Influence of Tall Fescue Epichloë Endophytes on Rhizosphere Soil Microbiome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Sampling and Tall Fescue Plants
2.3. DNA Extraction, PCR Amplification, and 16S rRNA Gene and ITS Gene Sequencing
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Chemical Properties
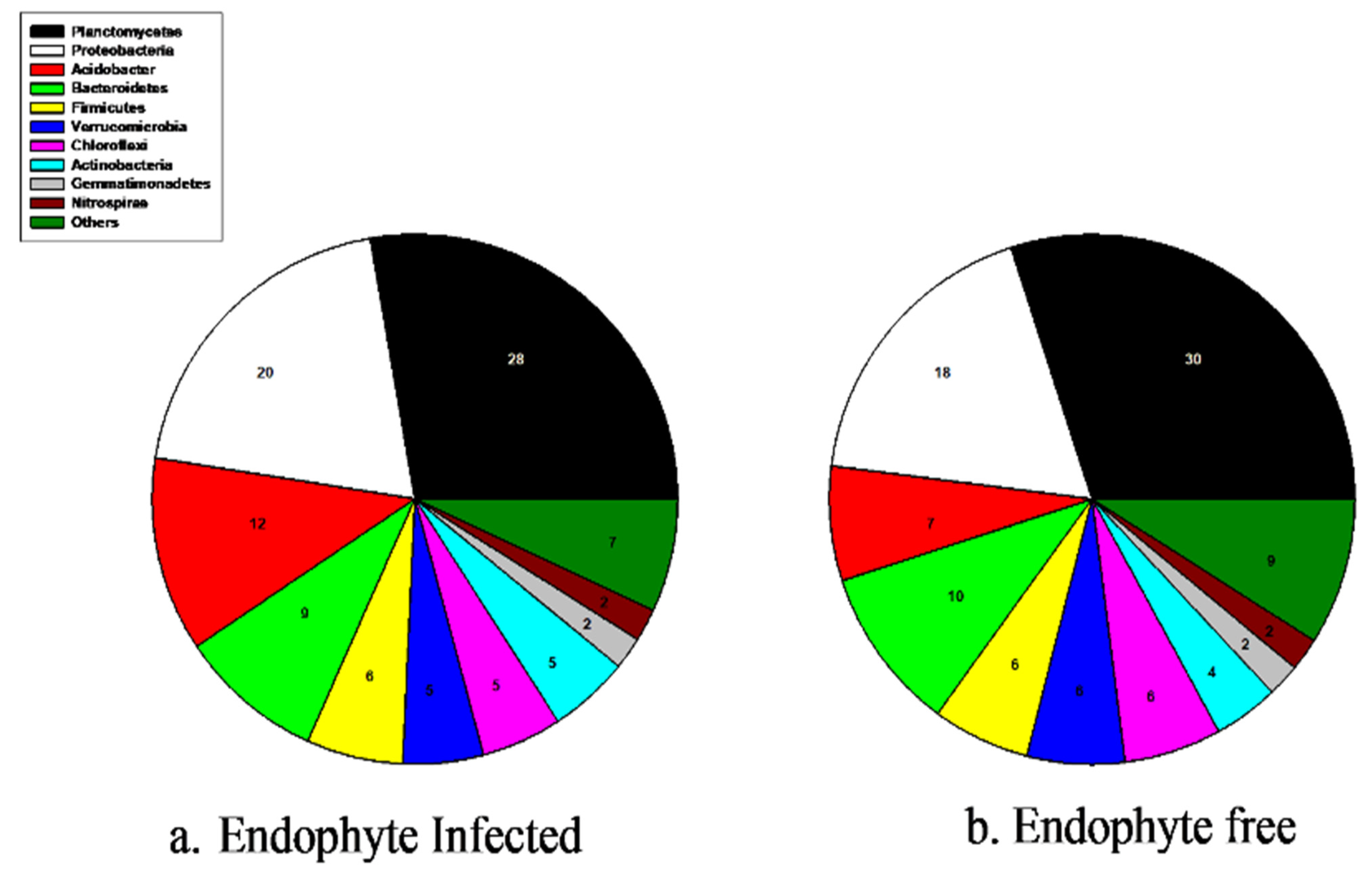
3.2. Soil Bacterial Abundance, Diversity, and Community Composition
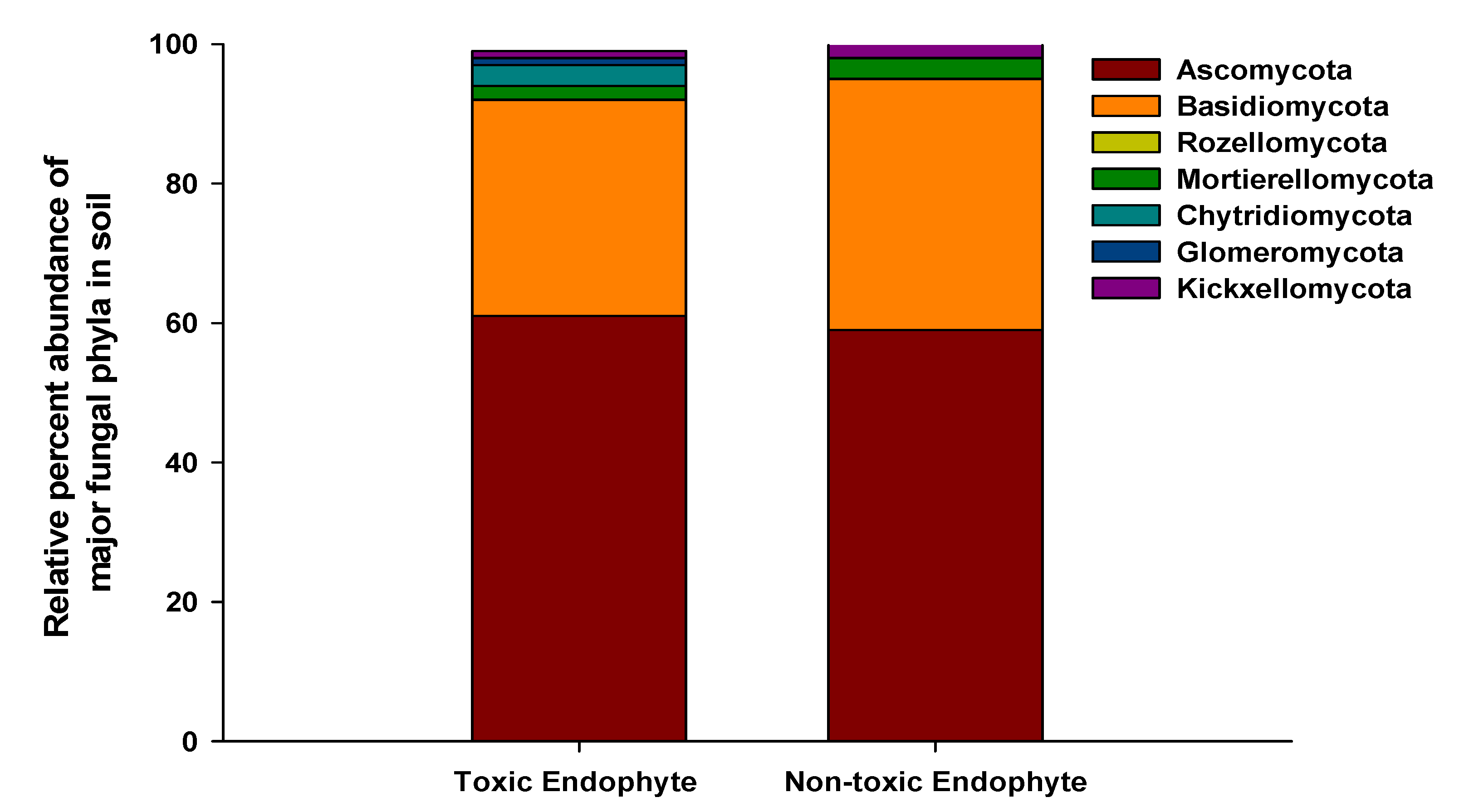
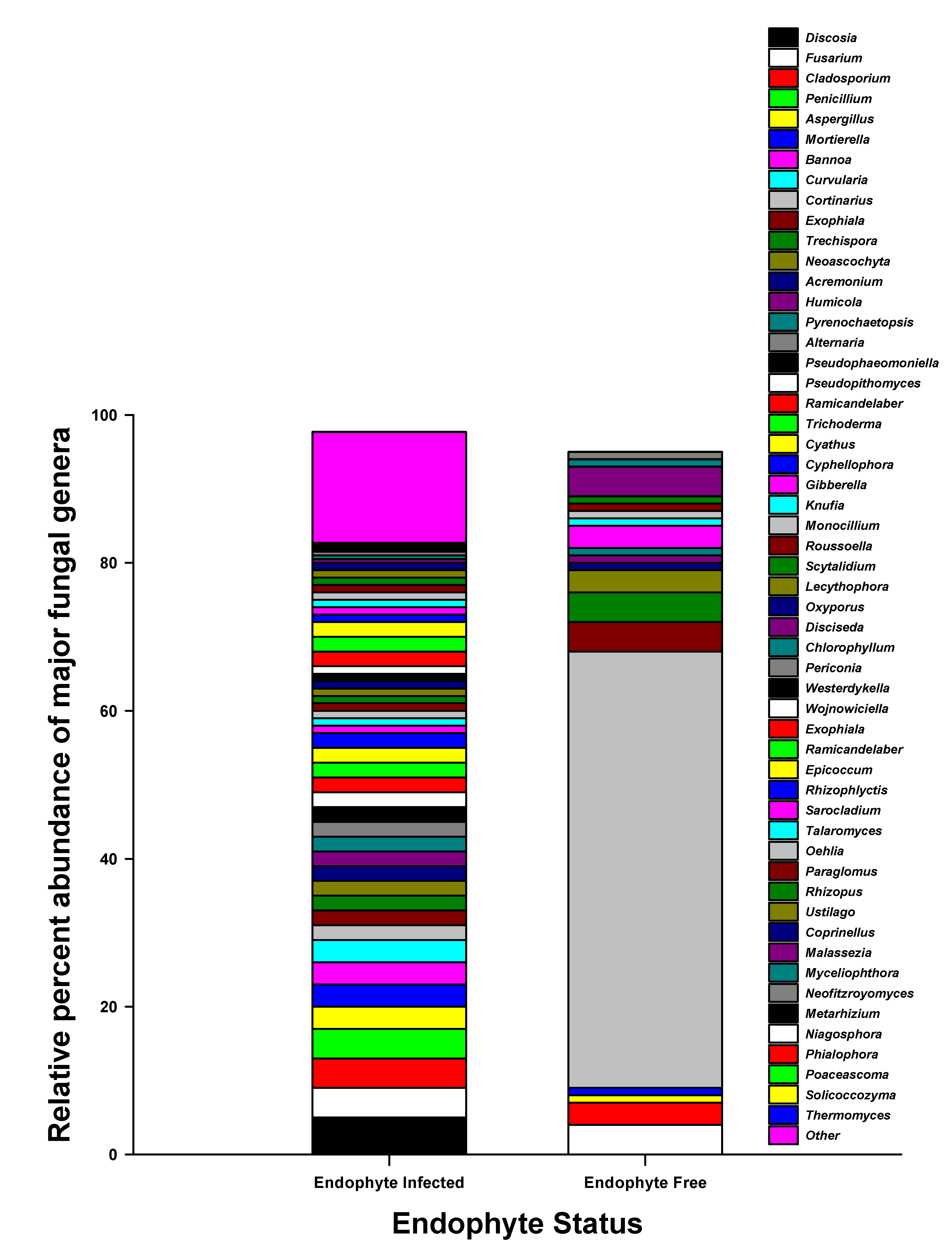
3.3. Soil Fungal Abundance, Diversity, and Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shantz, H. The place of grasslands in the Earth’s cover. Ecology 1954, 35, 143–145. [Google Scholar] [CrossRef]
- Dixon, A.; Faber-Langendoen, D.; Josse, C.; Morrison, J.; Loucks, C. Distribution mapping of world grassland types. J. Biogeogr. 2014, 41, 2003–2019. [Google Scholar] [CrossRef]
- Wedin, W.F. Grassland: Quietness and Strength for a New American Agriculture; ASA-CSSA-SSSA: Madison, WI, USA, 2009; Volume 142. [Google Scholar]
- Deng, L.; Shangguan, Z.-P.; Sweeney, S. “Grain for Green” driven land use change and carbon sequestration on the Loess Plateau, China. Sci. Rep. 2014, 4, 7039. [Google Scholar] [CrossRef]
- Endale, D.; Schomberg, H.; Franzluebbers, A.; Seman, D.; Franklin, D.; Stuedemann, J. Runoff nutrient losses from tall fescue pastures varying in endophyte association, fertilization, and harvest management. J. Soil Water Conserv. 2021, 76, 25–38. [Google Scholar] [CrossRef]
- Sala, O.E.; Yahdjian, L.; Havstad, K.; Aguiar, M.R. Rangeland ecosystem services: Nature’s supply and humans’ demand. In Rangeland Systems; Springer: Cham, Switzerland, 2017; pp. 467–489. [Google Scholar]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland ecosystem services: A systematic review of research advances and future directions. Landsc. Ecol. 2020, 35, 1–22. [Google Scholar] [CrossRef]
- Leuchtmann, A. Systematics, distribution, and host specificity of grass endophytes. Nat. Toxins 1993, 1, 150–162. [Google Scholar] [CrossRef]
- Field, K.J.; Pressel, S.; Duckett, J.G.; Rimington, W.R.; Bidartondo, M.I. Symbiotic options for the conquest of land. Trends Ecol. Evol. 2015, 30, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bouton, J.; Gates, R.; Belesky, D.; Owsley, M. Yield and persistence of tall fescue in the southeastern coastal plain after removal of its endophyte. Agron. J. 1993, 85, 52–55. [Google Scholar] [CrossRef]
- Young, C.A.; Charlton, N.D.; Takach, J.E.; Swoboda, G.A.; Trammell, M.A.; Huhman, D.V.; Hopkins, A.A. Characterization of Epichloë coenophiala within the US: Are all tall fescue endophytes created equal? Front. Chem. 2014, 2, 95. [Google Scholar] [CrossRef] [Green Version]
- Hoveland, C.S. Importance and economic significance of the Acremonium endophytes to performance of animals and grass plant. Agric. Ecosyst. Environ. 1993, 44, 3–12. [Google Scholar] [CrossRef]
- Stuedemann, J.A.; Hoveland, C.S. Fescue endophyte: History and impact on animal agriculture. J. Prod. Agric. 1988, 1, 39–44. [Google Scholar] [CrossRef]
- Hunt, M.G.; Newman, J.A. Reduced herbivore resistance from a novel grass–endophyte association. J. Appl. Ecol. 2005, 42, 762–769. [Google Scholar] [CrossRef]
- Parish, J.; McCann, M.; Watson, R.; Paiva, N.; Hoveland, C.; Parks, A.; Upchurch, B.; Hill, N.; Bouton, J. Use of nonergot alkaloid-producing endophytes for alleviating tall fescue toxicosis in stocker cattle. J. Anim. Sci. 2003, 81, 2856–2868. [Google Scholar] [CrossRef]
- Hill, N.; Bouton, J.; Hiatt, E.; Kittle, B. Seed maturity, germination, and endophyte relationships in tall fescue. Crop Sci. 2005, 45, 859–863. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Wang, R.; Wang, X.; Li, J. Conservation tillage increased soil bacterial diversity and improved soil nutrient status on the Loess Plateau in China. Arch. Agron. Soil Sci. 2020, 66, 1509–1519. [Google Scholar] [CrossRef]
- Koyama, A.; Wallenstein, M.D.; Simpson, R.T.; Moore, J.C. Soil bacterial community composition altered by increased nutrient availability in Arctic tundra soils. Front. Microbiol. 2014, 5, 516. [Google Scholar] [CrossRef] [Green Version]
- Lehman, R.M.; Acosta-Martinez, V.; Buyer, J.S.; Cambardella, C.A.; Collins, H.P.; Ducey, T.F.; Halvorson, J.J.; Jin, V.L.; Johnson, J.M.; Kremer, R.J. Soil biology for resilient, healthy soil. J. Soil Water Conserv. 2015, 70, 12A–18A. [Google Scholar] [CrossRef]
- Porter, S.S.; Bantay, R.; Friel, C.A.; Garoutte, A.; Gdanetz, K.; Ibarreta, K.; Moore, B.M.; Shetty, P.; Siler, E.; Friesen, M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2020, 34, 2075–2086. [Google Scholar] [CrossRef] [Green Version]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, K.; Missaoui, A.; Lee, K.C.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere Microbiome Manipulation for Sustainable Crop Production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Oki, Y.; Adachi, T. Mobilization and Acquisition of Sparingly Soluble P-Sources by Brassica Cultivars under P-Starved Environment II. Rhizospheric pH changes, Redesigned Root Architecture and Pi-Uptake Kinetics. J. Integr. Plant Biol. 2009, 51, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, I.; Holloway, R.; Armstrong, R.; McLaughlin, M. Chemical characteristics of phosphorus in alkaline soils from southern Australia. Soil Res. 2003, 41, 61–76. [Google Scholar] [CrossRef]
- Yang, X.J.; Finnegan, P.M. Regulation of phosphate starvation responses in higher plants. Ann. Bot. 2010, 105, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Chen, A.; Sun, S.; Xu, G. Complex regulation of plant phosphate transporters and the gap between molecular mechanisms and practical application: What is missing? Mol. Plant 2016, 9, 396–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, M.M.; Akhtar, K.; Karanja, J.K.; Haider, F.U. Understanding the Adaptive Mechanisms of Plant in Low Phosphorous Soil. In Plant Stress Physiology; IntechOpen: London, UK, 2020. [Google Scholar]
- Batool, S.; Iqbal, A. Phosphate solubilizing rhizobacteria as alternative of chemical fertilizer for growth and yield of Triticum aestivum (Var. Galaxy 2013). Saudi J. Biol. Sci. 2019, 26, 1400–1410. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, e0204408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, I.; Munné-Bosch, S. Linking phosphorus availability with photo-oxidative stress in plants. J. Exp. Bot. 2015, 66, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- Buyer, J.S.; Zuberer, D.A.; Nichols, K.A.; Franzluebbers, A.J. Soil microbial community function, structure, and glomalin in response to tall fescue endophyte infection. Plant Soil 2011, 339, 401–412. [Google Scholar] [CrossRef]
- Iqbal, J.; Siegrist, J.A.; Nelson, J.A.; McCulley, R.L. Fungal endophyte infection increases carbon sequestration potential of southeastern USA tall fescue stands. Soil Biol. Biochem. 2012, 44, 81–92. [Google Scholar] [CrossRef]
- Matthews, J.W.; Clay, K. Influence of fungal endophyte infection on plant–soil feedback and community interactions. Ecology 2001, 82, 500–509. [Google Scholar]
- Berg, G.; Smalla, K.J.F.m.e. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovira, A.J.P. Root excretions in relation to the rhizosphere effect. Plant Soil 1959, 11, 53–64. [Google Scholar] [CrossRef]
- Warembourg, F.; Paul, E.J.P. The use of C14O2 canopy techniques for measuring carbon transfer through the plant-soil system. Plant Soil 1973, 38, 331–345. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y.J.N.P. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Neumann, G.; Kania, A.; Weiskopf, L.; Lieberei, R.J.P. Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant Soil 2002, 246, 167–174. [Google Scholar] [CrossRef]
- Shi, S.; Richardson, A.E.; O’Callaghan, M.; DeAngelis, K.M.; Jones, E.E.; Stewart, A.; Firestone, M.K.; Condron, L.M.J.F.m.e. Effects of selected root exudate components on soil bacterial communities. FEMS Microbiol. Ecol. 2011, 77, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Chen, S.; Wu, X.; Syed, S.I.; Syed, I.U.S.; Huang, B.; Guan, P.; Wang, D. Grazing Affects Bacterial and Fungal Diversities and Communities in the Rhizosphere and Endosphere Compartments of Leymus chinensis through Regulating Nutrient and Ion Distribution. Microorganisms 2021, 9, 476. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Clay, K. An invasive plant–fungal mutualism reduces arthropod diversity. Ecol. Lett. 2008, 11, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, J.; Weigelt, A.; van Der Plas, F.; Laughlin, D.C.; Kuyper, T.W.; Guerrero-Ramirez, N.; Valverde-Barrantes, O.J.; Bruelheide, H.; Freschet, G.T.; Iversen, C.M. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 2020, 6, eaba3756. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; Van Der Heijden, M. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, P.; Xue, K.; Hao, Y.; Wang, Y.; Cui, X. Trait complementarity between fine roots of Stipa purpurea and their associated arbuscular mycorrhizal fungi along a precipitation gradient in Tibetan alpine steppe. J. Mt. Sci. 2019, 16, 542–547. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kim, Y.-H.; Choi, K.-S.; Lee, I.-J. Endophytic infection alleviates biotic stress in sunflower through regulation of defence hormones, antioxidants and functional amino acids. Eur. J. Plant Pathol. 2015, 141, 803–824. [Google Scholar] [CrossRef]
- Rana, K.L.; Kour, D.; Sheikh, I.; Dhiman, A.; Yadav, N.; Yadav, A.N.; Rastegari, A.A.; Singh, K.; Saxena, A.K. Endophytic fungi: Biodiversity, ecological significance, and potential industrial applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–62. [Google Scholar]
- Hacquard, S.; Kracher, B.; Hiruma, K.; Münch, P.C.; Garrido-Oter, R.; Thon, M.R.; Weimann, A.; Damm, U.; Dallery, J.-F.; Hainaut, M. Survival trade-offs in plant roots during colonization by closely related beneficial and pathogenic fungi. Nat. Commun. 2016, 7, 11362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daws, S.C.; Cline, L.A.; Rotenberry, J.; Sadowsky, M.J.; Staley, C.; Dalzell, B.; Kennedy, P.G. Do shared traits create the same fates? Examining the link between morphological type and the biogeography of fungal and bacterial communities. Fungal Ecol. 2020, 46, 100948. [Google Scholar] [CrossRef]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Qiu, W.; Su, H.; Yan, L.; Ji, K.; Liu, Q.; Jian, H. Organic Fertilization Assembles Fungal Communities of Wheat Rhizosphere Soil and Suppresses the Population Growth of Heterodera avenae in the Field. Front. Plant Sci. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, N.; Yadav, A.N.; Singh, J.; Rastegari, A.A.; Saxena, A.K. Agriculturally and industrially important fungi: Current developments and potential biotechnological applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–64. [Google Scholar]
- Kauppinen, M.; Saikkonen, K.; Helander, M.; Pirttilä, A.M.; Wäli, P.R. Epichloë grass endophytes in sustainable agriculture. Nat. Plants 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Service, N.R.C.; Department, A. Keys to Soil Taxonomy; Government Printing Office: Washington, DC, USA, 2010.
- Pacific Biosciences. Procedure & Checklist—Amplification of Full-Length 16S Gene with Barcoded Primers for Multiplexed SMRTbell® Library Preparation and Sequencing; Pacific Biosciences: Menlo Park, CA, USA, 2018; Available online: https://www.pacb.com/wp-content/uploads/Procedure-Checklist-%E2%80%93-Amplification-of-Full-Length-16S-Gene-with-Barcoded-Primers-for-Multiplexed-SMRTbell-Library-Preparation-and-Sequencing.pdf (accessed on 24 June 2021).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, E.; Erdogan, H.; Oztok, U. BIOQUERY-ASP: Querying biomedical ontologies using answer set programming. Proc. RuleML2011@ BRF Chall. 2011, 573–578. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.L.; Ferraro, A. Rhizosphere microbiome selection by Epichloë endophytes of Festuca arundinacea. Plant Soil 2015, 396, 229–239. [Google Scholar] [CrossRef]
- Franzluebbers, A.; Nazih, N.; Stuedemann, J.; Fuhrmann, J.; Schomberg, H.; Hartel, P. Soil carbon and nitrogen pools under low-and high-endophyte-infected tall fescue. Soil Sci. Soc. Am. J. 1999, 63, 1687–1694. [Google Scholar] [CrossRef] [Green Version]
- Franzluebbers, A.; Hill, N. Soil carbon, nitrogen, and ergot alkaloids with short-and long-term exposure to endophyte-infected and endophyte-free tall fescue. Soil Sci. Soc. Am. J. 2005, 69, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Franzluebbers, A.; Stuedemann, J. Soil carbon and nitrogen pools in response to tall fescue endophyte infection, fertilization, and cultivar. Soil Sci. Soc. Am. J. 2005, 69, 396–403. [Google Scholar] [CrossRef]
- Van Hecke, M.M.; Treonis, A.M.; Kaufman, J.R. How does the fungal endophyte Neotyphodium coenophialum affect tall fescue (Festuca arundinacea) rhizodeposition and soil microorganisms? Plant Soil 2005, 275, 101–109. [Google Scholar] [CrossRef]
- Vos, M.; Wolf, A.B.; Jennings, S.J.; Kowalchuk, G.A. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol. Rev. 2013, 37, 936–954. [Google Scholar] [CrossRef] [Green Version]
- Urbanová, M.; Kopecký, J.; Valášková, V.; Ságová-Marečková, M.; Elhottová, D.; Kyselková, M.; Moënne-Loccoz, Y.; Baldrian, P. Development of bacterial community during spontaneous succession on spoil heaps after brown coal mining. FEMS Microbiol. Ecol. 2011, 78, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Urbanová, M.; Šnajdr, J.; Baldrian, P. Composition of fungal and bacterial communities in forest litter and soil is largely determined by dominant trees. Soil Biol. Biochem. 2015, 84, 53–64. [Google Scholar] [CrossRef]
- Buckley, D.H.; Huangyutitham, V.; Nelson, T.A.; Rumberger, A.; Thies, J.E. Diversity of Planctomycetes in soil in relation to soil history and environmental heterogeneity. Appl. Environ. Microbiol. 2006, 72, 4522–4531. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, V.; Rehman, A.; Mishra, A.; Chauhan, P.S.; Nautiyal, C.S. Changes in bacterial community structure of agricultural land due to long-term organic and chemical amendments. Microb. Ecol. 2012, 64, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Ditterich, F.; Poll, C.; Pronk, G.J.; Heister, K.; Chandran, A.; Rennert, T.; Kögel-Knabner, I.; Kandeler, E. Succession of soil microbial communities and enzyme activities in artificial soils. Pedobiologia 2016, 59, 93–104. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupatini, M.; Korthals, G.W.; de Hollander, M.; Janssens, T.K.; Kuramae, E.E. Soil microbiome is more heterogeneous in organic than in conventional farming system. Front. Microbiol. 2017, 7, 2064. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, L.; Liu, J.; Yuan, M.; Liu, S.; Jiang, W.; Chen, J. Changes in bacterial community of soil induced by long-term straw returning. Sci. Agric. 2017, 74, 349–356. [Google Scholar] [CrossRef]
- Ramirez-Villanueva, D.A.; Bello-López, J.M.; Navarro-Noya, Y.E.; Luna-Guido, M.; Verhulst, N.; Govaerts, B.; Dendooven, L. Bacterial community structure in maize residue amended soil with contrasting management practices. Appl. Soil Ecol. 2015, 90, 49–59. [Google Scholar] [CrossRef]
- Mahmud, K.; Franklin, D.; Ney, L.; Cabrera, M.; Habteselassie, M.; Hancock, D.; Newcomer, Q.; Subedi, A.; Dahal, S. Improving inorganic nitrogen in soil and nutrient density of edamame bean in three consecutive summers by utilizing a locally sourced bio-inocula. Org. Agric. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, C.; Yu, W.; Turak, A.; Chen, D.; Huang, Y.; Ao, J.; Jiang, Y.; Huang, Z. Effects of nitrogen and phosphorus inputs on soil bacterial abundance, diversity, and community composition in Chinese fir plantations. Front. Microbiol. 2018, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Belesky, D.P. Epichloë (formerly Neotyphodium) fungal endophytes increase adaptation of cool-season perennial grasses to environmental stresses. Acta Agrobot. 2019, 2, 72. [Google Scholar] [CrossRef]
- Wang, J.; Hou, W.; Christensen, M.J.; Li, X.; Xia, C.; Li, C.; Nan, Z. Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J. Agric. Food Chem. 2020, 68, 6944–6955. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, D.P.; Belesky, D.P. Adaptations of endophyte-infected cool-season grasses to environmental stresses: Mechanisms of drought and mineral stress tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Eilers, K.G.; Lauber, C.L.; Knight, R.; Fierer, N. Shifts in bacterial community structure associated with inputs of low molecular weight carbon compounds to soil. Soil Biol. Biochem. 2010, 42, 896–903. [Google Scholar] [CrossRef]
- Joshi, S.; Jaggi, V.; Gangola, S.; Singh, A.; Sah, V.; Sahgal, M. Contrasting rhizosphere bacterial communities of healthy and wilted Dalbergia sissoo Roxb. forests. Rhizosphere 2021, 17, 100295. [Google Scholar] [CrossRef]
- Waller, J.C. Endophyte effects on cattle. Tall Fescue Twenty-First Century 2009, 53, 289–310. [Google Scholar]
- Fierer, N.; Ladau, J.; Clemente, J.C.; Leff, J.W.; Owens, S.M.; Pollard, K.S.; Knight, R.; Gilbert, J.A.; McCulley, R.L. Reconstructing the microbial diversity and function of pre-agricultural tallgrass prairie soils in the United States. Science 2013, 342, 621–624. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol. 2013, 21, 641–651. [Google Scholar] [CrossRef]
- Ding, J.; Jiang, X.; Guan, D.; Zhao, B.; Ma, M.; Zhou, B.; Cao, F.; Yang, X.; Li, L.; Li, J. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl. Soil Ecol. 2017, 111, 114–122. [Google Scholar] [CrossRef]
- Mueller, R.C.; Paula, F.S.; Mirza, B.S.; Rodrigues, J.L.; Nüsslein, K.; Bohannan, B.J. Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J. 2014, 8, 1548–1550. [Google Scholar] [CrossRef]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef]
- Hiscox, J.; Savoury, M.; Müller, C.T.; Lindahl, B.D.; Rogers, H.J.; Boddy, L. Priority effects during fungal community establishment in beech wood. ISME J. 2015, 9, 2246–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivlin, S.N.; Winston, G.C.; Goulden, M.L.; Treseder, K.K. Environmental filtering affects soil fungal community composition more than dispersal limitation at regional scales. Fungal Ecol. 2014, 12, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl. Soil Ecol. 2017, 113, 11–21. [Google Scholar] [CrossRef]
- Ponge, J.-F. Plant–soil feedbacks mediated by humus forms: A review. Soil Biol. Biochem. 2013, 57, 1048–1060. [Google Scholar] [CrossRef] [Green Version]
- Requena, N.; Jimenez, I.; Toro, M.; Barea, J. Interactions between plant-growth-promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi and Rhizobium spp. in the rhizosphere of Anthyllis cytisoides, a model legume for revegetation in mediterranean semi-arid ecosystems. New Phytol. 1997, 136, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Sláviková, E.; Košíková, B.; Mikulášová, M. Biotransformation of waste lignin products by the soil-inhabiting yeast Trichosporon pullulans. Can. J. Microbiol. 2002, 48, 200–203. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.; Semenov, A.M. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar] [CrossRef]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, M.G.; De Bruin, S.; Luckerhoff, L.; Van Logtestijn, R.S.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissinen, R.; Helander, M.; Kumar, M.; Saikkonen, K. Heritable Epichloë symbiosis shapes fungal but not bacterial communities of plant leaves. Sci. Rep. 2019, 9, 5253. [Google Scholar] [CrossRef] [PubMed]
- Dix, N.J. Fungal Ecology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Guo, J.; McCulley, R.; Phillips, T.; McNear, D., Jr. Fungal endophyte and tall fescue cultivar interact to differentially affect bulk and rhizosphere soil processes governing C and N cycling. Soil Biol. Biochem. 2016, 101, 165–174. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [Google Scholar] [CrossRef]
- Rostás, M.; Cripps, M.G.; Silcock, P. Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 2015, 177, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Parsons, A.J.; Fraser, K.; Xue, H.; Newman, J.A. Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiol. 2008, 146, 1440–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; McCulley, R.L.; McNear, D.H., Jr. Tall fescue cultivar and fungal endophyte combinations influence plant growth and root exudate composition. Front. Plant Sci. 2015, 6, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, X.; Guo, J.; Leff, J.W.; McNear, D.H.; Fierer, N.; McCulley, R.L. Infection with a shoot-specific fungal endophyte (Epichloë) alters tall fescue soil microbial communities. Microb. Ecol. 2016, 72, 197–206. [Google Scholar] [CrossRef]
- Ding, N.; Guo, H.; Kupper, J.V.; McNear, D.H., Jr. Phosphorus source and Epichloë coenophiala strain interact over time to modify tall fescue rhizosphere microbial community structure and function. Soil Biol. Biochem. 2021, 154, 108125. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A. Global diversity and geography of soil fungi. Science 2014, 346, 1256688. [Google Scholar] [CrossRef] [Green Version]
- García-García, N.; Tamames, J.; Linz, A.M.; Pedrós-Alió, C.; Puente-Sánchez, F. Microdiversity ensures the maintenance of functional microbial communities under changing environmental conditions. ISME J. 2019, 13, 2969–2983. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, B.S.; Philippot, L. Insights into the resistance and resilience of the soil microbial community. FEMS Microbiol. Rev. 2013, 37, 112–129. [Google Scholar] [CrossRef] [Green Version]
- Erkus, O.; De Jager, V.C.; Spus, M.; van Alen-Boerrigter, I.J.; Van Rijswijck, I.M.; Hazelwood, L.; Janssen, P.W.; Van Hijum, S.A.; Kleerebezem, M.; Smid, E.J. Multifactorial diversity sustains microbial community stability. ISME J. 2013, 7, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Read, J.S.; Youngblut, N.D.; Fierer, N.; Knight, R.; Kratz, T.K.; Lottig, N.R.; Roden, E.E.; Stanley, E.H.; Stombaugh, J. Lake microbial communities are resilient after a whole-ecosystem disturbance. ISME J. 2012, 6, 2153–2167. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Hsieh, M.; Kandel, S.L.; Cantillo, J.; Doty, S.L.; Kim, S.-H. Do endophytes promote growth of host plants under stress? A meta-analysis on plant stress mitigation by endophytes. Microb. Ecol. 2018, 75, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.N.; Menge, D.N. Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants 2018, 4, 655–661. [Google Scholar] [CrossRef]
- Kaschuk, G.; Kuyper, T.W.; Leffelaar, P.A.; Hungria, M.; Giller, K.E. Are the rates of photosynthesis stimulated by the carbon sink strength of rhizobial and arbuscular mycorrhizal symbioses? Soil Biol. Biochem. 2009, 41, 1233–1244. [Google Scholar] [CrossRef]
- Van Der Putten, W.H. Belowground drivers of plant diversity. Science 2017, 355, 134–135. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef] [Green Version]
- Lekberg, Y.; Bever, J.D.; Bunn, R.A.; Callaway, R.M.; Hart, M.M.; Kivlin, S.N.; Klironomos, J.; Larkin, B.G.; Maron, J.L.; Reinhart, K.O. Relative importance of competition and plant–soil feedback, their synergy, context dependency and implications for coexistence. Ecol. Lett. 2018, 21, 1268–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Whalen, J.K. A new perspective on functional redundancy and phylogenetic niche conservatism in soil microbial communities. Pedosphere 2020, 30, 18–24. [Google Scholar]


| Season | Maximum Atmospheric Temperature (°C) | Minimum Atmospheric Temperature (°C) | Daily Atmospheric Temperature (°C) | Daily Soil Temperature (°C) at 15 cm Depth | Average Rainfall (mm) |
|---|---|---|---|---|---|
| Summer 2015 | 32 | 21 | 26 | 28 | 391 |
| Summer 2016 | 33 | 21 | 27 | 30 | 295 |
| Summer 2017 | 30 | 20 | 25 | 28 | 517 |
| Summer 2018 | 31 | 20 | 25 | 28 | 392 |
| Summer 2019 | 31 | 20 | 25 | 29 | 304 |
| pH | NH4+- N | NO3− N | Ca | K | Mg | Mn | P | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| Endophyte-Free Soil | 6.53 a | 3 a | 244 a | 801 a | 40 a | 105 a | 17 a | 26 b | 1.0 a |
| Endophyte-Infected Soil (Toxic) | 6.59 a | 2 a | 270 a | 681 a | 43 a | 93 a | 17 a | 38 a | 1.17 a |
| Endophyte-Infected Soil (Non-toxic) | 6.59 a | 3 a | 245 a | 731 a | 45 a | 99 a | 16 a | 33 ab | 1.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmud, K.; Lee, K.; Hill, N.S.; Mergoum, A.; Missaoui, A. Influence of Tall Fescue Epichloë Endophytes on Rhizosphere Soil Microbiome. Microorganisms 2021, 9, 1843. https://doi.org/10.3390/microorganisms9091843
Mahmud K, Lee K, Hill NS, Mergoum A, Missaoui A. Influence of Tall Fescue Epichloë Endophytes on Rhizosphere Soil Microbiome. Microorganisms. 2021; 9(9):1843. https://doi.org/10.3390/microorganisms9091843
Chicago/Turabian StyleMahmud, Kishan, Kendall Lee, Nicholas S. Hill, Anaas Mergoum, and Ali Missaoui. 2021. "Influence of Tall Fescue Epichloë Endophytes on Rhizosphere Soil Microbiome" Microorganisms 9, no. 9: 1843. https://doi.org/10.3390/microorganisms9091843
APA StyleMahmud, K., Lee, K., Hill, N. S., Mergoum, A., & Missaoui, A. (2021). Influence of Tall Fescue Epichloë Endophytes on Rhizosphere Soil Microbiome. Microorganisms, 9(9), 1843. https://doi.org/10.3390/microorganisms9091843






