary vitamin E and fatty acid supplementation of pregnant and lactating ewes on placental and mammary transfer of vitamin E to the lamb. Br. J. Nutr. 2005, 93, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasapidou, E.; Enser, M.; Wood, J.; Richardson, R.; Wilkinson, R.; Sinclair, L. Influence of vitamin E supplementation and basal diet on the vitamin E status, performance and tissue fatty acid concentration in lambs. Anim. Int. J. Anim. Biosci. 2009, 3, 516. [Google Scholar] [CrossRef] [Green Version]
- Bertolín, J.; Joy, M.; Rufino-Moya, P.; Lobón, S.; Blanco, M. Simultaneous determination of carotenoids, tocopherols, retinol and cholesterol in ovine lyophilised samples of milk, meat, and liver and in unprocessed/raw samples of fat. Food Chem. 2018, 257, 182–188. [Google Scholar] [CrossRef]
- Yang, A.; Brewster, M.; Lanari, M.; Tume, R. Effect of vitamin E supplementation on α-tocopherol and β-carotene concentrations in tissues from pasture-and grain-fed cattle. Meat Sci. 2002, 60, 35–40. [Google Scholar] [CrossRef]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Lobón, S.; Blanco, M.; Sanz, A.; Ripoll, G.; Joy, M. Effects of feeding strategies during lactation and the inclusion of quebracho in the fattening on performance and carcass traits in light lambs. J. Sci. Food Agric. 2019, 99, 457–463. [Google Scholar] [CrossRef]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer Science & Business Media: Kluwer Academic Publishers: Dordrecht, The Nederlands, 2003. [Google Scholar]
- Fu, H.; Xie, B.; Ma, S.; Zhu, X.; Fan, G.; Pan, S. Evaluation of antioxidant activities of principal carotenoids available in water spinach (Ipomoea aquatica). J. Food Compos. Anal. 2011, 24, 288–297. [Google Scholar] [CrossRef]
- Lyan, B.; Azais-Braesco, V.; Cardinault, N.; Tyssandier, V.; Borel, P.; Alexandre-Gouabau, M.-C.; Grolier, P. Simple method for clinical determination of 13 carotenoids in human plasma using an isocratic high-performance liquid chromatographic method. J. Chromatogr. B Biomed. Sci. Appl. 2001, 751, 297–303. [Google Scholar] [CrossRef]
- Grabber, J.H.; Zeller, W.E.; Mueller-Harvey, I. Acetone enhances the direct analysis of procyanidin-and prodelphinidin-based condensed tannins in Lotus species by the butanol–HCl–iron assay. J. Agric. Food Chem. 2013, 61, 2669–2678. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.M.; Terrill, T.H.; Muir, J.P. Drying method and origin of standard affect condensed tannin (CT) concentrations in perennial herbaceous legumes using simplified butanol-HCl CT analysis. J. Sci. Food Agric. 2008, 88, 1060–1067. [Google Scholar] [CrossRef]
- Chauveau-Duriot, B.; Doreau, M.; Noziere, P.; Graulet, B. Simultaneous quantification of carotenoids, retinol, and tocopherols in forages, bovine plasma, and milk: Validation of a novel UPLC method. Anal. Bioanal. Chem. 2010, 397, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Ripoll, G.; Casasús, I.; Bertolín, J.R.; Joy, M. Carotenoids and tocopherol in plasma and subcutaneous fat colour to trace forage-feeding in growing steers. Livest. Sci. 2019, 219, 104–110. [Google Scholar] [CrossRef]
- Prache, S.; Priolo, A.; Grolier, P. Persistence of carotenoid pigments in the blood of concentrate-finished grazing sheep: Its significance for the traceability of grass-feeding. J. Anim. Sci. 2003, 81, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Salminen, H.; Estévez, M.; Kivikari, R.; Heinonen, M. Inhibition of protein and lipid oxidation by rapeseed, camelina and soy meal in cooked pork meat patties. Eur. Food Res. Technol. 2006, 223, 461. [Google Scholar] [CrossRef]
- Smith, C.W.; Creelman, R.A. Vitamin E concentration in upland cotton seeds. Crop Sci. 2001, 41, 577–579. [Google Scholar] [CrossRef]
- McDowell, L.; Williams, S.; Hidiroglou, N.; Njeru, C.; Hill, G.; Ochoa, L.; Wilkinson, N. Vitamin E supplementation for the ruminant. Anim. Feed Sci. Technol. 1996, 60, 273–296. [Google Scholar] [CrossRef]
- Debier, C.; Larondelle, Y. Vitamins A and E: Metabolism, roles and transfer to offspring. Br. J. Nutr. 2005, 93, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Sterndale, S.; Broomfield, S.; Currie, A.; Hancock, S.; Kearney, G.; Lei, J.; Liu, S.; Lockwood, A.; Scanlan, V.; Smith, G. Supplementation of Merino ewes with vitamin E plus selenium increases α-tocopherol and selenium concentrations in plasma of the lamb but does not improve their immune function. Animal 2018, 12, 998–1006. [Google Scholar] [CrossRef]
- González-Calvo, L.; Ripoll, G.; Molino, F.; Calvo, J.H.; Joy, M. The relationship between muscle α-tocopherol concentration and meat oxidation in light lambs fed vitamin E supplements prior to slaughter. J. Sci. Food Agric. 2015, 95, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shabtay, A.; Eitam, H.; Tadmor, Y.; Orlov, A.; Meir, A.; Weinberg, P.; Weinberg, Z.G.; Chen, Y.; Brosh, A.; Izhaki, I. Nutritive and antioxidative potential of fresh and stored pomegranate industrial byproduct as a novel beef cattle feed. J. Agric. Food Chem. 2008, 56, 10063–10070. [Google Scholar] [CrossRef] [PubMed]
- Judson, G.; Babidge, P.; Babidge, W. Plasma, liver and fat alpha-tocopherol concentrations in sheep given various oral and subcutaneous doses of vitamin E. Aust. J. Exp. Agric. 1991, 31, 45–50. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, H.; Wan, F.; Bi, Y.; Liu, G.; Liu, X.; Zhao, H.; You, W.; Liu, Y.; Tan, X. Effects of feeding β-carotene on levels of β-carotene and vitamin A in blood and tissues of beef cattle and the effects on beef quality. Meat Sci. 2015, 110, 293–301. [Google Scholar] [CrossRef]
- Luciano, G.; Roscini, V.; Mattioli, S.; Ruggeri, S.; Gravador, R.; Natalello, A.; Lanza, M.; De Angelis, A.; Priolo, A. Vitamin E is the major contributor to the antioxidant capacity in lambs fed whole dried citrus pulp. Animal 2017, 11, 411–417. [Google Scholar] [CrossRef]
- Quijada, J.; Drake, C.; Gaudin, E.; El-Korso, R.; Hoste, H.; Mueller-Harvey, I. Condensed tannin changes along the digestive tract in lambs fed with sainfoin pellets or hazelnut skins. J. Agric. Food Chem. 2018, 66, 2136–2142. [Google Scholar] [CrossRef]
- Rufino-Moya, P.J.; Blanco, M.; Lobón, S.; Joy, M.; Pérez-Jiménez, J. Effect of grazing on sainfoin or alfalfa during lactation on lambs’ muscle metabolites. A HPLC-ESI-QTOF MS approach. In Proceedings of 63rd International Congress of Meat Science and Technology (ICOMST), Cork, Ireland, 13–18 August 2017; pp. 826–828. [Google Scholar]
- Prache, S.; Priolo, A.; Grolier, P. Effect of concentrate finishing on the carotenoid content of perirenal fat in grazing sheep: Its significance for discriminating grass-fed, concentrate-fed and concentrate-finished grazing lambs. Anim. Sci. 2003, 77, 225–233. [Google Scholar] [CrossRef]
- Gladine, C.; Rock, E.; Morand, C.; Bauchart, D.; Durand, D. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br. J. Nutr. 2007, 98, 691–701. [Google Scholar] [CrossRef] [Green Version]
- Gessner, D.; Koch, C.; Romberg, F.-J.; Winkler, A.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. The effect of grape seed and grape marc meal extract on milk performance and the expression of genes of endoplasmic reticulum stress and inflammation in the liver of dairy cows in early lactation. J. Dairy Sci. 2015, 98, 8856–8868. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-González, I.; Chamorro, S.; Pérez-Jiménez, J.; López-Andrés, P.; Álvarez-Acero, I.; Herrero, A.M.; Nardoia, M.a.; Brenes, A.; Viveros, A.; Arija, I.; et al. Phenolic Metabolites in Plasma and Thigh Meat of Chickens Supplemented with Grape Byproducts. J. Agric. Food Chem. 2019, 67, 4463–4471. [Google Scholar] [CrossRef]
- Yagoubi, Y.; Joy, M.; Ripoll, G.; Mahouachi, M.; Bertolin, J.R.; Atti, N. Rosemary distillation residues reduce lipid oxidation, increase alpha-tocopherol content and improve fatty acid profile of lamb meat. Meat Sci 2018, 136, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Natalello, A.; Priolo, A.; Valenti, B.; Codini, M.; Mattioli, S.; Pauselli, M.; Puccio, M.; Lanza, M.; Stergiadis, S.; Luciano, G. Dietary pomegranate by-product improves oxidative stability of lamb meat. Meat Sci. 2020, 162, 108037. [Google Scholar] [CrossRef]
- Valenti, B.; Natalello, A.; Vasta, V.; Campidonico, L.; Roscini, V.; Mattioli, S.; Pauselli, M.; Priolo, A.; Lanza, M.; Luciano, G. Effect of different dietary tannin extracts on lamb growth performances and meat oxidative stability: Comparison between mimosa, chestnut and tara. Animal 2019, 13, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Muíño, I.; Apeleo, E.; de la Fuente, J.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Díaz, M.T.; Cañeque, V.; Lauzurica, S. Effect of dietary supplementation with red wine extract or vitamin E, in combination with linseed and fish oil, on lamb meat quality. Meat Sci. 2014, 98, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Larraín, R.; Schaefer, D.; Richards, M.; Reed, J. Finishing steers with diets based on corn, high-tannin sorghum or a mix of both: Color and lipid oxidation in beef. Meat Sci. 2008, 79, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, G.; Joy, M.; Muñoz, F.; Albertí, P. Meat and fat colour as a tool to trace grass-feeding systems in light lamb production. Meat Sci. 2008, 80, 239–248. [Google Scholar] [CrossRef]
- Lobón, S.; Joy, M.; Sanz, A.; Álvarez-Rodríguez, J.; Blanco, M. The fatty acid composition of ewe milk or suckling lamb meat can be used to discriminate between ewes fed different diets. Anim. Prod. Sci. 2019, 59, 1108–1118. [Google Scholar] [CrossRef]
- Prache, S.; Martin, B.; Coppa, M. Authentication of grass-fed meat and dairy products from cattle and sheep. Animal 2020, 14, 854–863. [Google Scholar] [CrossRef] [Green Version]




| Item | Control Concentrate | Quebracho Concentrate |
|---|---|---|
| Ingredients, g/kg dry matter | ||
| Corn | 350 | 400 |
| Soya bean meal | 238 | 263 |
| Wheat | 200 | 200 |
| Barley | 150 | 20 |
| Quebracho | - | 50 |
| Bran | 27 | - |
| Calcium carbonate | 15 | 15 |
| Palm oil | 12 | 29 |
| Cane molasses | - | 15 |
| Minerals and salt | 8 | 8 |
| Chemical composition, % | ||
| Neutral detergent fiber | 17.8 | 17.7 |
| Acid detergent fiber | 4.4 | 4.1 |
| Crude protein | 19.5 | 21.0 |
| Item | Ewe’s Feedstuffs | Lamb’s Concentrates | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TMR 1 | Alfalfa | Sainfoin | s.e. 2 | p-Value | Control | Quebracho | s.e. 2 | p-Value | |
| Carotenoids | |||||||||
| Neoxanthin, µg/g DM | n.d. | 33 | 28 | 2.3 | 0.29 | n.d. | n.d. | . | . |
| Violaxanthin, µg/g DM | n.d. | 35 | 30 | . | 0.37 | n.d. | n.d. | . | . |
| Zeaxanthin, µg/g DM | n.d. | 3.7b | 6.1a | . | 0.004 | 0.2 | 0.3 | 0.024 | 0.66 |
| Lutein, µg/g DM | 0.2b | 111a | 127a | 7.6 | 0.001 | 0.3 | 0.4 | 0.024 | 0.27 |
| 13-Z-β-carotene, µg/g DM | 0.2b | 3.4a | 4.1a | . | 0.0002 | n.d. | n.d. | . | . |
| 9-Z-β-carotene, µg/g DM | n.d. | 5.3a | 7.2a | 0.5 | 0.06 | n.d. | n.d. | . | . |
| All-β-carotene, µg/g DM | n.d. | 31b | 65a | 4.3 | 0.0003 | n.d. | n.d. | . | . |
| Tocopherols | |||||||||
| α-tocopherol, µg/g DM | 5.4c | 35b | 89a | 4.3 | 0.001 | 0.4 | 1.4 | 0.159 | 0.046 |
| γ-tocopherol, µg/g DM | 34a | 2.9c | 5.8b | . | 0.001 | 6.7 | 4.6 | 1.0 | 0.35 |
| Polyphenols, µg tannic acid/g DM | 6c | 11b | 32a | . | 0.001 | 3.8 | 34 | . | 0.001 |
| Total CT, eq 3/kg DM | n.d. | 2.0 | 25 | . | 0.001 | 12 | 76 | 2.98 | 0.001 |
| Item | Lactation (L) | Concentrate (C) | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensive 1 | Alfalfa | Sainfoin | Control | Quebracho | s.e. | L | C | LxC | |
| n | 21 | 21 | 21 | 31 | 32 | ||||
| Liver | |||||||||
| Lutein, ng/g FM | 1.6b | 5.4a | 4.9a | 4.2 | 3.1 | - | 0.001 | 0.26 | 0.30 |
| Retinol 2, µg/g FM | 1.74b | 2.79ab | 4.19a | 4.25a | 1.71b | - | <0.001 | <0.001 | 0.01 |
| α-tocopherol, µg/g FM | 0.25 | 0.31 | 0.30 | 0.41a | 0.17b | - | 0.07 | <0.001 | 0.87 |
| γ-tocopherol, µg/g FM | 0.14 | 0.15 | 0.12 | 0.17a | 0.11b | - | 0.36 | <0.001 | 0.27 |
| Muscle | |||||||||
| Retinol, µg/g FM | 0.032 | 0.035 | 0.033 | 0.032 | 0.034 | 0.001 | 0.19 | 0.35 | 0.96 |
| α-tocopherol, µg/g FM | 0.47c | 1.00b | 1.27a | 1.01a | 0.82b | 0.042 | <0.001 | 0.03 | 0.06 |
| γ-tocopherol 2, µg/g FM | 0.17 | 0.17 | 0.15 | 0.18a | 0.16b | 0.004 | 0.10 | 0.006 | 0.04 |
| Perirenal fat | |||||||||
| Retinol, µg/g FM | 0.61b | 1.35a | 1.43a | 1.04 | 1.16 | - | <0.001 | 0.25 | 0.17 |
| α-tocopherol, µg/g FM | 1.47c | 2.51b | 3.78a | 3.05a | 2.11b | 0.147 | <0.001 | 0.003 | 0.30 |
| γ-tocopherol, µg/g FM | 1.33 | 1.23 | 1.24 | 1.41a | 1.13b | 0.041 | 0.58 | 0.001 | 0.69 |
| Subcutaneous fat | |||||||||
| Retinol, µg/g FM | 0.48b | 1.01a | 1.08a | 0.80 | 0.91 | - | <0.001 | 0.27 | - |
| α-tocopherol, µg/g FM | 1.37c | 2.77b | 4.29a | 3.15 | 2.47 | 0.357 | <0.001 | 0.06 | 0.34 |
| γ-tocopherol, µg/g FM | 1.05 | 0.98 | 0.97 | 1.09a | 0.92b | 0.030 | 0.51 | 0.006 | 0.88 |
| Item | Intensive 1 | Alfalfa | Sainfoin | Intensive 1 | Grazing (Alfalfa + Sainfoin) |
|---|---|---|---|---|---|
| Plasma at weaning | 100% | 81% | 76% | 100% | 100% |
| Plasma at slaughter | 57% | 24% | 86% | 76% | 62% |
| Carcass | 95% | 67% | 62% | 100% | 88% |
| Liver | 67% | 48% | 52% | 86% | 62% |
| Muscle | 95% | 52% | 71% | 100% | 79% |
| Perirenal fat | 67% | 62% | 86% | 86% | 86% |
| Subcutaneous fat | 95% | 48% | 43% | 95% | 88% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rufino-Moya, P.J.; Joy, M.; Lobón, S.; Bertolín, J.R.; Blanco, M. Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Light Lambs Given Different Maternal Feedings and Fattening Concentrates. Animals 2020, 10, 1813. https://doi.org/10.3390/ani10101813
Rufino-Moya PJ, Joy M, Lobón S, Bertolín JR, Blanco M. Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Light Lambs Given Different Maternal Feedings and Fattening Concentrates. Animals. 2020; 10(10):1813. https://doi.org/10.3390/ani10101813
Chicago/Turabian StyleRufino-Moya, Pablo José, Margalida Joy, Sandra Lobón, Juan Ramón Bertolín, and Mireia Blanco. 2020. "Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Light Lambs Given Different Maternal Feedings and Fattening Concentrates" Animals 10, no. 10: 1813. https://doi.org/10.3390/ani10101813
APA StyleRufino-Moya, P. J., Joy, M., Lobón, S., Bertolín, J. R., & Blanco, M. (2020). Carotenoids and Liposoluble Vitamins in the Plasma and Tissues of Light Lambs Given Different Maternal Feedings and Fattening Concentrates. Animals, 10(10), 1813. https://doi.org/10.3390/ani10101813





