Effects of Dietary Andrographolide Levels on Growth Performance, Antioxidant Capacity, Intestinal Immune Function and Microbioma of Rice Field Eel (Monopterus Albus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Diets and Experimental Design
2.2. Sample Collection
2.3. Determination of Growth Parameters
2.4. Measurement of Digestive Enzyme Activities and Liver Antioxidant Parameters
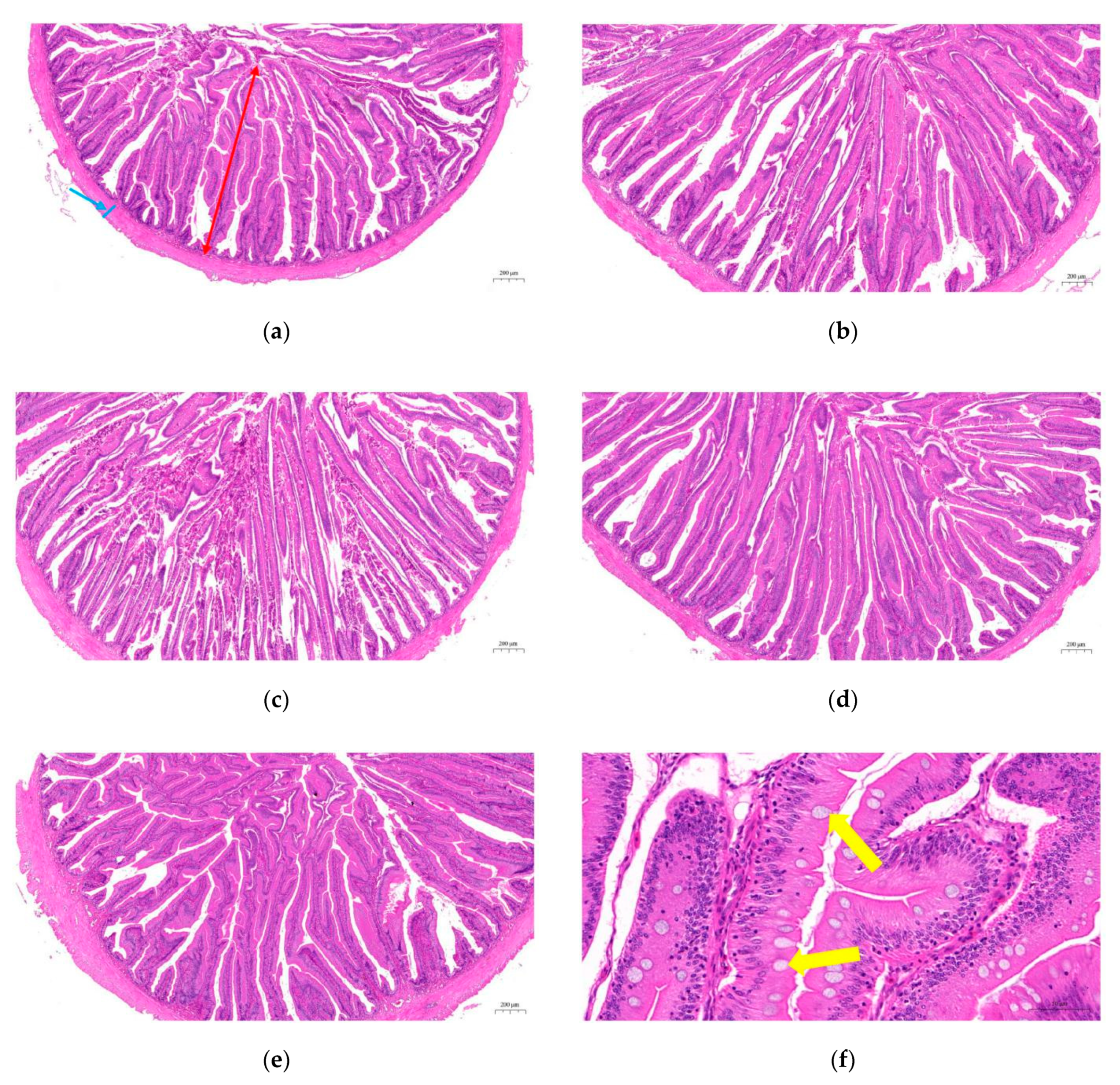
2.5. Intestinal Histological Structure
2.6. Reverse Transcription Real-Time Fluorescent Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.7. Intestinal Microbiology
2.8. Statistical Analysis
3. Results
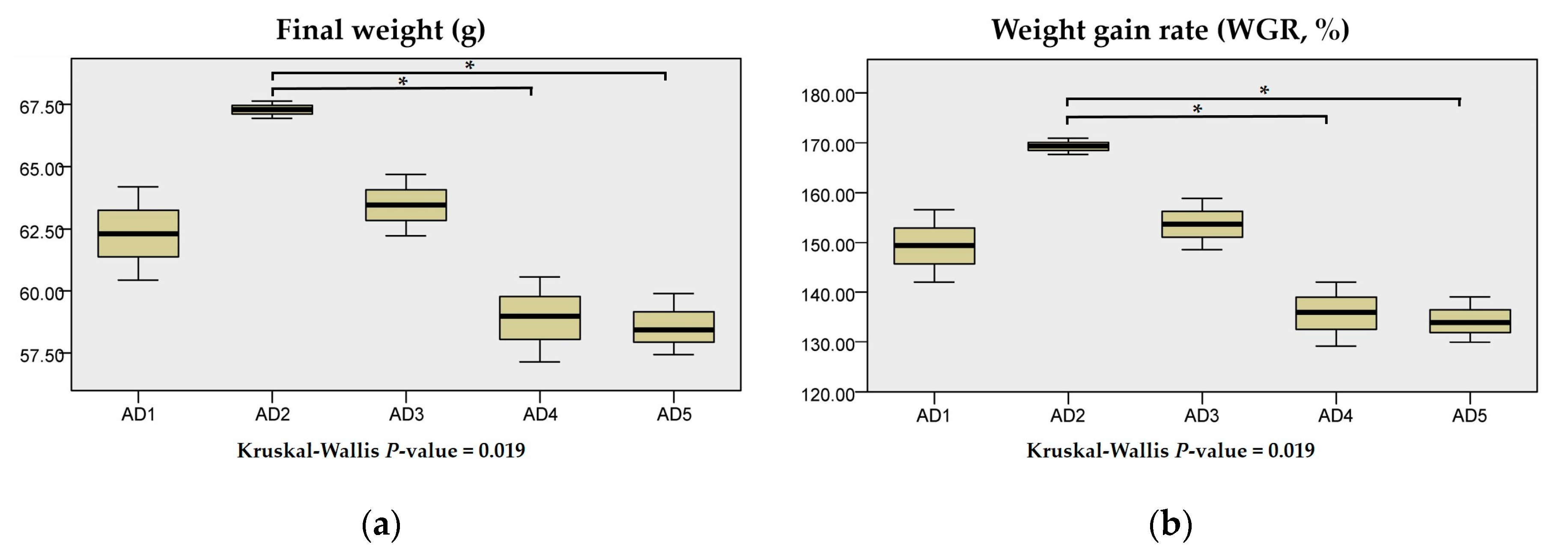
3.1. Growth Performance
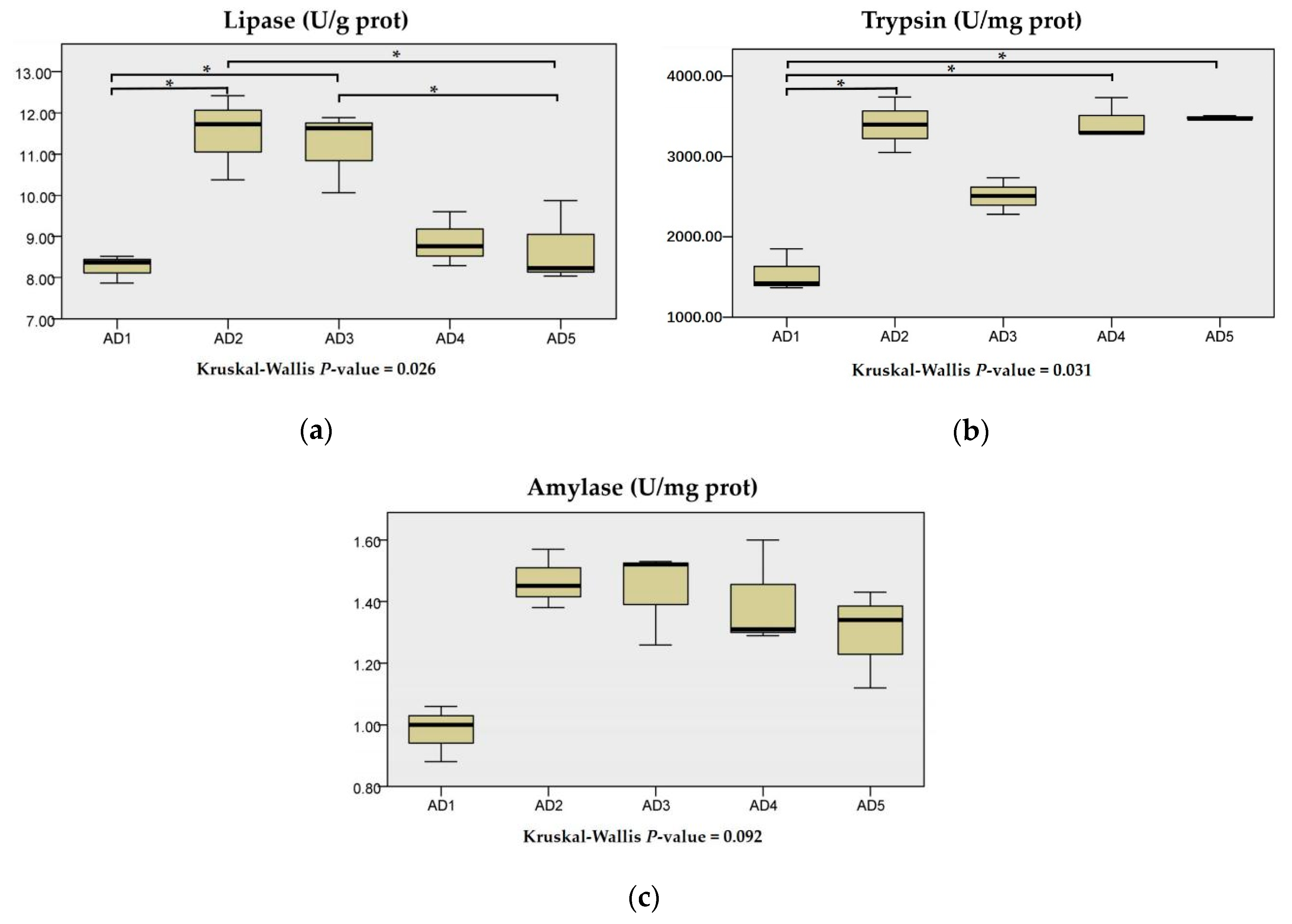
3.2. Digestive Enzyme Activities in Intestine
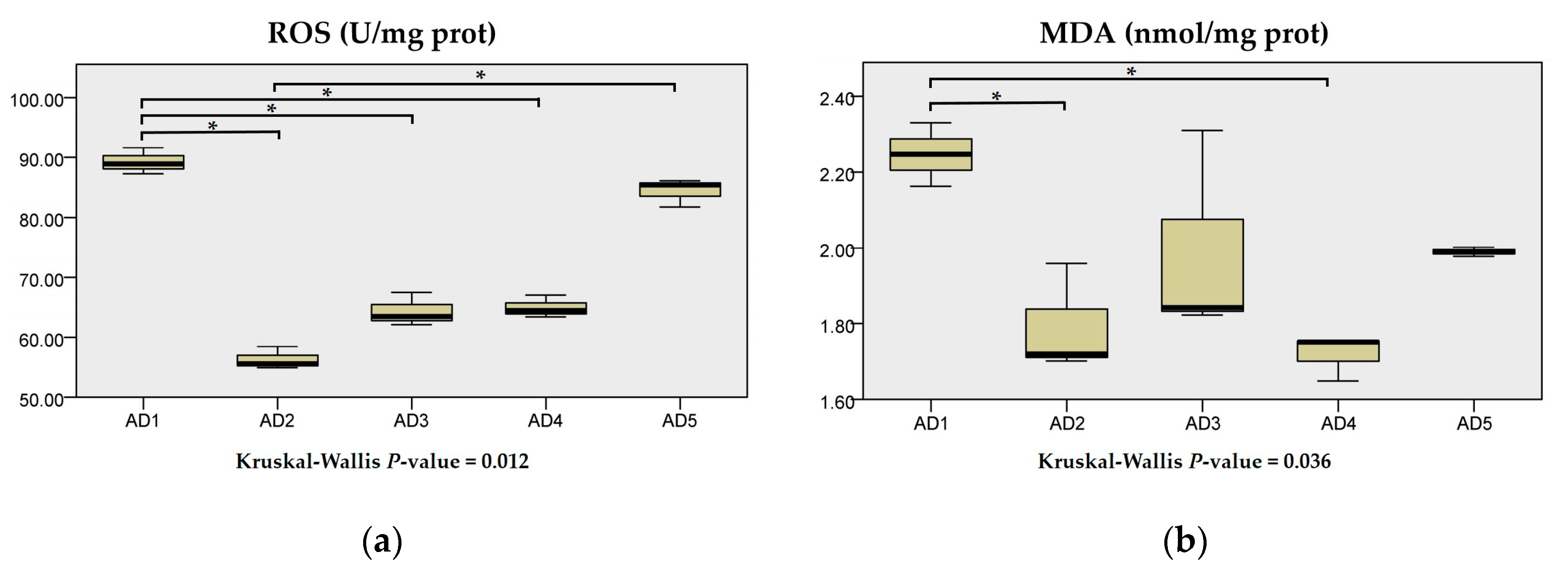
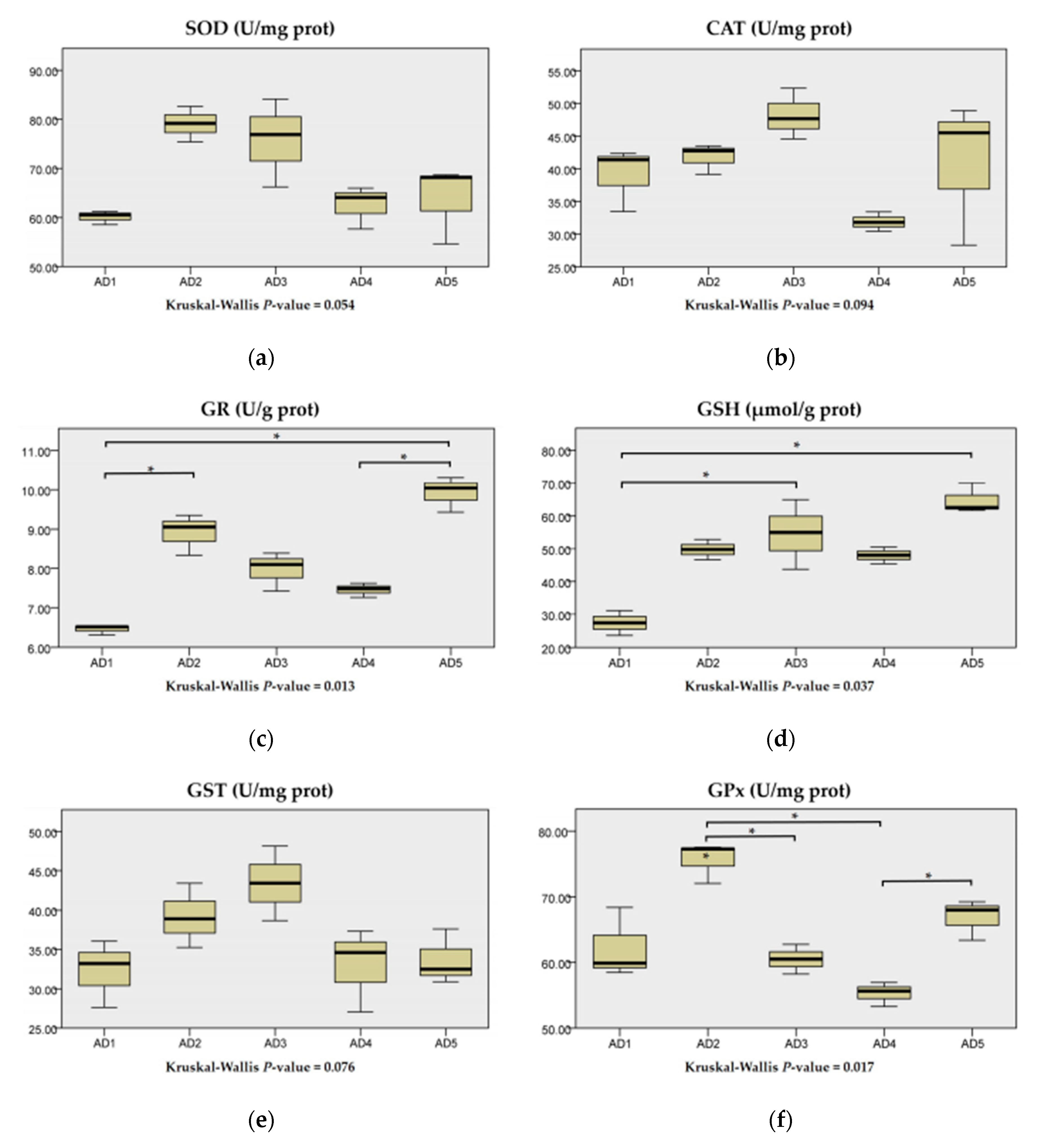
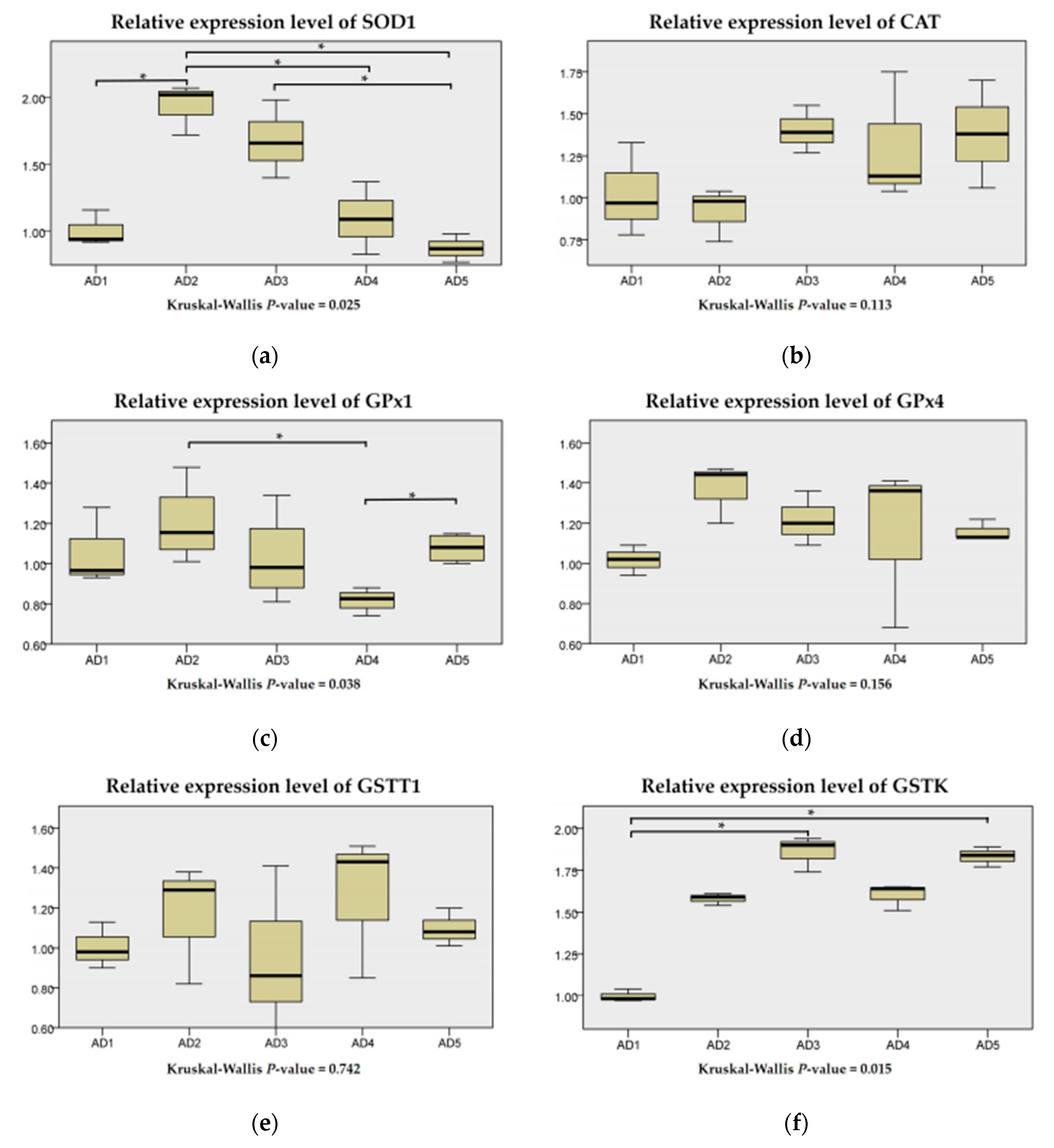
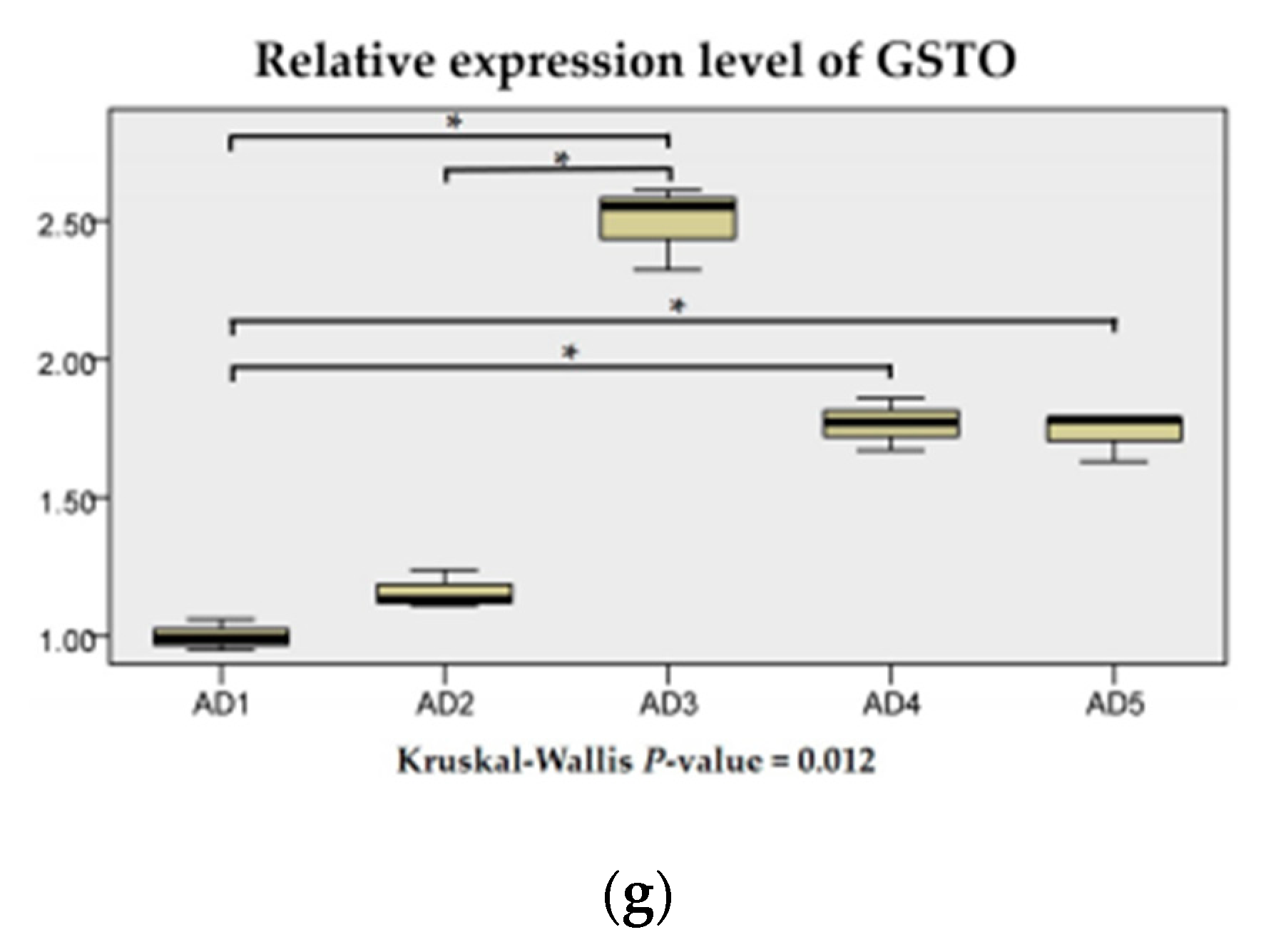
3.3. Antioxidant Enzyme Activities in the Liver
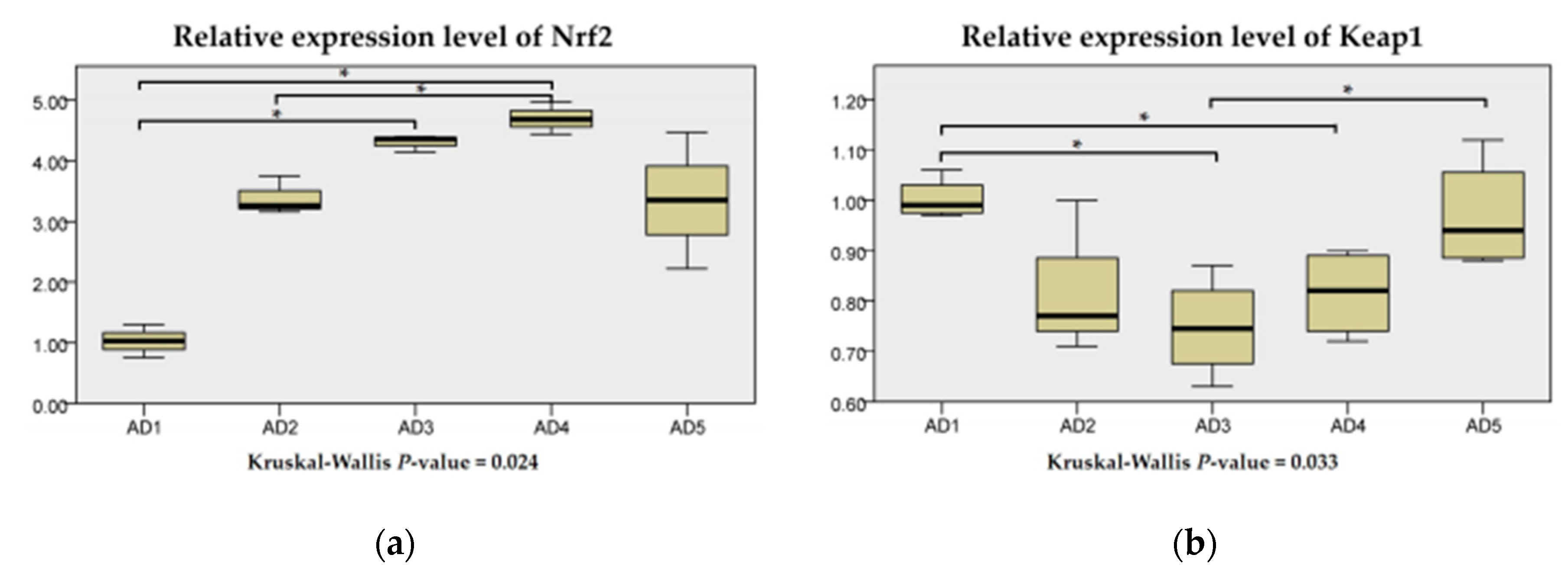
3.4. Antioxidant Related Gene Expression in the Liver
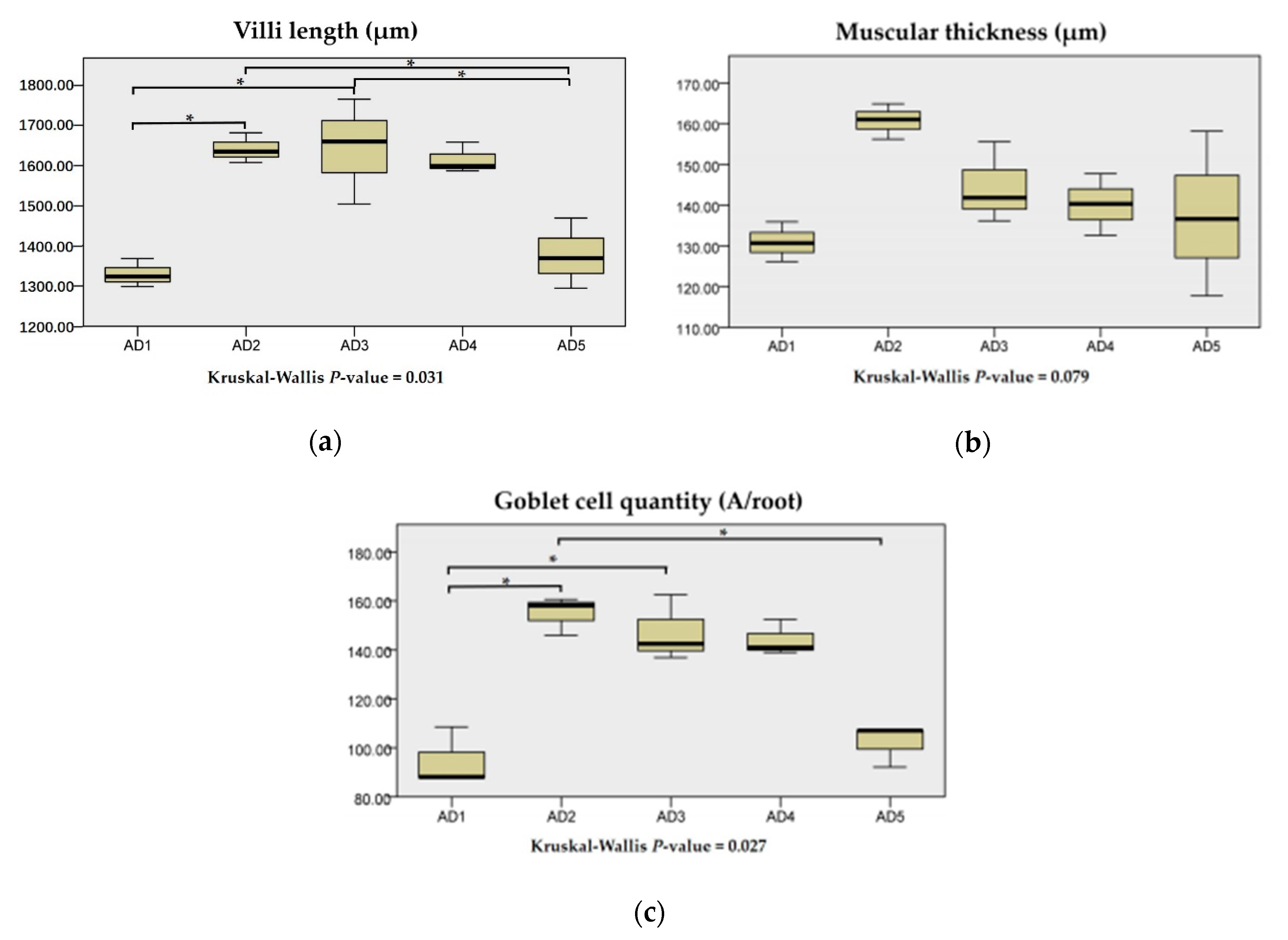
3.5. Histological Structure in the Intestine
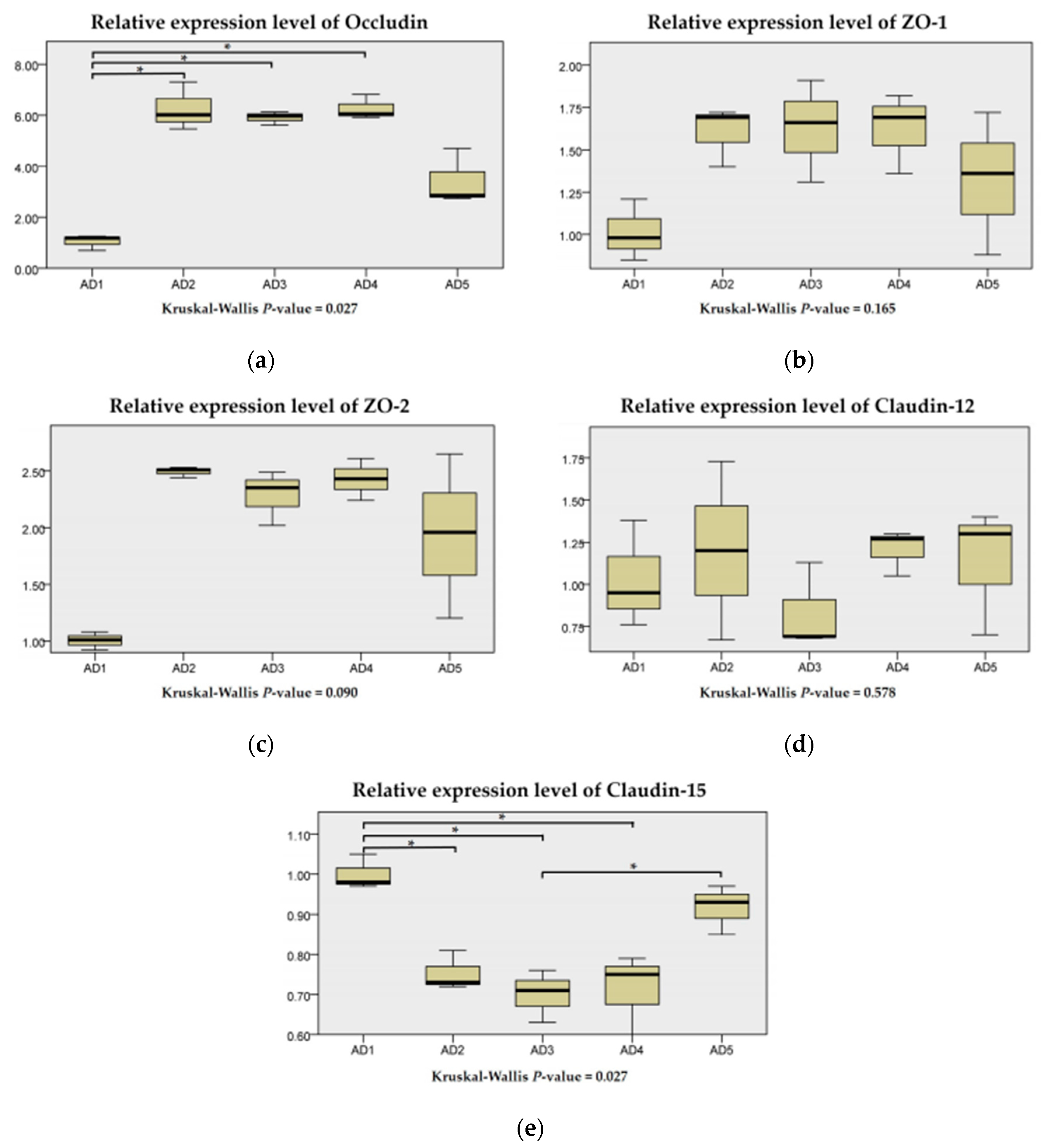
3.6. Expressions of Physical Barrier-Related Genes in the Intestine
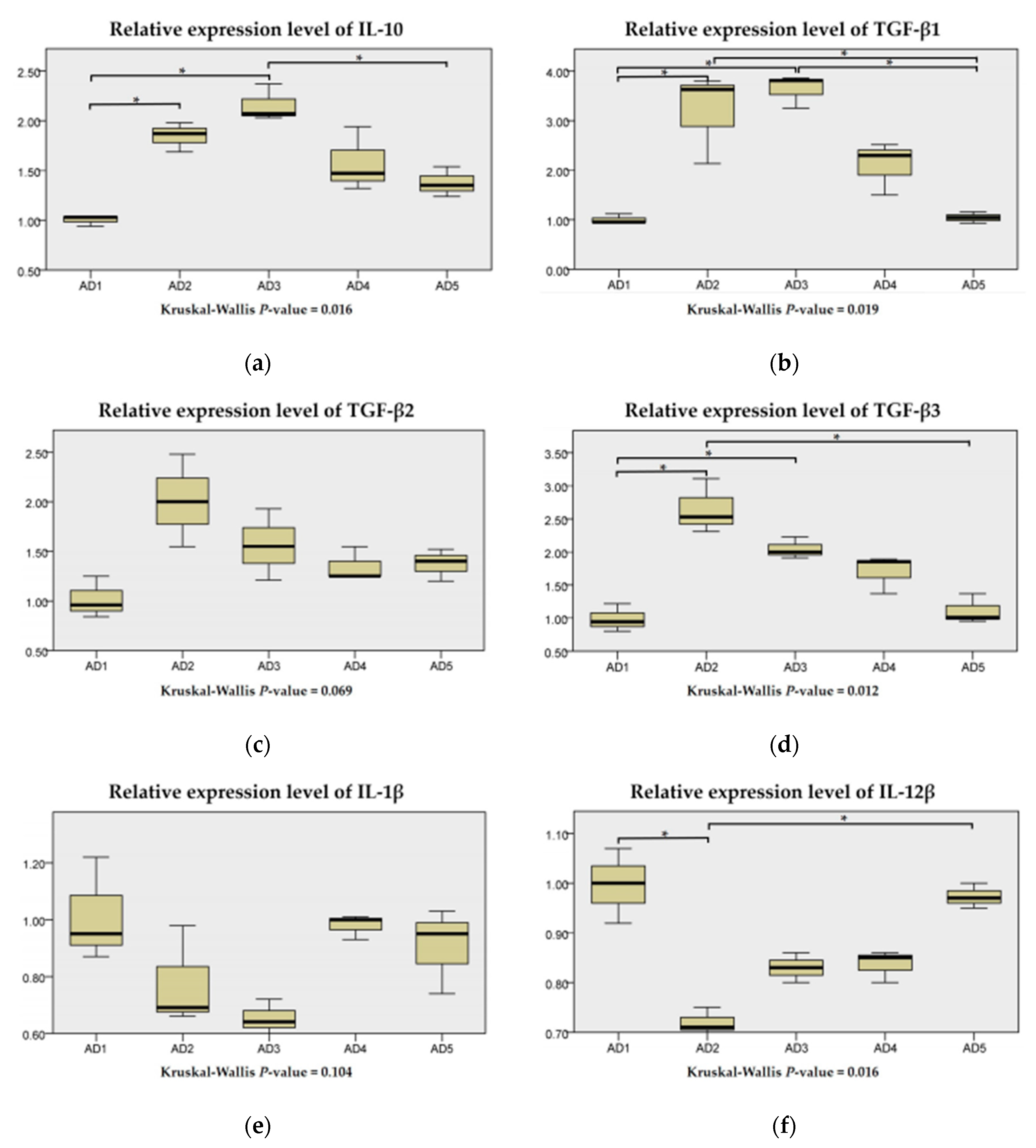
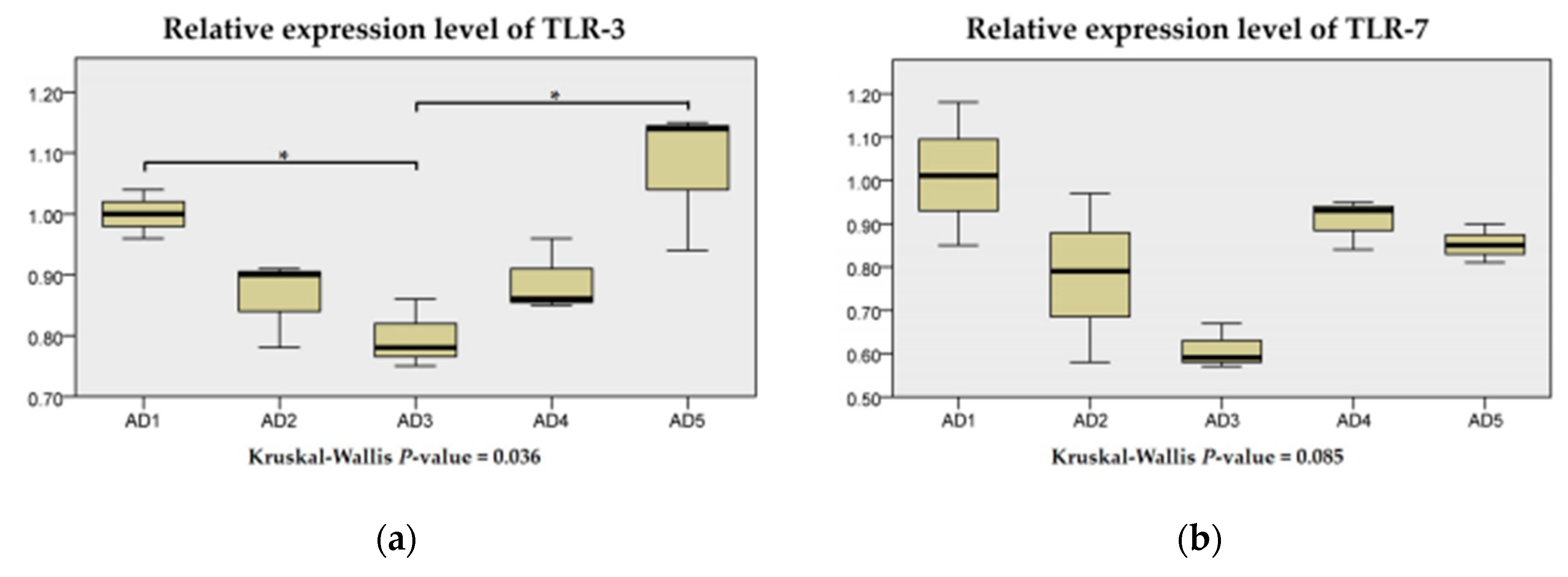
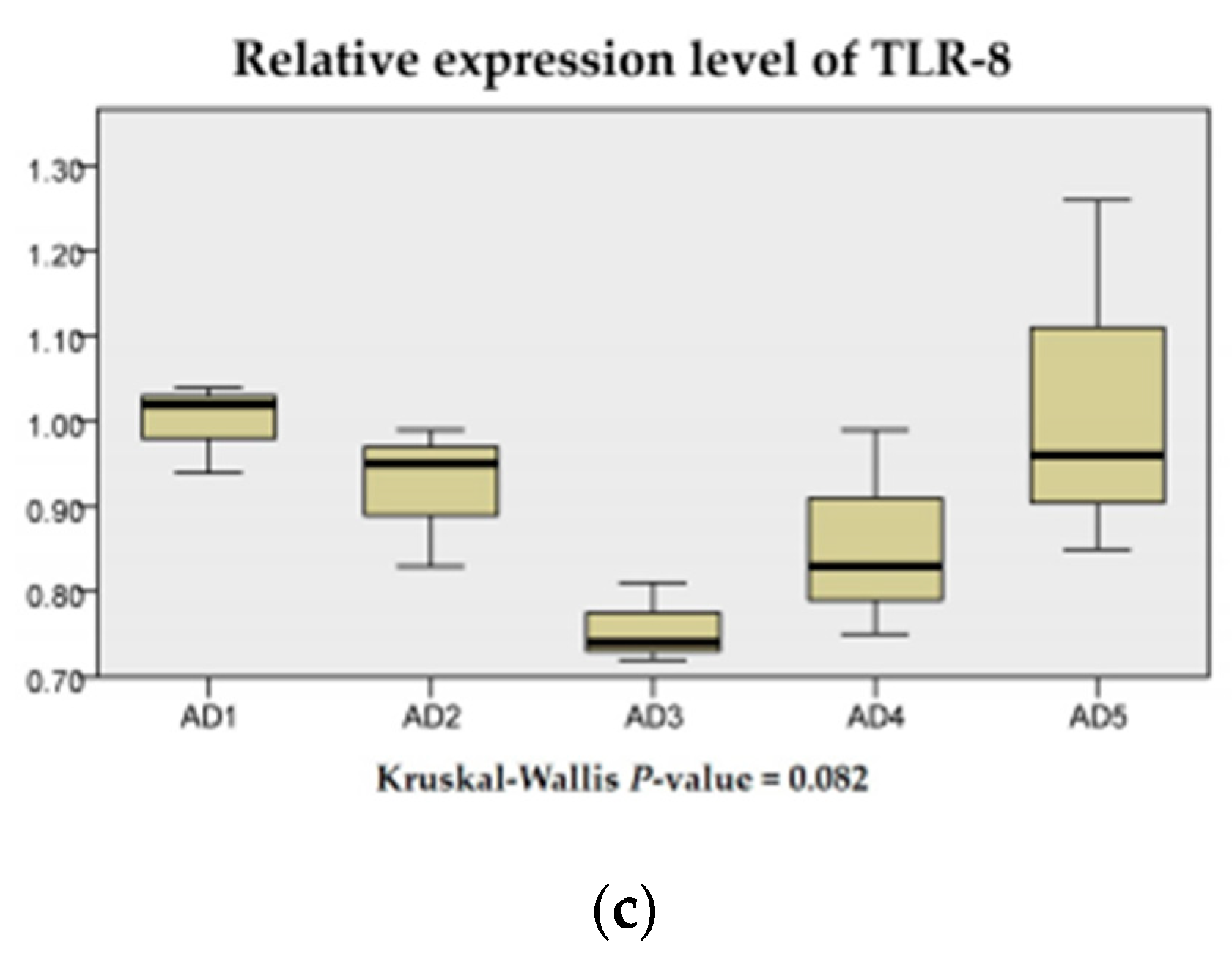
3.7. The mRNA Expression of Inflammation and the TLR Signaling Pathway in the Intestine
3.8. Intestinal Microbiology Analysis
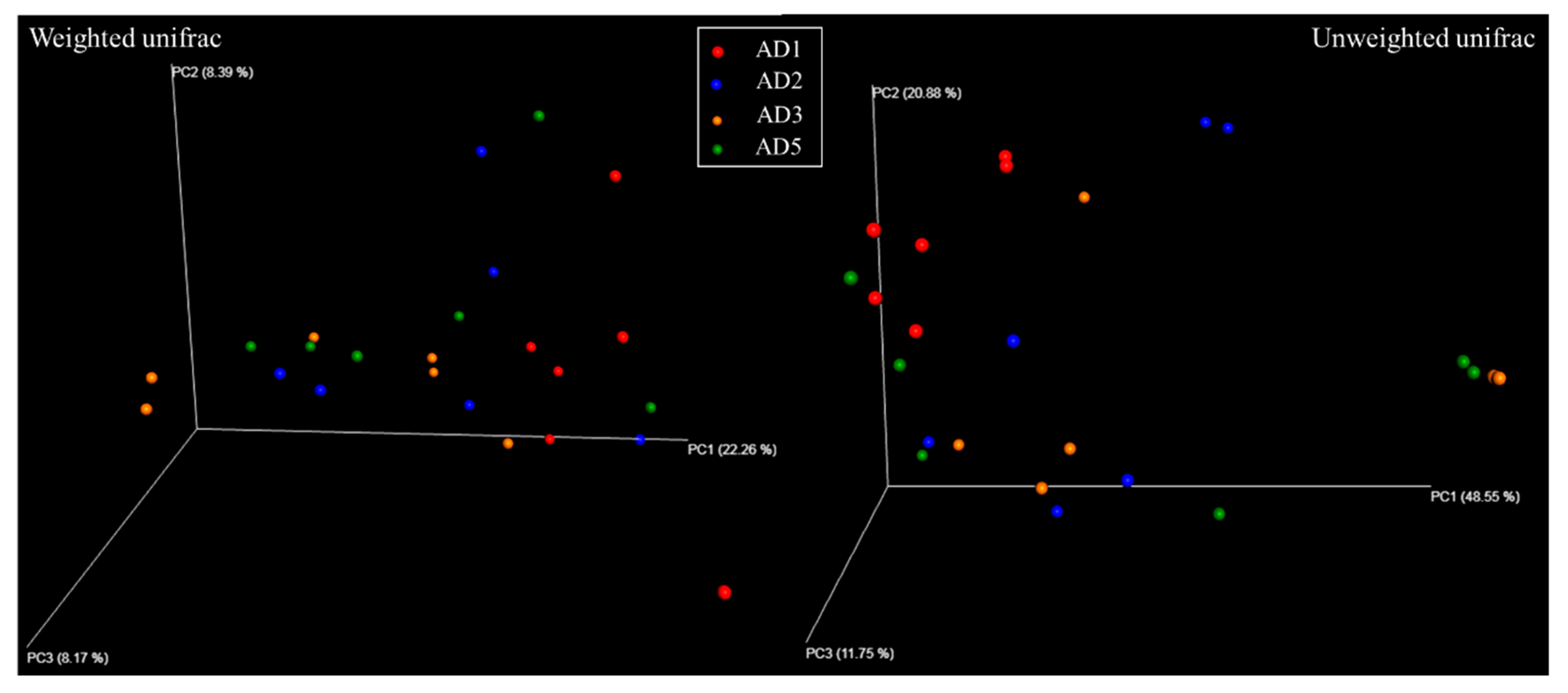
3.8.1. Diversity Analysis
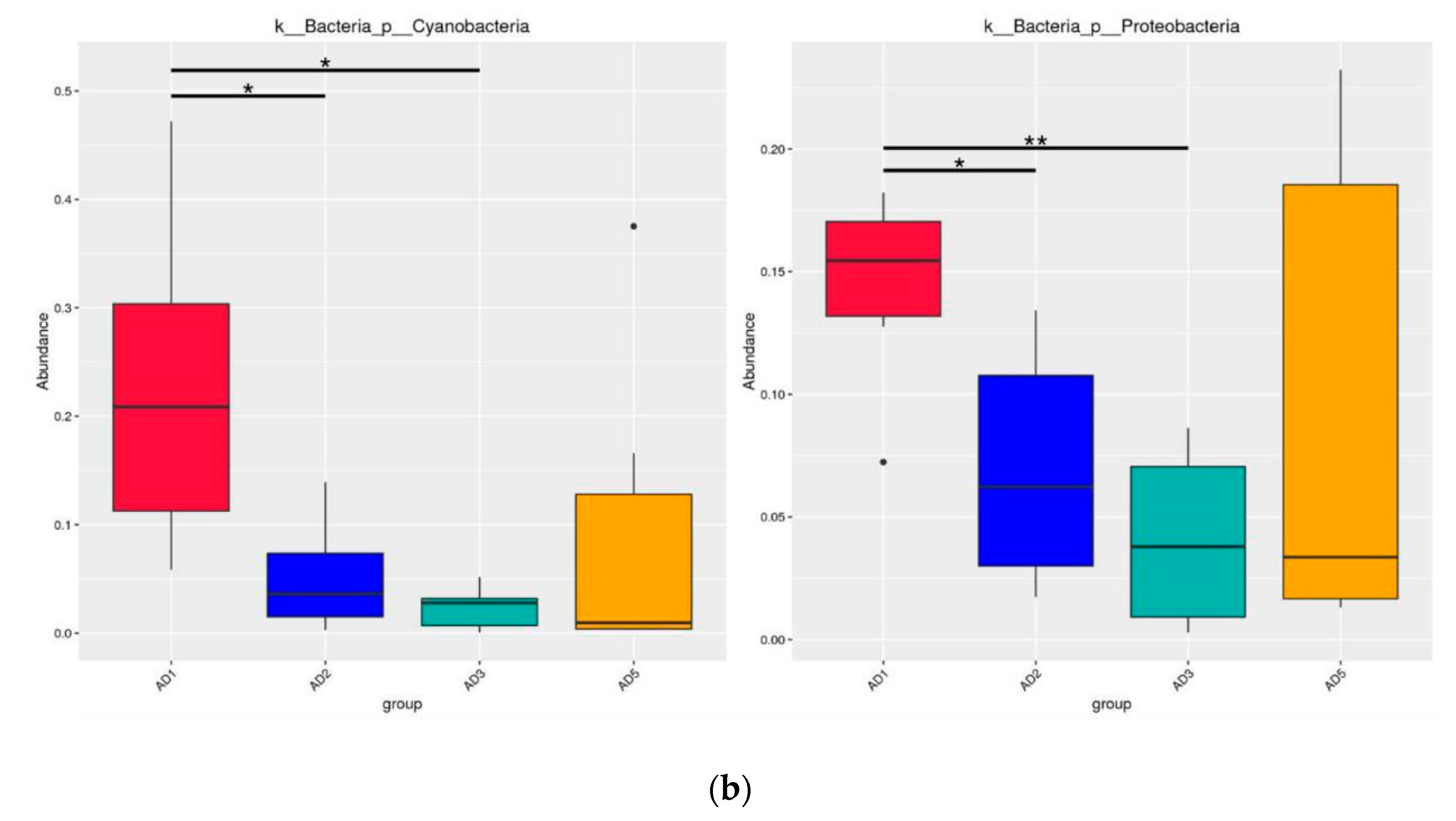
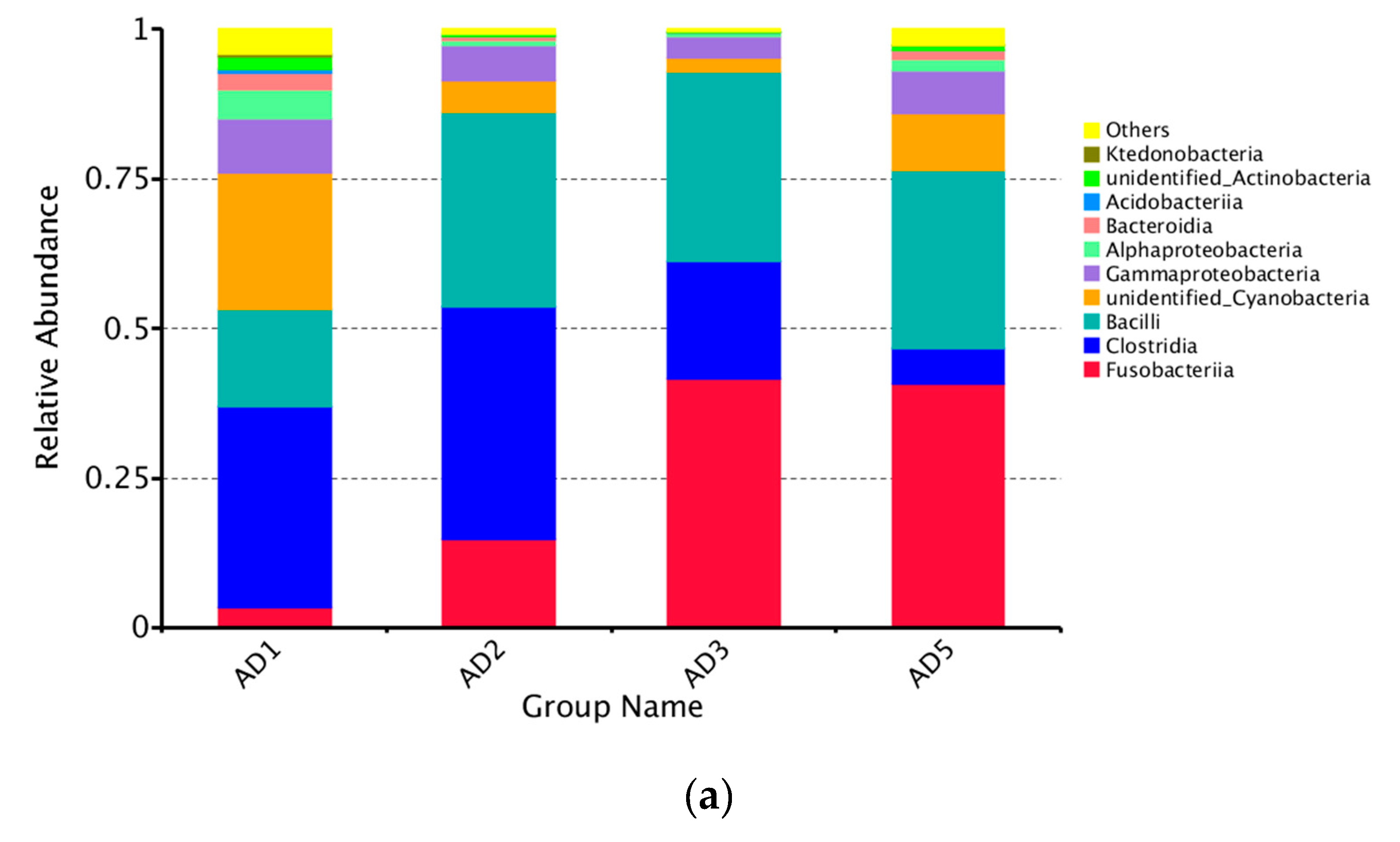
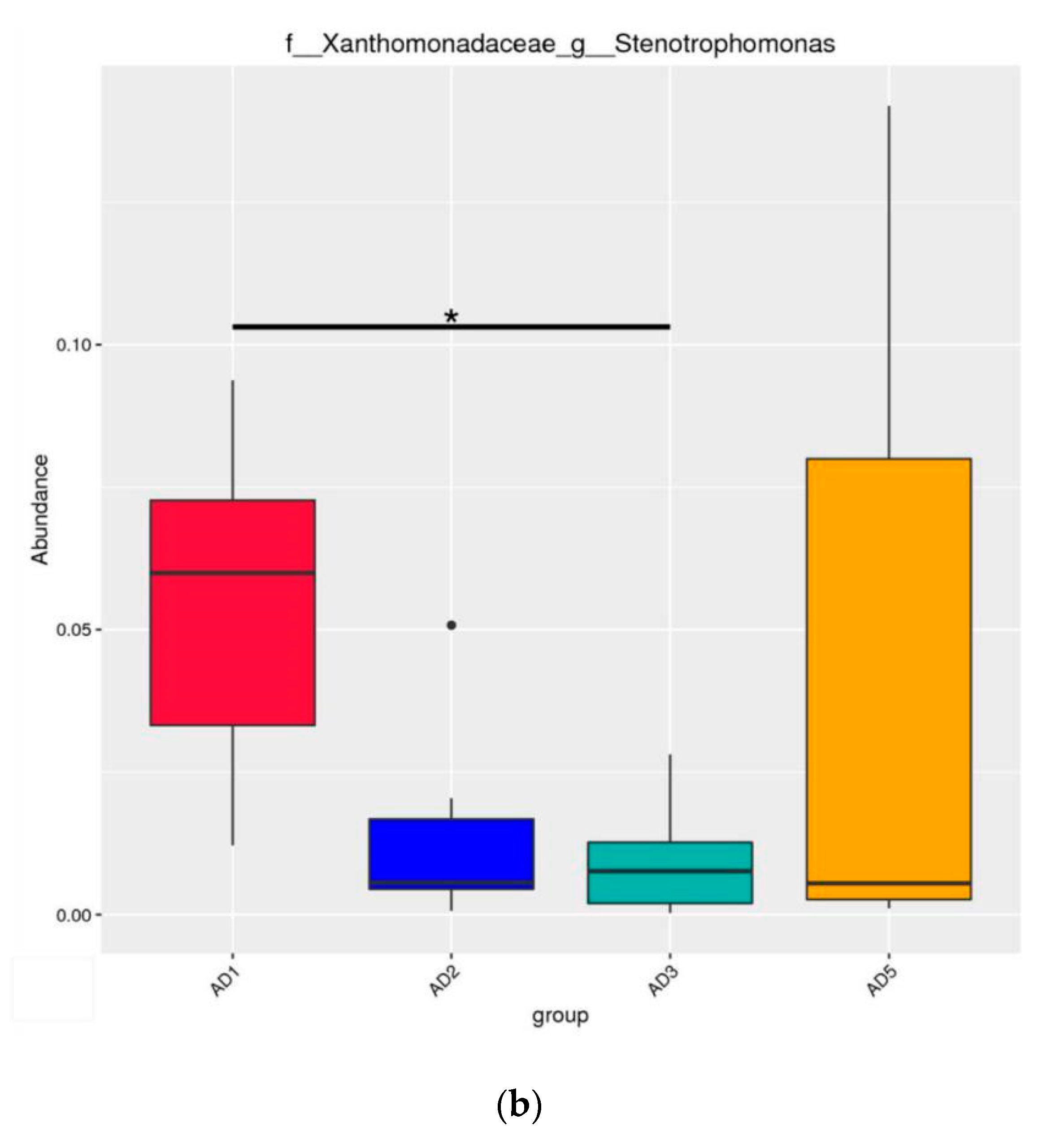
3.8.2. Microbial Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, X.; Hu, Y.; Wang, X.Q.; Ai, Q.H.; He, Z.G.; Feng, F.X.; Lu, X.Y. Effects of practical dietary protein to lipid levels on growth, digestive enzyme activities and body composition of juvenile rice field eel (Monopterus albus). Aquacult. Int. 2014, 22, 749–760. [Google Scholar] [CrossRef]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Boon, N.; Sorgeloos, P.; Verstraete, W.; Bossier, P. Alternatives to antibiotics to control bacterial infections: Luminescent vibriosis in aquaculture as an example. Trends Biotechnol. 2007, 25, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Parisien, A.; Allain, B.; Zhang, J.; Mandeville, R.; Lan, C.Q. Novel alternatives to antibiotics: Bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J. Appl. Microbiol. 2008, 104, 1–13. [Google Scholar] [CrossRef]
- Liu, B.; Ge, X.; Xie, J.; Xu, P.; He, Y.; Cui, Y.; Ming, J.; Zhou, Q.; Pan, L. Effects of anthraquinone extract from Rheum officinale Bail on the physiological responses and HSP70 gene expression of Megalobrama amblycephala under Aeromonas hydrophila infection. Fish Shellfish Immunol. 2012, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ojha, M.L.; Chadha, N.K.; Saini, V.P.; Damroy, S.; Chandraprakash, G.; Sawant, P.B. Effect of ethanolic extract of Mucuna pruriens on growth, metabolism and immunity of Labeo rohita (Hamilton, 1822) fingerlings. Int. J. Fauna Biol. Stud. 2014, 1, 1–9. [Google Scholar]
- Sharma, A.; Deo, A.D.; Riteshkumar, S.T.; Ibemcha Chanu, T.; Das, A. Effect of Withania somnifera (L. Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita (Hamilton) fingerlings. Fish Shellfish Immunol. 2010, 29, 508–512. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, X.W.; Zhu, J.Y.; Liu, L.L.; Liu, Y.C.; Zhu, H. Dietary sanguinarine affected immune response, digestive enzyme activity and intestinal microbiota of Koi carp (Cryprinus carpiod). Aquaculture 2019, 502, 72–79. [Google Scholar] [CrossRef]
- Chang, Z.Q.; Ge, Q.Q.; Sun, M.; Wang, Q. Immune responses by dietary supplement with Astragalus polysaccharides in the Pacific white shrimp, Litopenaeus vannamei. Aquacult. Nutr. 2018, 24, 702–711. [Google Scholar] [CrossRef]
- Zanuzzo, F.S.; Sabioni, R.E.; Montoya, L.N.F.; Favero, G.; Urbinati, E.C. Aloe vera, enhances the innate immune response of pacu (Piaractus mesopotamicus) after transport stress and combined heat killed, Aeromonas hydrophila, infection. Fish Shellfish Immunol. 2017, 65, 198–205. [Google Scholar] [CrossRef]
- Samad, A.P.A.; Santoso, U.; Lee, M.C.; Nan, F.H. Effects of dietary katuk (Sauropus androgynus L. Merr.) on growth, non-specific immune and diseases resistance against Vibrio alginolyticus infection in grouper Epinephelus coioides. Fish Shellfish Immunol. 2014, 36, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, H.; Zhang, Y.; Yang, Y.; Wang, D.; Ma, H. Research progress in pharmacological action of andrographolide. J. Trop. Med. 2019, 19, 518–522. [Google Scholar]
- Rajani, M.; Shrivastava, N.; Ravishankara, M.N. A rapid method for isolation of andrographolide from (Andrographis paniculata) Nees Kalmegh. Pharm. Biol. 2000, 38, 204–209. [Google Scholar] [CrossRef]
- Sheeja, K.; Kuttan, G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by andrographis paniculata extract and andrographolide. Immunopharmacol. Immunotoxicol. 2007, 29, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Peng, Y.; Yan, S.; Li, N.; Chen, Y.; Lan, T. Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation 2018, 41, 1681–1689. [Google Scholar] [CrossRef]
- Gupta, S.; Mishra, K.P.; Singh, S.B.; Ganju, L. Inhibitory effects of andrographolide on activated macrophages and adjuvant-induced arthritis. Inflammopharmacology 2018, 26, 447–456. [Google Scholar] [CrossRef]
- Fu, S.; Sun, C.; Tao, X.; Ren, Y. Anti-inflammatory effects of active constituents extracted from Chinese medicinal herbs against Propionibacterium acnes. Nat. Prod. Res. 2012, 26, 1746–1749. [Google Scholar] [CrossRef]
- Deng, Y.; Bi, R.; Guo, H.; Yang, J.; Du, Y.; Wang, C.; Wei, W. Andrographolide enhances TRAIL-induced apoptosis via p53-mediated death receptors up-regulation and suppression of the NF-кB pathway in bladder cancer cells. Int. J. Biol. Sci. 2019, 15, 688–700. [Google Scholar] [CrossRef]
- Chinnasri, P.; Ramphan, S.; Khongwichit, S.; Smith, D. Activity of andrographolide against dengue virus. Antivir. Res. 2016, 139, 69–78. [Google Scholar]
- Thisoda, P.; Rangkadilok, N.; Pholphana, N.; Worasuttayangkurn, L.; Ruchirawat, S.; Satayavivad, J. Inhibitory effect of Andrographis paniculata extract and its active diterpenoids on platelet aggregation. Eur. J. Pharmacol. 2006, 553, 39–45. [Google Scholar] [CrossRef]
- Chen, C.C.; Chuang, W.T.; Lin, A.H.; Tsai, C.W.; Huang, C.S.; Chen, Y.T.; Chen, H.W.; Lii, C.K. Andrographolide inhibits adipogenesis of 3T3-L1 cells by suppressing C/EBPβ expression and activation. Toxicol. Appl. Pharmacol. 2016, 307, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Basha, K.A.; Raman, R.P.; Prasad, K.P.; Kumar, K.; Nilavan, E.; Kumar, S. Effect of dietary supplemented andrographolide on growth, non-specific immune parameters and resistance against Aeromonas hydrophila in Labeo rohita (Hamilton). Fish Shellfish Immunol. 2013, 35, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture Fisheries and Fisheries Administration. China Fisheries Statistical Yearbook; China Agriculture Press: Beijing, China, 2020; pp. 32–33.
- Ou, T.; Zhu, R.; Chen, Z.; Zhang, Q. Isolation and identification of a lethal rhabdovirus from farmed rice field eels Monopterus albus. Dis. Aquat. Organ. 2013, 106, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, L.; Huan, Z.; Liu, Z.; Hu, Y.; Wang, P.; Xie, J. Effects of fish meal replacement by different proportions of extruded soybean meal on growth performance, body composition, intestinal digestive enzyme activities and serum biochemical indices of rice filed eel (Monopterus albus). Chin. J. Anim. Nutr. 2015, 27, 3567–3576. [Google Scholar]
- Kwmar, H.; Kuwai, T.; Akwa, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef]
- Xavier, R.; Podolsky, D. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018, 361, k2179. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Ma, X.; Liu, Y.; Tang, T.; Hu, Y. Effect of replacing fishmeal with stickwater hydrolysate on the growth, serum biochemical indexes, immune indexes, intestinal histology and microbiota of rice field eel (Monopterus albus). Aquacult. Rep. 2019, 15, 100223. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, L.; Chi, S.; Chu, W.; Liu, Y.; Hu, Y. Sodium butyrate supplementation in high-soybean meal diets for juvenile rice field eel (Monopterus albus): Effects on growth, immune response and intestinal health. Aquaculture 2020, 520, 734952. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Liu, L.; Li, Y.; Huang, X.; Zhong, Y.; Tang, T.; Zhang, J.; Chu, W.; Shen, Y. Effects of high-fat diet on muscle textural properties, antioxidant status and autophagy of Chinese soft-shelled turtle (Pelodiscus sinensis). Aquaculture 2019, 511, 734228. [Google Scholar] [CrossRef]
- Ramos, M.A.; Batista, S.; Pires, M.A.; Silva, A.P.; Pereira, L.F.; Saavedra, M.J.; Ozorio, R.O.A.; Rema, P. Dietary probiotic supplementation improves growth and the intestinal morphology of Nile tilapia. Animal 2017, 11, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of dietary Lactobacillus plantarum in different treatments on growth performance and immune gene expression of white shrimp Litopenaeus vannamei under normal condition and stress of acute low salinity. Fish Shellfish Immunol. 2017, 62, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Peng, M.; Chu, W.; Hu, Y.; Zhong, L. Effects of high-fat diet on growth performance, lipid accumulation and lipid metabolism-related MicroRNA/gene expression in the liver of grass carp (Ctenopharyngodon idella). Comp. Biochem. Phys. B 2019, 234, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ni, J.J.; Li, X.J.; Chen, F.; Wu, H.H.; Xu, M.Y. Community structure and potential nitrogen metabolisms of subtropical aquaculture pond microbiota. Appl. Ecol. Environ. Res. 2018, 16, 7687–7697. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, S.; Xiao, T.Y.; Cao, Y.K.; Xie, M.; Yin, Z.F. Cellular biological and eumelanin-related gene expressional bases of pigment deviation of Leptobotia taeniops. Appl. Ecol. Environ. Res. 2019, 17, 12181–12189. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhong, L.; Chen, T.; Shi, Y.; Hu, Y.; Zeng, J.G.; Liu, H.Y.; Xu, S.D. Dietary sanguinarine supplementation on the growth performance, immunity and intestinal health of grass carp fed cottonseed and rapeseed meal diets. Aquaculture 2020, 528, 735521. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical analysis. Q. Rev. Biol. 1999, 18, 797–799. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Aftabuddin, S.; Siddique, M.A.M.; Romkey, S.S.; Shelton, W.L. Antibacterial function of herbal extracts on growth, survival and immunoprotection in the black tiger shrimp, Penaeus monodon. Fish Shellfish Immunol. 2017, 65, 52–58. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, K.W.; Jung, H.S.; Kim, J. Effects of dietary inclusion of yacon, ginger and blueberry on growth, body composition and challenge test of juvenile rockfish (Sebastes schlegeli) against Edwardsiella tarda. Aquacult. Nutr. 2017, 24, 1048–1055. [Google Scholar] [CrossRef]
- Hassanin, M.E.; Hakim, Y.; Badawi, M.E. Dietary effect of ginger (Zingiber officinale Roscoe) on growth performance, immune response of Nile tilapia (Oreochromis niloticus) and disease resistance against Aeromonas hydrophila. Abbassa Int. J. Aqua. 2014, 7, 35–52. [Google Scholar]
- Ribeiro, L.; Zambonino-Infante, J.; Cahu, C.; Dinis, M. Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 1999, 179, 465–473. [Google Scholar] [CrossRef]
- Khosravi, S.; Rahimnejad, S.; Herault, M.; Fournier, V.; Lee, C.R.; Dio Bui, H.T.; Jeong, J.B.; Lee, K.J. Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol. 2015, 45, 858–868. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Urea, E.; Sanz, A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 1999, 170, 267–283. [Google Scholar] [CrossRef]
- Zeng, C.; Xie, C.X.; Chen, X.M.; Fu, H.; Li, Q.; Lin, C.; Liu, X.X. Periphery olfactory system in rice field eel, Monopterus Albus (pisces: Synbranchidae), a new model in anatomy in vertebrate. Acta Hydrob. Sin. 2012, 36, 220–228. [Google Scholar]
- Ye, L.; Wang, T.; Tang, L.; Liu, W.; Yang, Z.; Zhou, J.; Zheng, Z.; Cai, Z.; Hu, M.; Liu, Z. Poor oral bioavailability of a promising anticancer agent andrographolide is due to extensive metabolism and efflux by P-glycoprotein. J. Pharm. Sci. 2011, 100, 5007–5017. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, 31–38. [Google Scholar] [CrossRef]
- Rudneva, I.I. Blood antioxidant system of Black Sea elas-mobranch and teleost. Comp. Biochem. Physiol. C Pharmacol. Toxicol. 1997, 118, 255–260. [Google Scholar]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish. Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Li, B.; Jiang, T.; Liu, H.; Miao, Z.; Fang, D.; Zheng, L.; Zhao, J. Andrographolide protects chondrocytes from oxidative stress injury by activation of the Keap1-Nrf2-Are signaling pathway. J. Cell Physiol. 2019, 234, 561–571. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2-Keap1 pathway in the European eel Anguilla anguilla: Role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef]
- Jung, K.A.; Kwak, M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 2010, 15, 7266–7291. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Rymuszka, A.; Adaszek, Ł. Pro- and anti-inflammatory cytokine expression in carp blood and head kidney leukocytes exposed to cyanotoxin stress-an in vitro study. Fish Shellfish Immunol. 2012, 33, 382–388. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Targan, S.R. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, J.T.; Fan, Q.X.; Geng, J.G. Andrographolide inhibits NF-κB activation and attenuates neointimal hyperplasia in arterial restenosis. Cell Res. 2007, 17, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T.; Fukata, M.; Arditi, M. TLR signaling in the gut in health and disease. J. Immunol. 2005, 174, 4453–4460. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ji, R.; Cui, K.; Chen, Q.; Chen, Q.; Fang, W.; Mai, K.; Zhang, Y.; Xu, W.; Ai, Q. High percentage of dietary palm oil suppressed growth and antioxidant capacity and induced the inflammation by activation of TLR-NF-κB signaling pathway in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019, 87, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Cario, E.; Gerken, G.; Podolsky, D.K. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Chasiotis, H.; Kolosov, D.; Bui, P.; Kelly, S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: A review. Respir. Physiol. Neurobiol. 2012, 184, 269–281. [Google Scholar] [CrossRef]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar]
- Tarnecki, A.M.; Burgos, F.A.; Ray, C.L.; Arias, C.R. Fish intestinal microbiome: Diversity and symbiosis unraveled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef]
- Yang, G.; Jian, S.Q.; Cao, H.; Wen, C.; Hu, B.; Peng, M.; Peng, L.S.; Yuan, J.G.; Liang, L.F. Changes in microbiota along the intestine of grass carp (Ctenopharyngodon idella): Community, interspecific interactions, and functions. Aquaculture 2019, 498, 151–161. [Google Scholar] [CrossRef]
- Peng, M.; Xue, J.J.; Hu, Y.; Wen, C.G.; Hu, B.Q.; Jian, S.Q.; Liang, L.F.; Yang, G. Disturbance in the homeostasis of intestinal microbiota by a high-fat diet in the rice field eel (Monopterus albus). Aquaculture 2019, 502, 347–355. [Google Scholar] [CrossRef]
- Yang, X.; Xu, M.; Huang, G.; Zhang, C.; Pang, Y.; Cheng, Y. Effect of dietary L-tryptophan on the survival, immune response and gut microbiota of the Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2018, 84, 1007–1017. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Wladis, E.J. Dacryocystitis secondary to Stenotrophomonas maltophilia infection. Ophthal. Plast. Reconstr. Surg. 2011, 27, e116. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.; Knudsen, C.; Beaumont, M.; Rodriguez, J.; Neyrinck, A.; Bindels, L. Contribution of the gut microbiota to the regulation of host metabolism and energy balance: A focus on the gut–liver axis. Proc. Nutr. Soc. 2019, 78, 319–328. [Google Scholar] [CrossRef]
- Singha, P.K.; Roy, S.; Dey, S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 2003, 74, 692–694. [Google Scholar] [CrossRef]


| Ingredients | g/kg | Proximate Composition | g/kg |
|---|---|---|---|
| Fish meal | 360.0 | Crude protein 4 | 431.6 |
| Soybean meal | 200.0 | Crude lipid 4 | 51.6 |
| Corn gluten meal | 80.0 | Ash 4 | 111.4 |
| Soy protein concentrate | 50.0 | ||
| Beer yeast | 50.0 | ||
| Fish oil | 20.0 | ||
| Choline chloride | 5.0 | ||
| Premix 1 | 10.0 | ||
| Monocalcium phosphate | 15.0 | ||
| α-starch | 209.6 | ||
| Antioxidants 2 | 0.1 | ||
| Mold inhibitor 3 | 0.3 |
| Gene | Forward Sequences (5′–3′) | Reverse Sequences (5′–3′) | Accession No. 1 | PCR Efficiency (%) | Product Length |
|---|---|---|---|---|---|
| Occludin | TGTCGGGGAGTGGGTAAA | TCCAGGCAAATAAAGAGGCT | XM_020599328.1 | 97 | 130 |
| ZO-1 | GGCATCATCCCCAACAAA | GCGAAGACCACGGAACCT | XM_020621576.1 | 96 | 111 |
| ZO-2 | AGCCGAGGTCGCACTTTA | GCTTTGCTTCTGTGGTTGAT | XM_020615114.1 | 98 | 246 |
| Claudin-12 | TCACCTTCAATCGCAACG | ATGTCTGGCTCAGGCTTATCT | XM_020607277.1 | 99 | 250 |
| Claudin-15 | CTCGCTGCTTGCTTTGACT | TTGAAGGCGTACCAGGACA | XM_020611334.1 | 96 | 225 |
| IL-10 | TTTGCCTGCCAAGTTATGAG | CATTTGGTGACATCGCTCTT | XM_020593225.1 | 100 | 158 |
| IL-1β | GAGATGTGGAGCCCAAACTT | CTGCCTCTGACCTTCTGGACTT | KM113037.1 | 97 | 127 |
| IL-12β | CAAGTCAGTTGCCAAAATCC | CCAAGCAGCTCAGGGTCT | XM_020594580.1 | 99 | 103 |
| TGF-β1 | AACCCACTACCTCACTACCCG | GCCGAAGTTGGAAACCCT | XM_020605575.1 | 96 | 128 |
| TGF-β2 | ATTACGCCAAGGAGGTGC | GGGTTTTGAAGACGGAAGAT | XM_020622328.1 | 98 | 178 |
| TGF-β3 | AGTTTGTCGCTATCCACTTGC | GATGAGTTCCTTGGTGCTGTTA | XM_020590885.1 | 95 | 180 |
| TLR-3 | TATTTAGAGCCATACAGGG | CACAATCAAGAACGCACA | XM_020614353.1 | 100 | 244 |
| TLR-7 | ATCCTCACGACTTCCCTC | TTTCTTTCATCACCCACT | XM_020596482.1 | 97 | 205 |
| TLR-8 | AAGTGAAGCAGGATGAAG | AAGTCCCAGATTGAGTGA | XM_020596483.1 | 96 | 139 |
| Nrf2 | CTTCAGACAGCGGTGACAGG | GCCTCATTCAGTTGGTGCTT | XM_020596409.1 | 96 | 260 |
| Keap1 | AGCCTGGGTGCGATACGA | CAAGAAATGACTTTGGTGGG | XM_020597068.1 | 98 | 198 |
| SOD1 | AGCTGGCTAAGTTCTCATTCAC | GCAGTAACATTGCCCAAGTCT | XM_020598413.1 | 99 | 227 |
| CAT | GTCCAAGTCTAAGGCATCTCC | CTCCTCTTCGTTCAGCACC | XM_020624985.1 | 96 | 106 |
| GPx1 | GTTCACCGCCAAACTCTT | TTCCCATTCACATCTACCTT | XM_020607739.1 | 98 | 303 |
| GPx4 | ATTTATGACTTCTCAGCGACAG | CCTTCAGCCACTTCCACA | XM_020612291.1 | 100 | 325 |
| GSTK | TTGATGTTCCCCTGCGTTAT | CACCTGCTCTACCTGCTTGTC | XM_020610780.1 | 97 | 131 |
| GSTO | GGGAGAAATAAAGGTGAGGATG | CAGATGAGTTGACAAGGCAGTT | XM_020600427.1 | 99 | 199 |
| RPL-17 | GTTGTAGCGACGGAAAGGGAC | GACTAAATCATGCAAGTCGAGGG | XM_020587712.1 | 98 | 160 |
| Index | AD1 | AD2 | AD3 | AD5 |
|---|---|---|---|---|
| Observed species | 453.33 ± 58.21 b | 276.67 ± 35.79 a | 195.5 ± 44.83 a | 279.83 ± 59.10 a |
| Shannon | 4.45 ± 0.37 b | 3.02 ± 0.54 a | 2.83 ± 0.39 a | 3.46 ± 0.49 ab |
| Chao1 | 478.56 ± 64.49 b | 308.9 ± 30.79 a | 218.57 ± 48.57 a | 296.75 ± 58.01 a |
| ACE | 478.4 ± 66.62 b | 318.77 ± 29.11 a | 228.69 ± 46.86 a | 302.55 ± 57.53 a |
| PD whole tree | 55.86 ± 6.65 b | 38.71 ± 4.34 a | 28.13 ± 4.74 a | 37.39 ± 6.08 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zhong, L.; Liu, Y.; Zhang, J.; Lv, Z.; Li, Y.; Hu, Y. Effects of Dietary Andrographolide Levels on Growth Performance, Antioxidant Capacity, Intestinal Immune Function and Microbioma of Rice Field Eel (Monopterus Albus). Animals 2020, 10, 1744. https://doi.org/10.3390/ani10101744
Shi Y, Zhong L, Liu Y, Zhang J, Lv Z, Li Y, Hu Y. Effects of Dietary Andrographolide Levels on Growth Performance, Antioxidant Capacity, Intestinal Immune Function and Microbioma of Rice Field Eel (Monopterus Albus). Animals. 2020; 10(10):1744. https://doi.org/10.3390/ani10101744
Chicago/Turabian StyleShi, Yong, Lei Zhong, Yanli Liu, Junzhi Zhang, Zhao Lv, Yao Li, and Yi Hu. 2020. "Effects of Dietary Andrographolide Levels on Growth Performance, Antioxidant Capacity, Intestinal Immune Function and Microbioma of Rice Field Eel (Monopterus Albus)" Animals 10, no. 10: 1744. https://doi.org/10.3390/ani10101744
APA StyleShi, Y., Zhong, L., Liu, Y., Zhang, J., Lv, Z., Li, Y., & Hu, Y. (2020). Effects of Dietary Andrographolide Levels on Growth Performance, Antioxidant Capacity, Intestinal Immune Function and Microbioma of Rice Field Eel (Monopterus Albus). Animals, 10(10), 1744. https://doi.org/10.3390/ani10101744





