Uphill Shifts of Fungal Fruiting Due to Climate Change at the Polar Urals
Abstract
:1. Introduction
2. Material and Methods
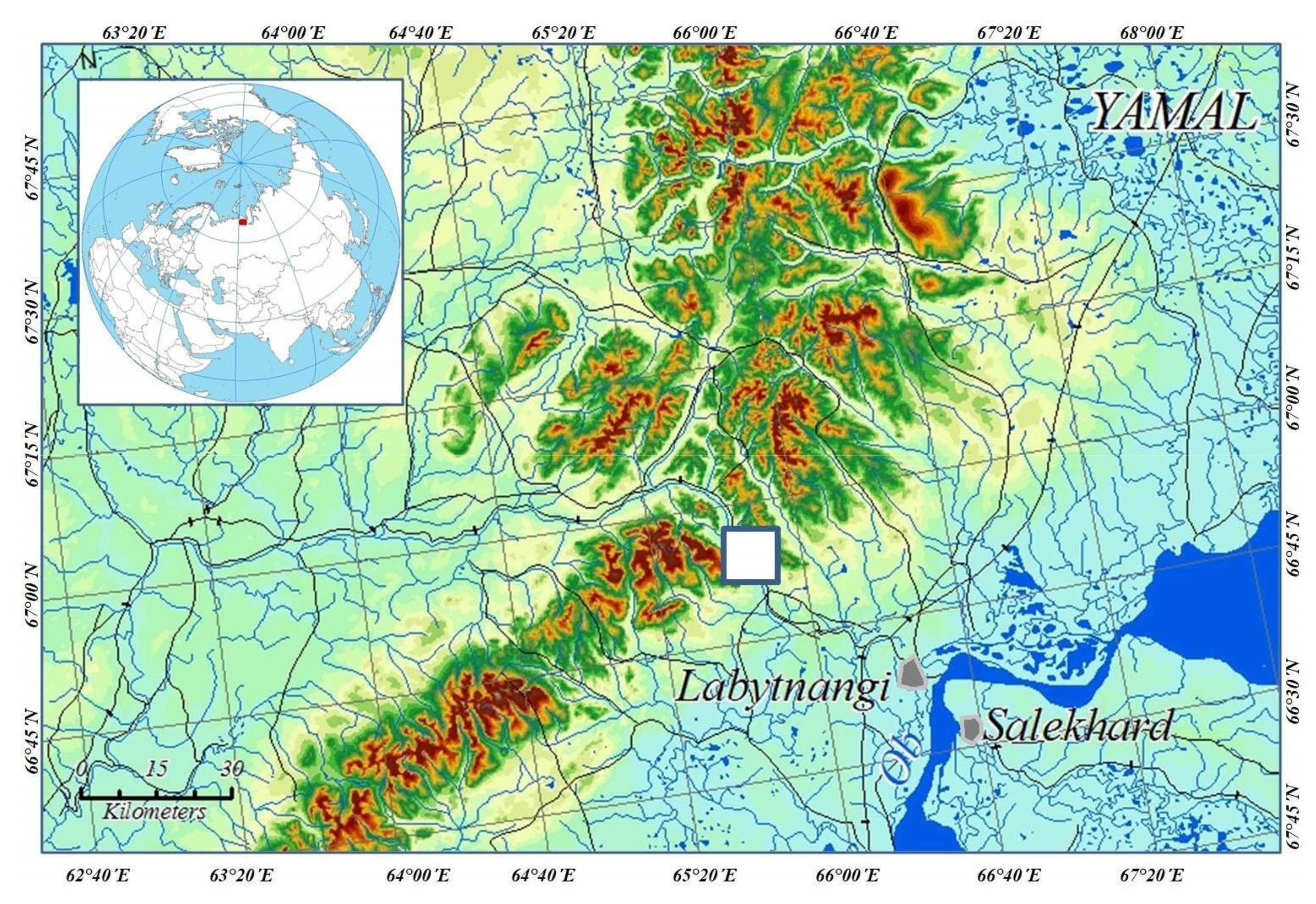
2.1. Area of Investigation
2.2. Fungi Sampling
2.3. Statistical Analysis
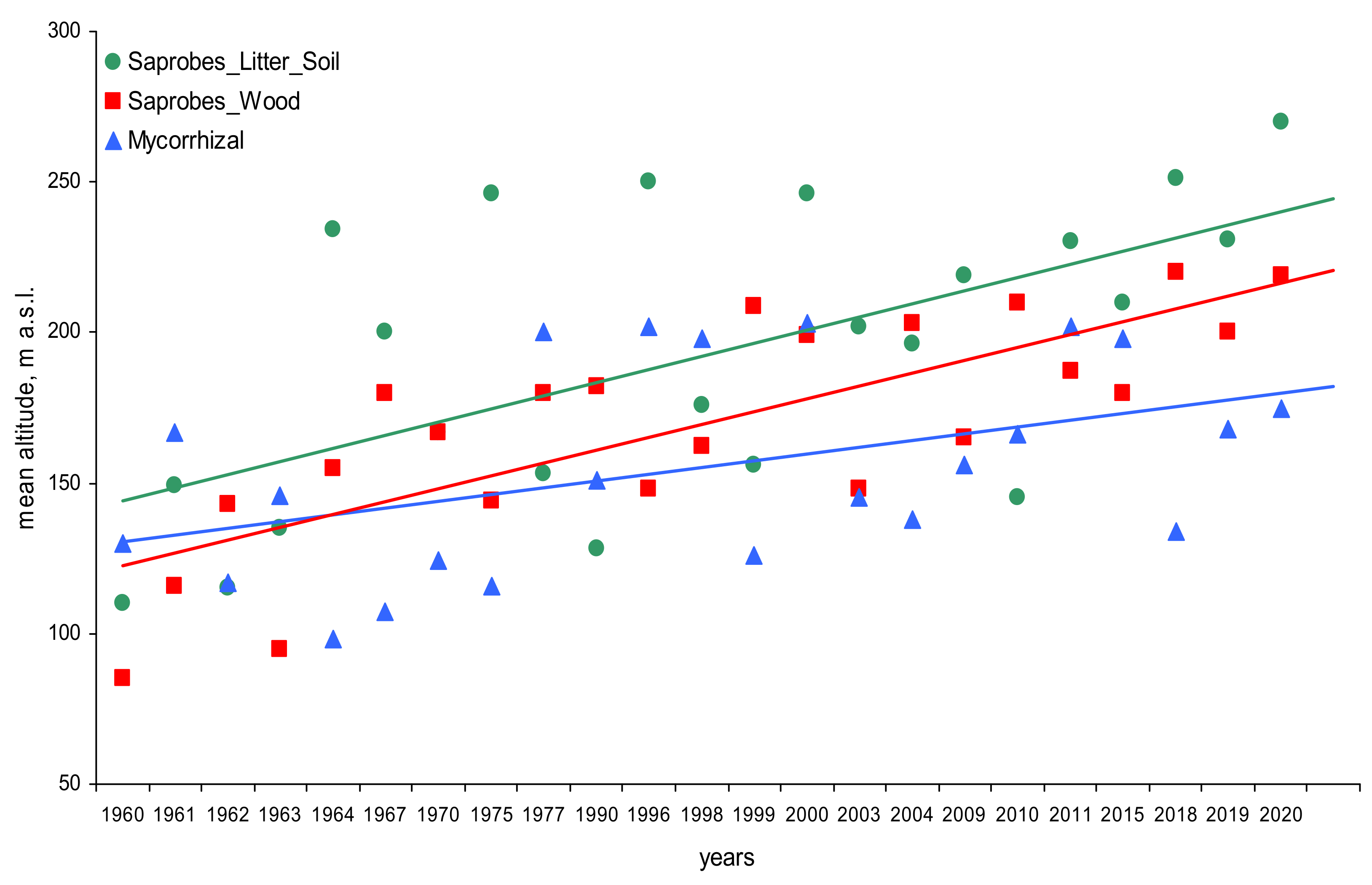
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Working Group, II. Climate Change 2014: Impacts, Adaptation, and Vulnerability. 2014. Available online: https://www.ipcc.ch/working-group/wg2/ (accessed on 20 August 2019).
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.-J.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Forbes, B.C.; Fauria, M.M.; Zetterberg, P. Russian Arctic warming and “greening” are closely tracked by tundra shrub willows. Glob. Chang. Biol. 2010, 16, 1542–1554. [Google Scholar] [CrossRef]
- Kauserud, H.; Stige, L.C.; Vik, J.O.; Økland, R.H.; Høiland, K.; Stenseth, N.C. Mushroom fruiting and climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 3811–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez, J.; Kauserud, H.; Andrew, C.; Heegaard, E.; Krisai-Greilhuber, I.; Senn-Irlet., B.; Høiland, K.; Egli, S.; Büntgen, U. Altitudinal upwards shifts in fungal fruiting in the Alps. Proc. R. Soc. B 2020, 287, 20192348. [Google Scholar] [CrossRef] [Green Version]
- Susan, G.; Woodward, S.; Taylor, A.F.S. Strong altitudinal partitioning in the distributions of ectomycorrhizal fungi along a short (300 m) elevation gradient. New Phytol. 2015, 206, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Kauserud, H.; Heegaard, E.; Buntgen, U.; Halvorsen, R.; Egli, S.; Senn-Irlet, B.; Krisai-Greilhuber, I.; Dämon, J.; Sparks, T.; Norden, J.; et al. Warming-induced shift in European mushroom fruiting phenology. Proc. Natl. Acad. Sci. USA 2012, 109, 14488–14493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahram, M.; Polme, S.; Koljalg, U.; Zarre, S.; Tedersoo, L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol. 2012, 193, 465–473. [Google Scholar] [CrossRef]
- Geml, J.; Pastor, N.; Fernandez, L.; Pacheco, S.; Semenova, T.A.; Becerra, A.G.; Wicaksono, C.Y.; Nouhra, E.R. Large-scale fungal diversity assessment in the Andean Yungas forests reveals strong community turnover among forest types along an altitudinal gradient. Mol. Ecol. 2014, 23, 2452–2472. [Google Scholar] [CrossRef] [PubMed]
- Kazantseva, L.K. Mycoflora of the eastern slope of Polar Ural. Zap. Sverdl. Dep. Bot. Soc. UrFAN 1966, 4, 162–166. (In Russian) [Google Scholar]
- Stepanova, N.T.; Sirko, A.V. To flora of Agaricoid and Gasteroid fungi of the Urals. Mycol. Stud. Ural. 1977, 1, 51–106. (In Russian) [Google Scholar]
- Karatygin, I.V.; Nezdoyminogo, E.L.; Novozhilov, Y.K.; Zhurbenko, M.P. Fungi of Russian Arctic; SPBKPA Pub.: Saint Petersburg, Russia, 1999. (In Russian) [Google Scholar]
- Nezdojminogo, E.L. Basidial macrofungi in mountain tundra of Polar Urals. Mykol. Phytopatologia 2001, 35, 26–29. (In Russian) [Google Scholar]
- Kotiranta, H.; Penzina, T. Notes on the North Urals aphyllophorales. In Arctic and Alpine Mycology; Yekaterinburg Pub.: Yekaterinburg, Russia, 1998; Volume 5, pp. 67–81. [Google Scholar]
- Shiryaev, A.G.; Moiseev, P.A.; Peintner, U.; Devi, N.M.; Kukarskih, V.V.; Elsakov, V.V. Arctic greening caused by warming contributes to compositional changes of mycobiota at the Polar Urals. Forests 2019, 10, 1112. [Google Scholar] [CrossRef] [Green Version]
- Shiryaev, A.G.; Peintner, U.; Elsakov, V.V.; Sokovnina, S.Y.; Kosolapov, D.A.; Shiryaeva, O.S.; Devi, N.M.; Grigoriev, A.A. Relationship between species richness, biomass and structure of vegetation and mycobiota along an altitudinal transect in the Polar Urals. J. Fungi 2020, 6, 353. [Google Scholar] [CrossRef] [PubMed]
- Shiyatov, S.G. Dynamics of Woody and Shrubby Vegetation in the Mountains of the Polar Urals under the Influence of Modern Climate Changes; Yekaterinburg Pub.: Yekaterinburg, Russia, 2009. [Google Scholar]
- Byalt, V.V.; Egorov, A.A.; Pismarkina, E.V.; Galanina, O.V. Addition to the flora of Northern Asia: Alien vascular plant records in the Yamal-Nenets Autonomous district (Russia). Check-List 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Degteva, S.V. (Ed.) Red Data Book of Komi Republic, 3rd ed.; Ministry of Natural Resources and Environment: Syktyvkar, Russia, 2019. (In Russian)
- Dyachenko, A.P. Bryoflora of the Urals. Study history, Conspectus and Taxonomy; Ekaterinburg State Ped. Univ.: Ekaterinburg, Russia, 1997. (In Russian) [Google Scholar]
- Kelly, A.E.; Goulden, M.L. Rapid shifts in plant distribution with recent climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 11823–11826. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.A.; Leafloor, J.O.; Meltofte, H.; Lanctot, R.B.; Fox, A.D.; Soloviev, M.; Franke, A.; Falk, K.; Golovatin, M.; Sokolov, V.; et al. Status and trends of tundra birds across the Circumpolar Arctic. Ambio 2020, 49, 732–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, H.; Mukhin, V.A. The arctic-alpine agaric element in the Polar Urals and Yamal, Western Siberia. In Arctic and Alpine Mycology; Yekaterinburg Pub.: Yekaterinburg, Russia, 1998; Volume 5, pp. 152–162. [Google Scholar]
- IndexFungorum. CABI, Kew, 2021. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 11 July 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 4 December 2019).
- Koropachinski, I.Y. North Asian Woody Plants; Geo Pub.: Novosibirsk, Russia, 2015; Volume 1–2. [Google Scholar]
- Shiryaev, A.G. Clavarioid fungi of the Urals. III. Arctic zone. Mycol. Phytopathol. 2006, 40, 294–307. [Google Scholar]
- Shiryaev, A.G. Changes in mycobiota of Ural-Siberian region under global warming and anthropogenic impact. Bull. Ecol. For. Landsc. 2008, 9, 37–47. (In Russian) [Google Scholar]
- Solly, E.F.; Djukic, I.; Moiseev, P.A.; Andreyashkina, N.I.; Devi, N.M.; Göransson, H.; Mazepa, V.S.; Shiyatov, S.G.; Trubina, M.R.; Schweingruber, F.H.; et al. Treeline advances and associated shifts in the ground vegetation alter fine root dynamics and mycelia production in the South and Polar Urals. Oecologia 2017, 183, 571–586. [Google Scholar] [CrossRef]
- Verheiten, K.; Chećko, M.; Chudomelova, M.; Decocq, P.; de Frenne, P.; Czortek, P.; Decocq, G.; De Frenne, P.; De Keersmaeker, L.; Carcía, C.E.; et al. Observer and relocation errors matter in resurveys of historical vegetation plots. J. Veg. Sci. 2018, 29, 812–823. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, T.; Tkachenko, O.; Kiriaki, M.; Yumoto, I.; Matsumoto, N. Winter damage caused by Typhula ishikariensis biological species I on conifer seedlings and hop roots collected in the Volga–Ural regions of Russia. Can. J. Plant Pathol. 2004, 26, 391–396. [Google Scholar] [CrossRef]
- Shiryaev, A.G.; Mukhin, V.A. Abundance dynamics of Subarctic mycobiota of the Yamal peninsula under climate change. Plant World Asian Russ. 2020, 40, 79–88. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiryaev, A.G. Uphill Shifts of Fungal Fruiting Due to Climate Change at the Polar Urals. Microorganisms 2021, 9, 1892. https://doi.org/10.3390/microorganisms9091892
Shiryaev AG. Uphill Shifts of Fungal Fruiting Due to Climate Change at the Polar Urals. Microorganisms. 2021; 9(9):1892. https://doi.org/10.3390/microorganisms9091892
Chicago/Turabian StyleShiryaev, Anton G. 2021. "Uphill Shifts of Fungal Fruiting Due to Climate Change at the Polar Urals" Microorganisms 9, no. 9: 1892. https://doi.org/10.3390/microorganisms9091892
APA StyleShiryaev, A. G. (2021). Uphill Shifts of Fungal Fruiting Due to Climate Change at the Polar Urals. Microorganisms, 9(9), 1892. https://doi.org/10.3390/microorganisms9091892





