Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement and Composition of MLPs
2.2. Experimental Design and Ethical Statement
2.3. Feeding Management and Sampling
2.4. Immune Parameters Analysis
2.4.1. Determination of Serum Levels of Cytokines and Immunoglobulins
2.4.2. Peripheral Blood Lymphocyte Proliferation and Nitric Oxide Production
2.4.3. The mRNA Abundances of IGF1 in Liver and Longissimus dorsi
2.5. Data Analysis
3. Results
3.1. Effects of MLPs on the Growth Performance and Immune Organ Weights of the Early Weaning Piglets
3.2. Effects of MLPs on the Serum Immunoglobulins and Cytokines of Weanling Pigs
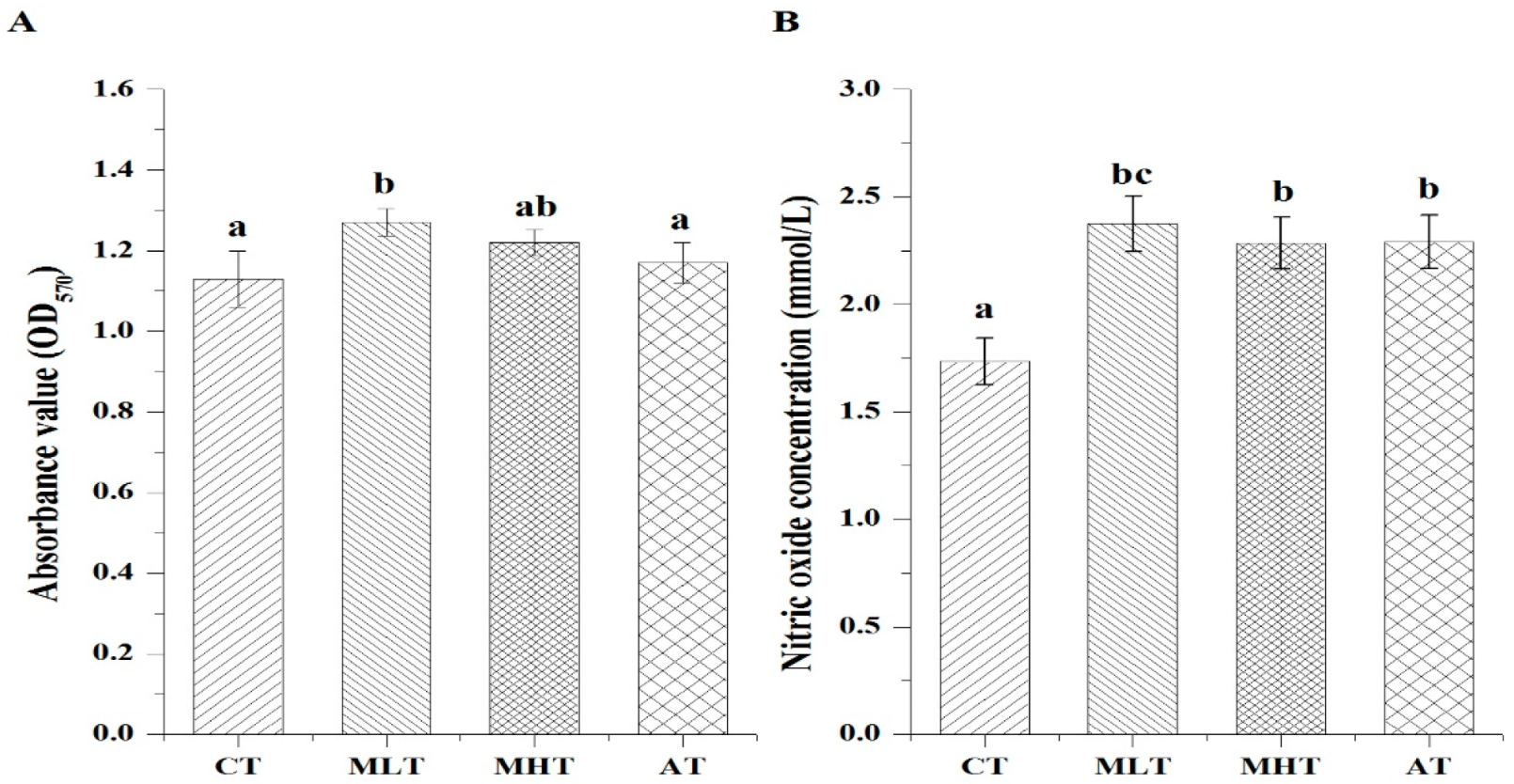
3.3. Effects of MLPs on the Lymphocyte Proliferation and NO Production of Weanling Pigs
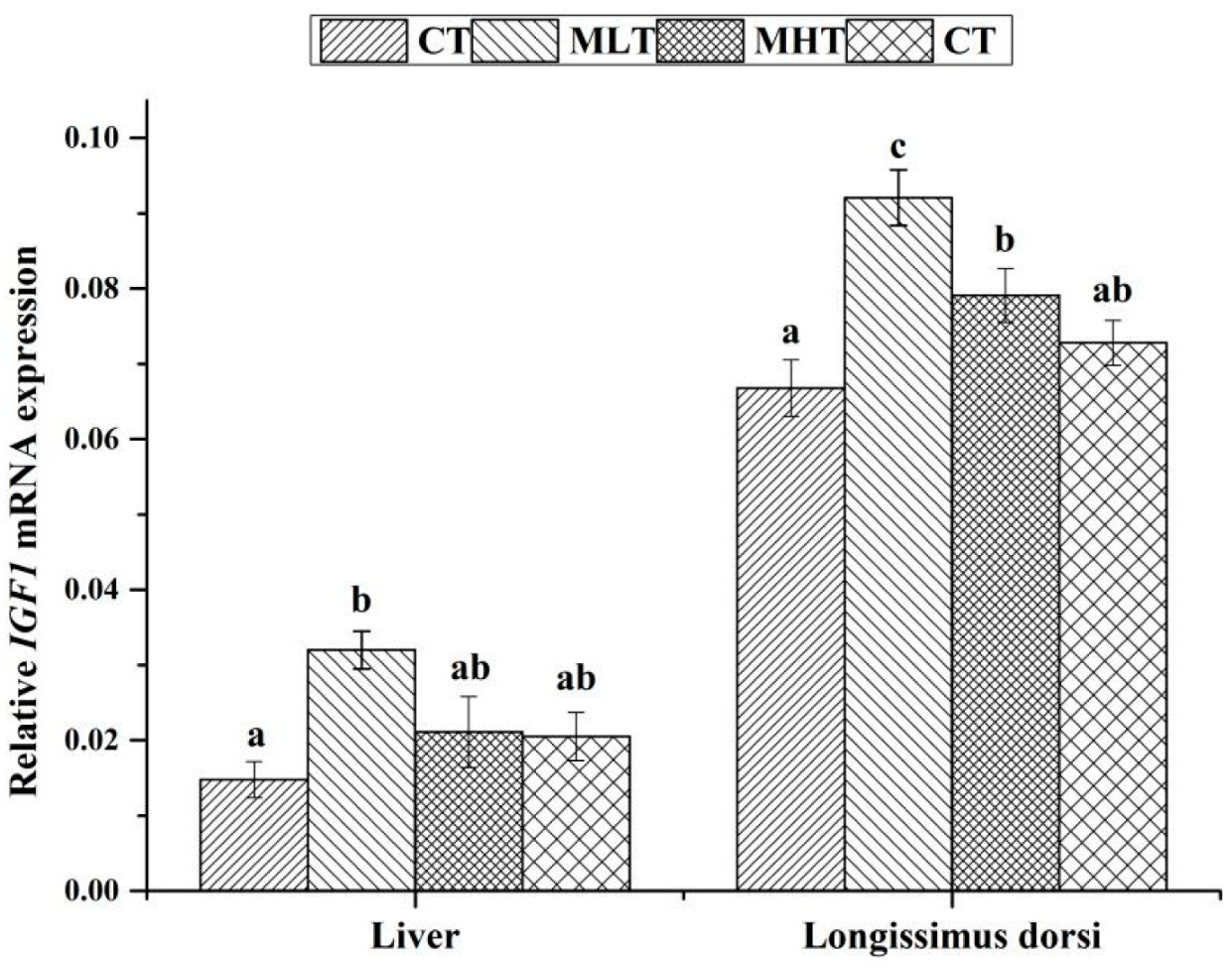
3.4. Effects of MLPs on the Expression Levels of IGF1 mRNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schmitt, O.; O’Driscoll, K.; Boyle, L.A.; Baxter, E.M. Artificial rearing affects piglets pre-weaning behaviour, welfare and growth performance. Appl. Anim. Behav. Sci. 2019, 210, 16–25. [Google Scholar] [CrossRef]
- Duan, Y.H.; Li, F.N.; Tan, B.; Lin, B.B.; Kong, X.F.; Li, Y.H.; Yin, Y.L. Myokine interleukin-15 expression profile is different in suckling and weaning piglets. Anim. Nutr. 2015, 1, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, L.B.; Nie, Y.F.; Cen, J.W.; Zheng, W.Y.; Wang, X.K.; Xie, C.L.; Zhen, Z.L.; Wang, Z.C.; Yang, T.; et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe 2018, 24, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Zhang, C.Y.; Wang, L.X.; Shang, Q.H.; Zhang, G.G.; Yang, W.R. Effects of dietary supplementation of Enterococcus faecium on growth performance, intestinal morphology, and selected microbial populations of piglets. Livest. Sci. 2018, 210, 111–117. [Google Scholar] [CrossRef]
- Li, J.L.; Shi, B.K.; Yan, S.M.; Jin, L.; Guo, Y.W.; Xu, Y.Q.; Li, T.Y.; Guo, X.Y. Effects of dietary supplementation of chitosan on humoral and cellular immune function in weaned piglets. Anim. Feed Sci. Technol. 2013, 186, 204–208. [Google Scholar] [CrossRef]
- Xi, Q.Y.; Jiang, Y.; Zhao, S.; Zeng, B.; Wang, F.; Wang, L.N.; Jiang, Q.Y.; Zhang, Y.L. Effect of ginseng polysaccharides on the immunity and growth of piglets by dietary supplementation during late pregnancy and lactating sows. Anim. Sci. J. 2017, 88, 863–872. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gan, L.P.; Du, M.Y.; Shang, Q.H.; Xie, Y.H.; Zhang, G.G. Effects of dietary supplementation of alfalfa polysaccharides on growth performance, small intestinal enzyme activities, morphology, and large intestinal selected microbiota of piglets. Livest. Sci. 2019, 223, 47–52. [Google Scholar] [CrossRef]
- Li, L.L.; Yin, F.G.; Zhang, B.; Peng, H.Z.; Li, F.N.; Zhu, F.N.; Zhu, N.S.; Hou, D.X.; Yin, Y.L.; Luo, J.J.; et al. Dietary supplementation with Atractylodes Macrophala Koidz polysaccharides ameliorate metabolic status and improve immune function in early-weaned pigs. Livest. Sci. 2011, 142, 33–41. [Google Scholar] [CrossRef]
- Li, X.L.; He, L.P.; Yang, Y.; Liu, F.J.; Cao, Y.; Zuo, J.J. Effects of extracellular polysaccharides of Ganoderma lucidum supplementation on the growth performance, blood profile, and meat quality in finisher pigs. Livest. Sci. 2015, 178, 187–194. [Google Scholar] [CrossRef]
- Qiao, J.; Li, H.H.; Zheng, C.J.; Feng, Z.Y.; Wang, W. Dietary supplementation with Aloe vera polysaccharide enhances the growth performance and immune function of weaned piglets. J. Anim. Feed Sci. 2013, 22, 329–334. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Jin, J.; Shi, L.G. Antioxidant activity of the derivatives of polysaccharide extracted from a Chinese medical herb. Food Sci. Technol. Res. 2008, 14, 160–168. [Google Scholar] [CrossRef]
- Hou, R.H.; Liao, S.T.; Liu, F.; Zou, Y.X.; Deng, Y.Y. Immunomodulatory effect of polysaccharides from mulberry leaves (PML) in mice. Food Sci. 2011, 32, 280–283. (In Chinese) [Google Scholar]
- Zhang, Z.F.; Shi, L.G. Anti-inflammatory and antinociceptive properties of cis-mulberroside A from Ramuls mori. Fitoterapia 2010, 81, 214–218. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Swine; National Academy of Sciences: Washington, DC, USA, 1998. [Google Scholar]
- Yin, Y.L.; Tang, Z.R.; Sun, Z.H.; Liu, Z.Q.; Li, T.J.; Huang, R.L.; Ruan, Z.; Deng, Z.Y.; Gao, B.; Chen, L.X.; et al. Effect of galacto-mannan-oligosaccharides or chitosan supplementation on cytoimmunity and humoral immunity response in early-weaned piglets. Asian-Australas. J. Anim. Sci. 2008, 21, 723–731. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Guo, Y.W.; Shi, B.L.; Yan, S.M.; Guo, X.Y. Dietary arginine supplementation enhances the growth performance and immune status of broiler chickens. Livest. Sci. 2018, 209, 8–13. [Google Scholar] [CrossRef]
- Kloubert, V.; Blaabjerg, K.; Dalgaard, T.S.; Poulsen, H.D.; Rink, L.; Wessels, I. Influence of zinc supplementation on immune parameters in weaned pigs. J. Trace Elem. Med. Biol. 2018, 49, 231–240. [Google Scholar] [CrossRef]
- Wang, J.P.; Jung, J.H.; Kim, I.H. Effects of dietary supplementation with delta-aminolevulinic acid on growth performance, hematological status, and immune responses of weanling pigs. Livest. Sci. 2011, 140, 131–135. [Google Scholar] [CrossRef]
- Küskü-Kiraz, Z.; Genc, S.; Bekpınar, S.; Ünlücerci, Y.; Çevik, A.; Olgaç, V.; Gürdöl, F.; Uysal, M. Effects of betaine supplementation on nitric oxide metabolism, atherosclerotic parameters, and fatty liver in guinea pigs fed a high cholesterol plus methionine diet. Nutrition 2018, 45, 41–48. [Google Scholar] [CrossRef]
- Zhao, X.J.; Li, L.; Luo, Q.L.; Ye, M.Q.; Luo, G.Q.; Kuang, Z.S. Effects of mulberry (Morus alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livest. Sci. 2015, 177, 88–94. [Google Scholar] [CrossRef]
- Marin, D.E.; Pistol, G.C.; Neagoe, I.V.; Calin, L.; Taranu, I. Effects of zearalenone on oxidative stress and inflammation in weanling piglets. Food Chem. Toxicol. 2013, 58, 408–415. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Wei, X.H.; Zi, Z.H.; Zou, B.J.; Xiaa, S.S.; Lu, N.S.; Lei, H.L.; Lu, Y.; Parvizi, N.; Xia, D. Potassium diformate influences gene expression of GH/IGF-I axis and glucose homeostasis in weaning piglets. Livest. Sci. 2015, 172, 85–90. [Google Scholar] [CrossRef]
- Lei, X.J.; Park, J.W.; Baek, D.H.; Kim, J.K.; Kim, I.H. Feeding the blend of organic acids and medium chain fatty acids reduces the diarrhea in piglets orally challenged with enterotoxigenic Escherichia coli K88. Anim. Feed Sci. Technol. 2017, 224, 46–51. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, X.; Peng, H.Z.; Fan, M.Z.; Hou, Z.P.; Kong, X.F.; Yin, Y.L.; Zhang, B.; Li, T.J.; Hou, Y.Q.; et al. The effect of dietary addition of a polysaccharide from Atractylodes Macrophala Koidz on growth performance, immunoglobulin concentration and IL-1β expression in weaned piglets. J. Agric. Sci. 2009, 147, 625–631. [Google Scholar] [CrossRef]
- Li, X.B. Effect of mulberry leaves polysaccharide on immune function in mice with long-term heavy training. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 269–272. (In Chinese) [Google Scholar]
- Wu, Y.; Pan, L.; Shang, Q.H.; Ma, X.K.; Long, S.F.; Xu, Y.T.; Piao, X.S. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed Sci. Technol. 2017, 230, 126–135. [Google Scholar] [CrossRef]
- Huntley, N.F.; Nyachoti, C.M.; Patience, J.F. Lipopolysaccharide immune stimulation but not β-mannanase supplementation affects maintenance energy requirements in young weaned pigs. J. Anim. Sci. Biotechnol. 2018, 9, 47. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Poutahidis, T.; Tallarico, N.; Hardas, A.; Teliousis, K.; Arsenos, G.; Fortomaris, P.D. Dietary supplementation of encapsulated organic acids enhances performance and modulates immune regulation and morphology of jejunal mucosa in piglets. Res. Veter. Sci. 2017, 115, 174–182. [Google Scholar] [CrossRef]
- Kuang, Y.; Wang, Y.; Zhang, Y.; Song, Y.; Zhang, X.; Lin, Y.; Che, L.; Xu, S.; Wu, D.; Xue, B.; et al. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Anim. Feed Sci. Technol. 2015, 208, 145–157. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Park, J.W.; Kim, I.H. Effect of replacement of de-hulled barley with water-soaked barley in corn–soybean meal-based diet on growth performance, blood characteristics, and meat quality in finishing pigs. J. Appl. Anim. Res. 2017, 45, 164–169. [Google Scholar] [CrossRef]
- Liu, D.; Sun, Q.W.; Xu, J.; Li, N.; Lin, J.N.; Chen, S.; Li, F. Purification, characterization, and bioactivities of a polysaccharide from mycelial fermentation of Bjerkandera fumosa. Carbohydr. Polym. 2017, 167, 115–122. [Google Scholar] [CrossRef]
- Badovinac, V.; Trajkovic, V.; Mostarica-Stojkovic, M. Nitric oxide promotes growth and major histocompatibility complex-unrestricted cytotoxicity of interleukin-2-activated rat lymphocytes. Scand. J. Immunol. 2000, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała, A.M.; Pareek, C.S.; Urbański, P.; Goluch, D.; Kamyczek, M.; Różycki, M.; Smoczynski, R.; Horbańczuk, J.O.; Kuryłet, J. Study of the differential transcription in liver of growth hormone receptor (GHR), insulin-like growth factors (IGF1, IGF2) and insulin-like growth factor receptor (IGF1R) genes at different postnatal developmental ages in pig breeds. Mol. Biol. Rep. 2011, 39, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W. The endocrine regulation of chicken growth. Asian-Australas. J. Anim. Sci. 2010, 23, 1668–1676. [Google Scholar] [CrossRef]
- Katsumata, M.; Kawakami, S.; Kaji, Y.; Takada, R.; Dauncey, M.J. Differential regulation of porcine hepatic IGF-I mRNA expression and plasma IGF-I concentration by a low lysine diet. J. Nutr. 2002, 132, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Lo, L.L.; Chung, M.T.; Li, H.L.; Tu, C.F.; Tsou, H.L.; Huang, M.C.; Yu, Y.C.; Huang, T.H.; Lin, E.C. Effects of single-nucleotide polymorphisms in insulin-like growth factor-1 and insulin-like growth factor-2 genes on growth performance of centrally tested Duroc boars using segregated early weaning entrance. Livest. Sci. 2012, 144, 290–293. [Google Scholar] [CrossRef]
| Ingredients | Content | Nutrition Composition | Content |
|---|---|---|---|
| Maize [%] | 60.50 | Metabolizable energy [MJ/kg] | 14.20 |
| Fish Meal [%] | 3.50 | Crude protein [%] | 18.75 |
| Soybean Meal [%] | 26.00 | Total Ca [%] | 0.91 |
| Soybean Oil [%] | 2.00 | Total P [%] | 0.61 |
| Whey Powder [%] | 5.00 | Digestible Lys [%] | 1.21 |
| Calcium Carbonate | 0.55 | ||
| Di-Calcium Phosphate [%] | 1.00 | ||
| L-Lys·HCl, 78% [%] | 0.15 | ||
| Salt [%] | 0.30 | ||
| Mineral Premix [%] | 1.00 |
| Parameters | Treatment | |||
|---|---|---|---|---|
| CT | MLT | MHT | AT | |
| Growth Performance | ||||
| Initial Body Weight, g | 9.45 ± 0.15 | 8.92 ± 0.26 | 9.26 ± 0.20 | 8.98 ± 0.16 |
| Final Body Weight, g | 17.8 ± 0.26 | 17.62 ± 0.35 | 17.77 ± 0.23 | 17.22 ± 0.14 |
| ADG, g | 441.0 ± 5.67 a | 462.81 ± 5.40 b | 447.8 ± 1.89 ab | 433.68 ± 1.90 a |
| ADFI, g | 737.59 ± 7.65 | 729.4 ± 10.54 | 736.47 ± 8.96 | 712.94 ± 2.70 |
| F/G, g/g | 1.670 ± 0.004 b | 1.576 ± 0.012 a | 1.640 ± 0.011 ab | 1.640 ± 0.012 ab |
| Immune organ Indexes | ||||
| Thymus Index | 2.59 ± 0.15 a | 2.94 ± 0.04 ab | 3.15 ± 0.15 b | 2.61 ± 0.08 a |
| Spleen Index | 1.69 ± 0.04 a | 1.90 ± 0.07 b | 1.90 ± 0.06 b | 1.87 ± 0.09 b |
| Parameters | Treatment | |||
|---|---|---|---|---|
| CT | MLT | MHT | AT | |
| IgM [mg/mL] | 0.64 ± 00.03 ab | 0.68 ± 00.06 ab | 0.55 ± 00.01 a | 0.66 ± 00.07 ab |
| IgG [mg/mL] | 3.97 ± 00.06 a | 4.98 ± 00.27 c | 3.70 ± 00.20 ab | 4.05 ± 00.22 abc |
| IL-1β [pg/mL] | 156.36 ± 7.47 a | 210.39 ± 07.76 b | 164.84 ± 00.67 a | 161.59 ± 3.36 a |
| IL-2 [pg/mL] | 352.51 ± 4.52 a | 450.41 ± 09.16 c | 415.39 ± 08.62 bc | 361.13 ± 5.98 a |
| IL-6 [pg/mL] | 53.80 ± 1.76 a | 62.02 ± 04.19 ab | 70.68 ± 01.56 bc | 57.05 ± 01.48 a |
| IL-8 [pg/mL] | 222.18 ± 9.46 a | 271.07 ± 10.15 b | 231.14 ± 11.22 a | 233.61 ± 13.60 a |
| IFN-γ [pg/mL] | 44.87 ± 0.94 a | 50.42 ± 01.98 bc | 52.73 ± 01.87 c | 49.71 ± 01.13 bc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Yang, R.; Bi, Y.; Bilal, M.; Kuang, Z.; Iqbal, H.M.N.; Luo, Q. Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs. Animals 2020, 10, 35. https://doi.org/10.3390/ani10010035
Zhao X, Yang R, Bi Y, Bilal M, Kuang Z, Iqbal HMN, Luo Q. Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs. Animals. 2020; 10(1):35. https://doi.org/10.3390/ani10010035
Chicago/Turabian StyleZhao, Xiangjie, Rongling Yang, Yanhong Bi, Muhammad Bilal, Zheshi Kuang, Hafiz M. N. Iqbal, and Qiulan Luo. 2020. "Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs" Animals 10, no. 1: 35. https://doi.org/10.3390/ani10010035
APA StyleZhao, X., Yang, R., Bi, Y., Bilal, M., Kuang, Z., Iqbal, H. M. N., & Luo, Q. (2020). Effects of Dietary Supplementation with Mulberry (Morus alba L.) Leaf Polysaccharides on Immune Parameters of Weanling Pigs. Animals, 10(1), 35. https://doi.org/10.3390/ani10010035






