Identification and Isolation Pattern of Globisporangium spp. from a Sanionia Moss Colony in Ny-Ålesund, Spitsbergen Is., Norway from 2006 to 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation
2.2. rDNA-ITS Analysis
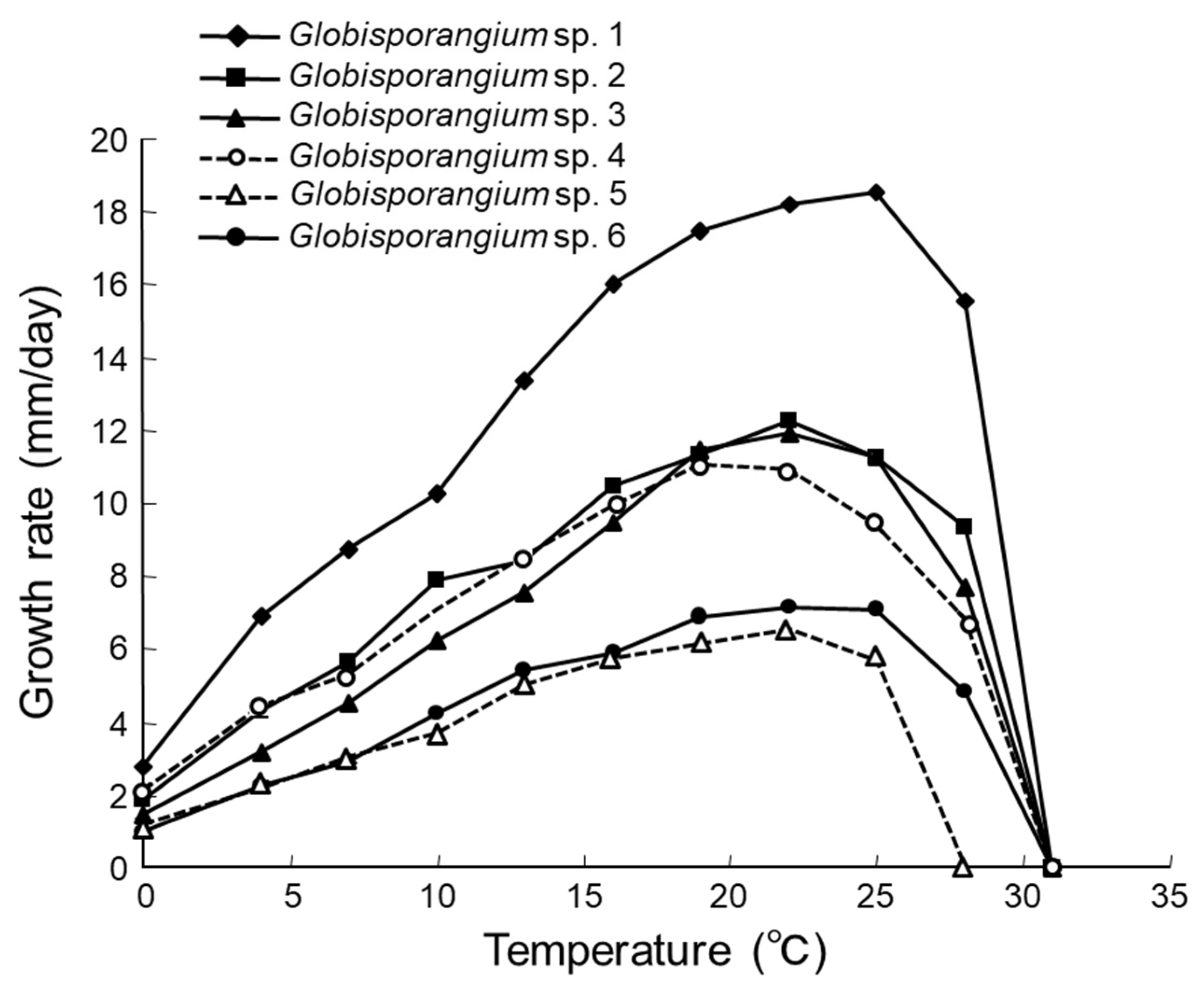
2.3. Characterizations of Morphology and Hyphal Growth Speed
2.4. Isolation Pattern
2.5. Infectivity to Sanionia Moss
3. Results and Discussion
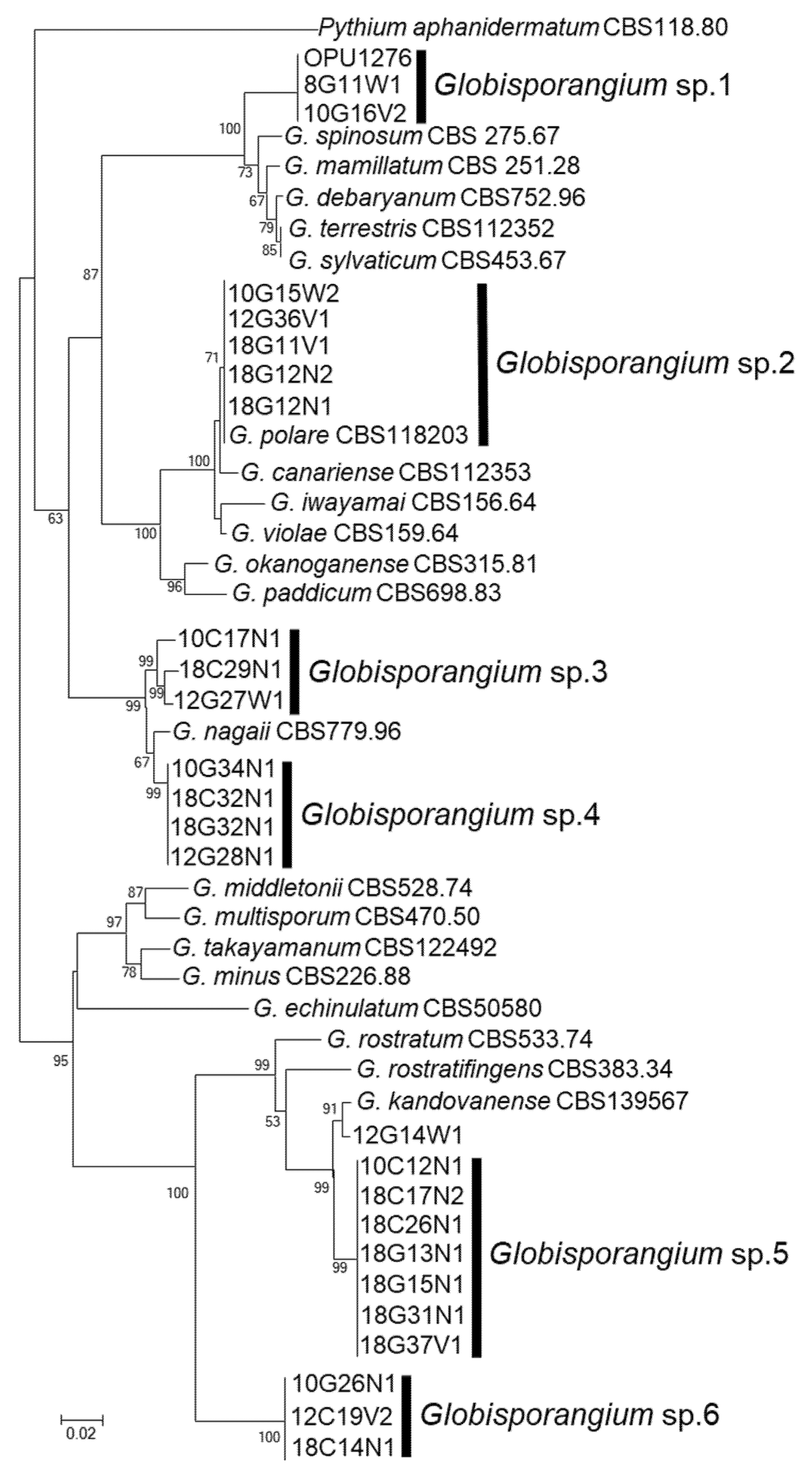
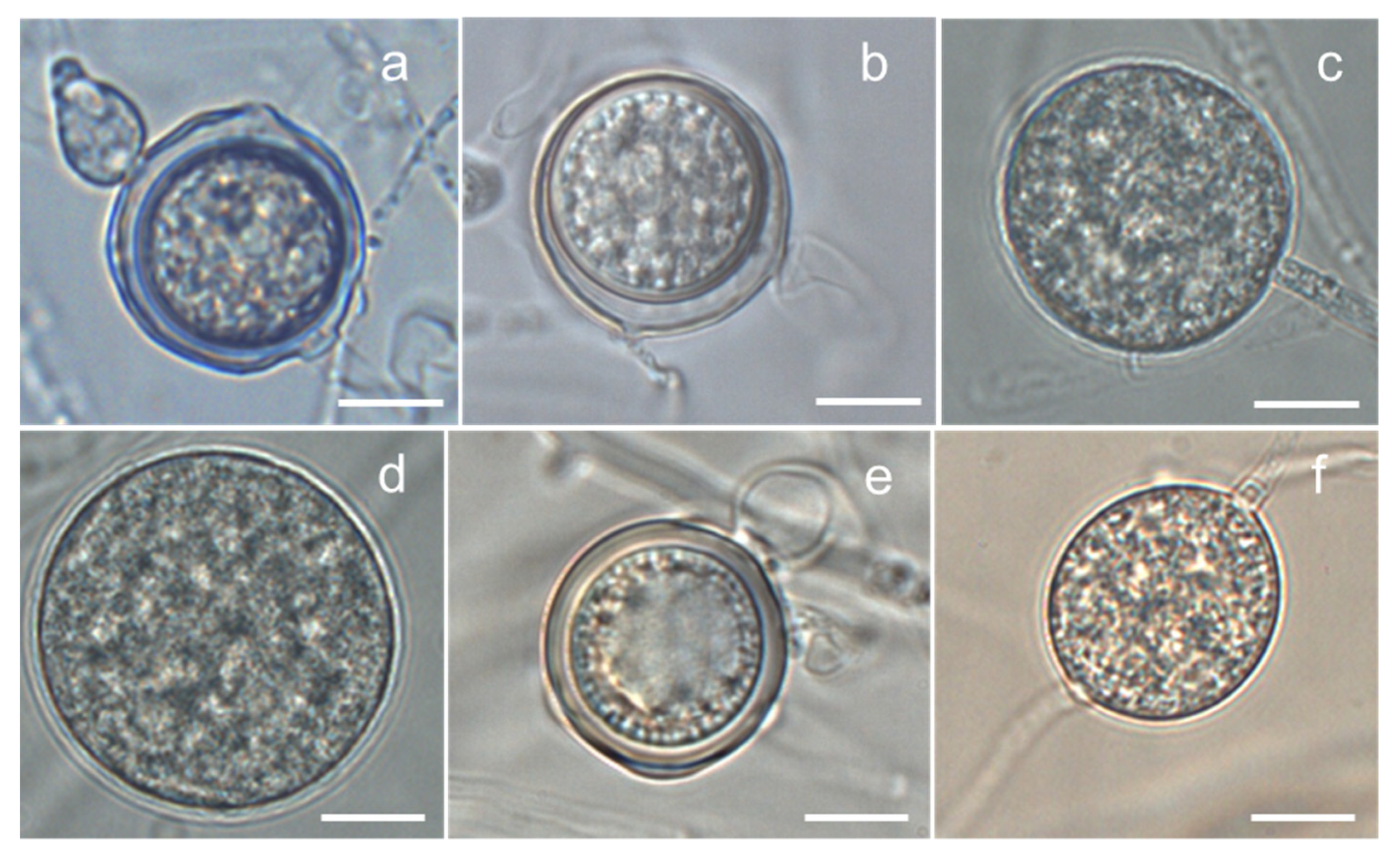
3.1. Isolation and Identification
3.2. Infectivity to Sanionia Moss
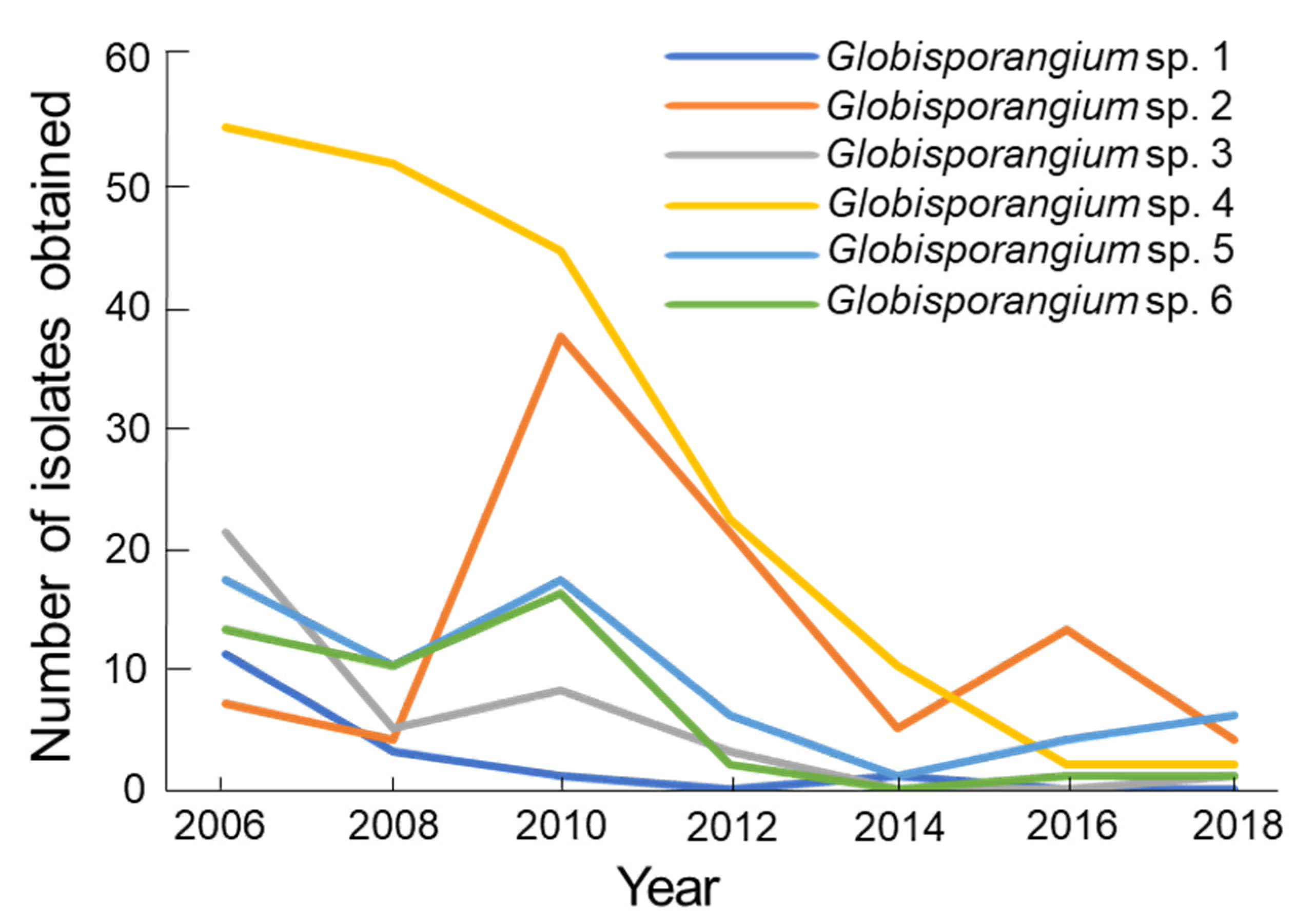
3.3. Isolation Pattern
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, G.S. Evolutionary ecology of plant disease in natural ecosystems. Annu. Rev. Phytopathol. 2002, 40, 13–43. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Guerra, G.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Romero, F.; Cazzato, S.; Walder, F.; Vogelgsang, S.; Bender, S.F.; van der Heijden, M.G.A. Humidity and high temperature are important for predicting fungal disease outbreaks worldwide. New Phytol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Tojo, M.; Newsham, K.K. Snow mould in polar environments. Fungal Ecol. 2012, 5, 395–402. [Google Scholar] [CrossRef]
- Karsten, P.A. Fungi in insulis Spetsbergen et Beeren eiland collecti, in Öfvers. Kungliga Vetenskapsakademien. Förhandlingar 1872, 2, 81–108. [Google Scholar]
- Lind, J. The Micromycetes of Svalbard. Skr Svalbard Ishavet 1928, 13, 1–61. [Google Scholar]
- Tojo, M.; Masumoto, S.; Hoshino, T. Phytopathogenic fungi and fungal-like microbes in Svalbard. In Plant and Microbe Adaptations to Cold in a Changing World; Imai, R., Yoshida, M., Matsumoto, N., Eds.; Springer: New York, NY, USA, 2013; pp. 263–284. [Google Scholar]
- Tsuji, M.; Uetake, J.; Tanabe, Y. Changes in the fungal community of Austre Brøggerbreen deglaciation area, Ny-Ålesund, Svalbard, High Arctic. Mycoscience 2016, 57, 448–451. [Google Scholar] [CrossRef]
- IPCC Sixth Assessment Report. 2021. Available online: https://www.ipcc.ch/assessment-report/ar6/ (accessed on 7 September 2021).
- Cornelissen, J.H.C.; Callaghan, T.V.; Alatalo, J.M.; Michelsen, A.; Graglia, E.; Hartley, A.E.; Hik, D.S.; Hobbie, S.E.; Press, M.C.; Robinson, C.H.; et al. Global change and arctic ecosystems: Is lichen decline a function of increases in vascular plant biomass? Ecol. J. 2001, 89, 984–994. [Google Scholar] [CrossRef]
- Hollister, R.D.; Webber, P.J.; Tweedie, C.E. The response of Alaskan arctic tundra to experimental warming: Differences between short-and long-term responses. Glob. Chang. Biol. 2005, 11, 525–536. [Google Scholar] [CrossRef]
- Zhang, W.; Miller, P.A.; Smith, B.; Wania, R.; Koenigk, T.; Döscher, R. Tundra shrubification and tree-line advance amplify arctic climate warming: Results from an individual-based dynamic vegetation model. Environ. Res. Lett. 2013, 8, 034023. [Google Scholar] [CrossRef] [Green Version]
- Sturm, M.; McFadden, J.P.; Liston, G.E.; Chapin, F.S.; Racine, C.H.; Holmgren, J. Snow–shrub interactions in arctic tundra: A hypothesis with climatic implications. J. Clim. 2001, 14, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Miles, J.; Walton, D.W.H. Primary succession revisited. In Primary Succession on Land; Miles, J., Walton, D.W.H., Eds.; Blackwell Science: Oxford, UK, 1993; pp. 295–302. [Google Scholar]
- Smith, R.I.L. Terrestrial and freshwater biotic components of the western Antarctic Peninsula. In Foundations of Ecological Research West of the Antarctic Peninsula Antarctic Research Series; Ross, R.M., Hofmann, E.E., Quentin, L.B., Eds.; American Geophysical Union: Washington DC, USA, 1996; Volume 70, pp. 15–59. [Google Scholar]
- Virtanen, R.J.; Lundberg, P.A.; Moen, J.; Oksanen, L. Topographic and altitudinal patterns in plant communities on European arctic islands. Polar Biol. 1997, 17, 95–113. [Google Scholar] [CrossRef]
- Fenton, J.H.C. Concentric fungal rings in Antarctic moss communities. Trans. Br. Mycol. Soc. 1983, 80, 413–420. [Google Scholar] [CrossRef]
- Longton, R.E. The occurrence of radial infection patterns in colonies of polar bryophytes. BAS Bull. 1973, 32, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.H.; Wookey, P.A. Microbial ecology, decomposition and nutrient cycling. In Ecology of Arctic Environments; Woodin, S.J., Marquis, M., Eds.; Blackwell Science: Oxford, UK, 1997; pp. 41–68. [Google Scholar]
- Rosa, L.H.; de Sousa, J.R.P.; de Menezes, G.C.A.; da Costa Coelho, L.; Carvalho-Silva, M.; Convey, P.; Câmara, P.E.A.S. Opportunistic fungi found in fairy rings are present on different moss species in the Antarctic Peninsula. Polar Biol. 2020, 43, 587–596. [Google Scholar] [CrossRef]
- Rosa, L.H.; da Silva, T.H.; Ogaki, M.B.; Pinto, O.H.B.; Stech, M.; Convey, P.; Carvalho-Silva, M.; Rosa, C.A.; Câmara, P.E.A.S. DNA metabarcoding uncovers fungal diversity in soils of protected and non-protected areas on Deception Island, Antarctica. Sci. Rep. 2020, 10, 21986. [Google Scholar] [CrossRef]
- Tojo, M.; Van West, P.; Hoshino, T.; Kida, K.; Fujii, H.; Hakoda, H.; Kawaguchi, Y.; Mühlhauser, H.A.; Van den Berg, A.H.; Küpper, F.C.; et al. Pythium polare, a new heterothallic Oomycete causing brown discoloration of Sanionia uncinata in the Arctic and Antarctic. Fungal Biol. 2012, 116, 756–768. [Google Scholar] [CrossRef]
- Uzuhashi, S.; Tojo, M.; Kakishima, M. Phylogeny of the genus Pythium and description of new genera. Mycoscience 2010, 51, 337–365. [Google Scholar] [CrossRef]
- Molin, C.; Ribeiro, N.R.; Matsumoto, M.N.; Giasson, N.F.; Brollo, J.; Zanardo, B.; Pelissoni, M.; Capitanio, S.; Comín, T.; Deuner, C.C.; et al. Damping-off of soybean in southern Brazil can be associated with different species of Globisporangium spp. and Pythium spp. Plant Pathol. 2021, 70, 1686–1694. [Google Scholar] [CrossRef]
- Lipps, P.E.; Bruehl, G.W. Snow rot of winter wheat in Washington. Phytopathology 1978, 68, 1120–1127. [Google Scholar] [CrossRef]
- Bridge, P.D.; Newsham, K.K.; Denton, G.J. Snow mould caused by a Pythium sp.: A potential vascular plant pathogen in the maritime Antarctic. Plant Pathol. 2008, 57, 1066–1072. [Google Scholar] [CrossRef]
- Hoshino, T.; Terami, F.; Tkachenko, O.B.; Tojo, M.; Matsumoto, N. Mycelial growth of the snow mold fungus, Sclerotinia borealis improved at low water potentials: An adaptation to frozen environment. Mycoscience 2010, 51, 98–102. [Google Scholar] [CrossRef]
- Murakami, R.; Yajima, Y.; Kida, K.; Tokura, K.; Tojo, M.; Hoshino, T. Surviving freezing in plant tissues by oomycetous snow molds. Cryobiology 2015, 70, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Nakagawa, T.; Yajima, Y.; Uchida, M.; Tojo, M. Note on a snow mold and a fungus-like microbe from Kuujjuarapik-Whapmagoostui, Quebec, subarctic Canada. Polar Sci. 2021, 27, 100559. [Google Scholar] [CrossRef]
- Ali-shtayeh, M.S.; Lim-ho, C.L.; Dick, N.W. An improved method and medium for quantitative estimates of populations of Pythium species from soil. Trans. Brit. Mycol. Soc. 1986, 86, 39–47. [Google Scholar] [CrossRef]
- Morita, Y.; Tojo, M. Modifications of PARP medium using fluazinam, miconazole and nystatin for detection of Pythium spp. in soil. Plant. Dis. 2007, 91, 1591–1599. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.M. V8 juice agar as a general purpose medium for fungi and bacteria. Phytopathology 1955, 45, 461–462. [Google Scholar] [CrossRef] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA gene for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Martin, F.N. Pythium. In Methods for Research on Soilborne Phytopathogenic Fungi; Singleton, L.L., Mihail, J.D., Rush, C.M., Eds.; APS Press: Saint Paul, MN, USA, 1992; pp. 39–49. [Google Scholar]
- van der Plaats-Niterink, A.J. Monograph of the genus Pythium. Stud. Mycol. 1981, 21, 1–242. [Google Scholar]
- Nehira, K. Germination and protonemata. In Methods in Bryology; Glime, J., Ed.; Hattori Botanical Laboratory: Nichinan, Japan, 1988; pp. 113–117. [Google Scholar] [CrossRef] [Green Version]
- Hyde, K.D.; Nilsson, R.H.; Alias, S.A.; Ariyawansa, H.A.; Blair, J.E.; Cai, L.; de Cock, A.W.A.M.; Dissanayake, A.J.; Glockling, S.L.; Goonasekara, I.D.; et al. One stop shop: Backbones trees for important phytopathogenic genera: I. Fungal Divers. 2014, 67, 21–125. [Google Scholar] [CrossRef] [Green Version]
- Robideau, G.P.; De Cock, A.W.; Coffey, M.D.; Voglmayr, H.; Brouwer, H.; Bala, K.; Chitty, D.W.; Désaulniers, N.; Eggertson, Q.A.; Gachon, C.M.; et al. DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol. Ecol. Resour. 2011, 11, 1002–1011. [Google Scholar] [CrossRef]
- Bouket, A.C.; Arzanlou, M.; Tojo, M.; Babai-Ahari, A. Pythium kandovanense sp. nov., a fungus-like eukaryotic microorganism (Stramenopila, Pythiales) isolated from snow covered ryegrass leaves. Int. J. Syst. Evol. Microbiol. 2015, 65, 2500–2506. [Google Scholar] [CrossRef]
- Lévesque, C.A.; de Cock, A.W.A.M. Molecular phylogeny and taxonomy of the genus Pythium. Mycol. Res. 2004, 108, 1363–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Box, J.E.; Colgan, W.T.; Christensen, T.R.; Schmidt, N.M.; Lund, M.; Parmentier, F.J.W.; Brown, R.; Bhatt, U.S.; Euskirchen, E.S.; Romanovsky, V.E.; et al. Key indicators of Arctic climate change: 1971–2017. Environ. Res. Lett. 2019, 14, 045010. [Google Scholar] [CrossRef]
- Overland, J.E.; Hanna, E.; Hanssen-Bauer, I.; Kim, S.J.; Walsh, J.E.; Wang, M.; Bhatt, U.S. An RL Surface air temperature. In Arctic Report Card; Richter-Menge, J., Overland, J.E., Mathis, J.T., Osborne, E., Eds.; National Oceanic and Atmospheric Administration (NOAA): Miami, FL, USA, 2017; pp. 5–12. [Google Scholar] [CrossRef]
- Pearson, R.G.; Phillips, S.J.; Loranty, M.M.; Beck, P.S.; Damoulas, T.; Knight, S.J.; Goetz, S.J. Shifts in Arctic vegetation and associated feedbacks under climate change. Nat. Clim. Change 2013, 3, 673–677. [Google Scholar] [CrossRef]
- Walker, M.D.; Wahren, C.H.; Hollisterc, R.D.; Henry, G.H.R.; Ahlquist, L.E.; Alatalo, J.M.; Bret-Harte, M.S.; Cale, M.P.; Callaghan, T.V.; Carroll, A.B.; et al. Plant community responses to experimental warming across the tundra biome. Proc. Natl. Acad. Sci. USA 2006, 103, 1342–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Taxonomic Group | Strain | Temperature | Infection into the Host Plant Cells with; | Recovery from the Host Plant | |
|---|---|---|---|---|---|
| (°C) | Hyphae | Oospores or Sporangia | |||
| Globisporangium sp. 1 | 10G16V2 | 4 | + | ++ | + |
| Globisporangium sp. 2 (G. polare) | 10G15W2 | 4 | + | + | + |
| 18G12N1 | 10 | + | + | + | |
| Globisporangium sp. 3 | 10C17N1 | 4 | + | ++ | + |
| 18C32N1 | 10 | + | + | + | |
| Globisporangium sp. 4 | 10G34N1 | 4 | + | + | + |
| 18C32N1 | 10 | + | + | + | |
| Globisporangium sp. 5 | 10C12N1 | 4 | − | − | − |
| 18G13N1 | 10 | + | + | + | |
| Globisporangium sp. 6 | 10G26N1 | 4 | + | − | + |
| 18C14N1 | 10 | + | + | + | |
| Uninoculated | 4 | − | − | − | |
| 10 | − | − | − | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tojo, M.; Fujii, N.; Yagi, H.; Yamashita, Y.; Tokura, K.; Kida, K.; Hakoda, A.; Herrero, M.-L.; Hoshino, T.; Uchida, M. Identification and Isolation Pattern of Globisporangium spp. from a Sanionia Moss Colony in Ny-Ålesund, Spitsbergen Is., Norway from 2006 to 2018. Microorganisms 2021, 9, 1912. https://doi.org/10.3390/microorganisms9091912
Tojo M, Fujii N, Yagi H, Yamashita Y, Tokura K, Kida K, Hakoda A, Herrero M-L, Hoshino T, Uchida M. Identification and Isolation Pattern of Globisporangium spp. from a Sanionia Moss Colony in Ny-Ålesund, Spitsbergen Is., Norway from 2006 to 2018. Microorganisms. 2021; 9(9):1912. https://doi.org/10.3390/microorganisms9091912
Chicago/Turabian StyleTojo, Motoaki, Natsumi Fujii, Hironori Yagi, Yuki Yamashita, Katsuyuki Tokura, Kenichi Kida, Akiho Hakoda, María-Luz Herrero, Tamotsu Hoshino, and Masaki Uchida. 2021. "Identification and Isolation Pattern of Globisporangium spp. from a Sanionia Moss Colony in Ny-Ålesund, Spitsbergen Is., Norway from 2006 to 2018" Microorganisms 9, no. 9: 1912. https://doi.org/10.3390/microorganisms9091912
APA StyleTojo, M., Fujii, N., Yagi, H., Yamashita, Y., Tokura, K., Kida, K., Hakoda, A., Herrero, M.-L., Hoshino, T., & Uchida, M. (2021). Identification and Isolation Pattern of Globisporangium spp. from a Sanionia Moss Colony in Ny-Ålesund, Spitsbergen Is., Norway from 2006 to 2018. Microorganisms, 9(9), 1912. https://doi.org/10.3390/microorganisms9091912






