High Occurrence of Shiga Toxin-Producing Escherichia coli in Raw Meat-Based Diets for Companion Animals—A Public Health Issue
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Screening for Stx Genes
2.3. Recovery of STEC
2.4. DNA Extraction and Whole Genome Sequencing
3. Results
3.1. Real-Time Screening for Stx Genes and Isolation of STEC
3.2. Serotypes and Stx Subtypes
3.3. Additional Virulence Factor Genes
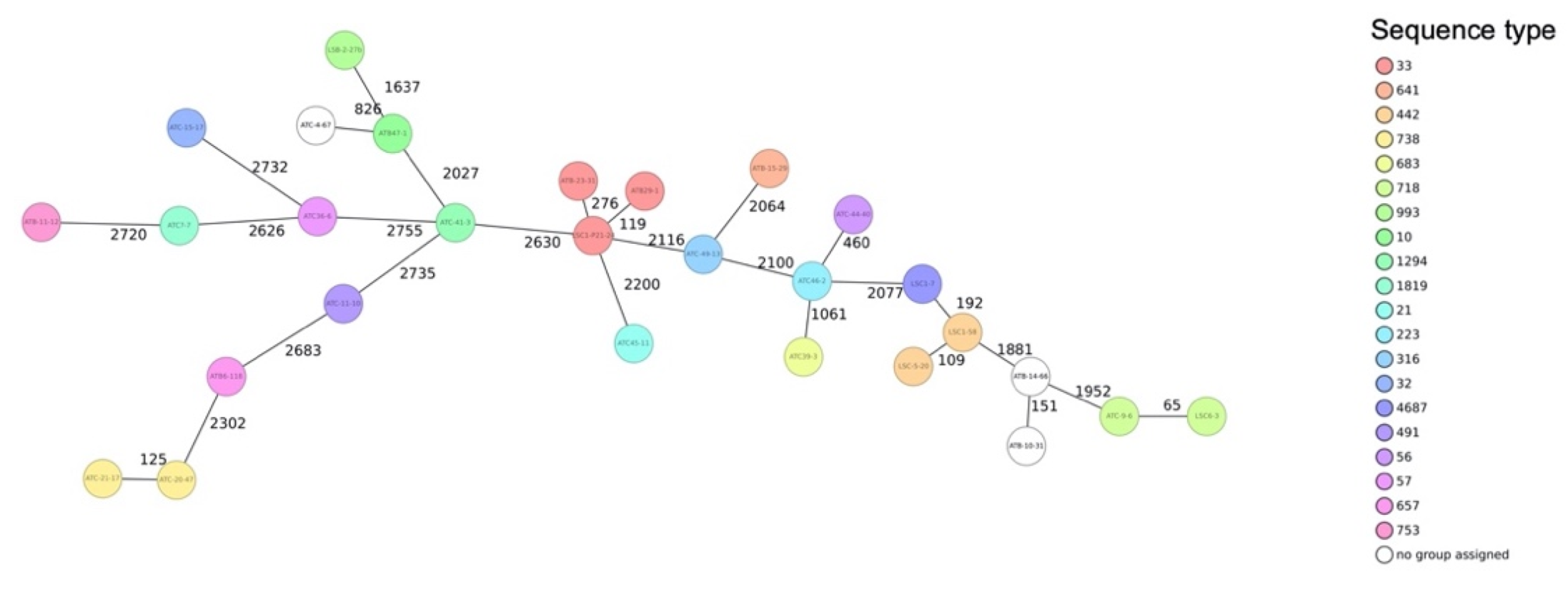
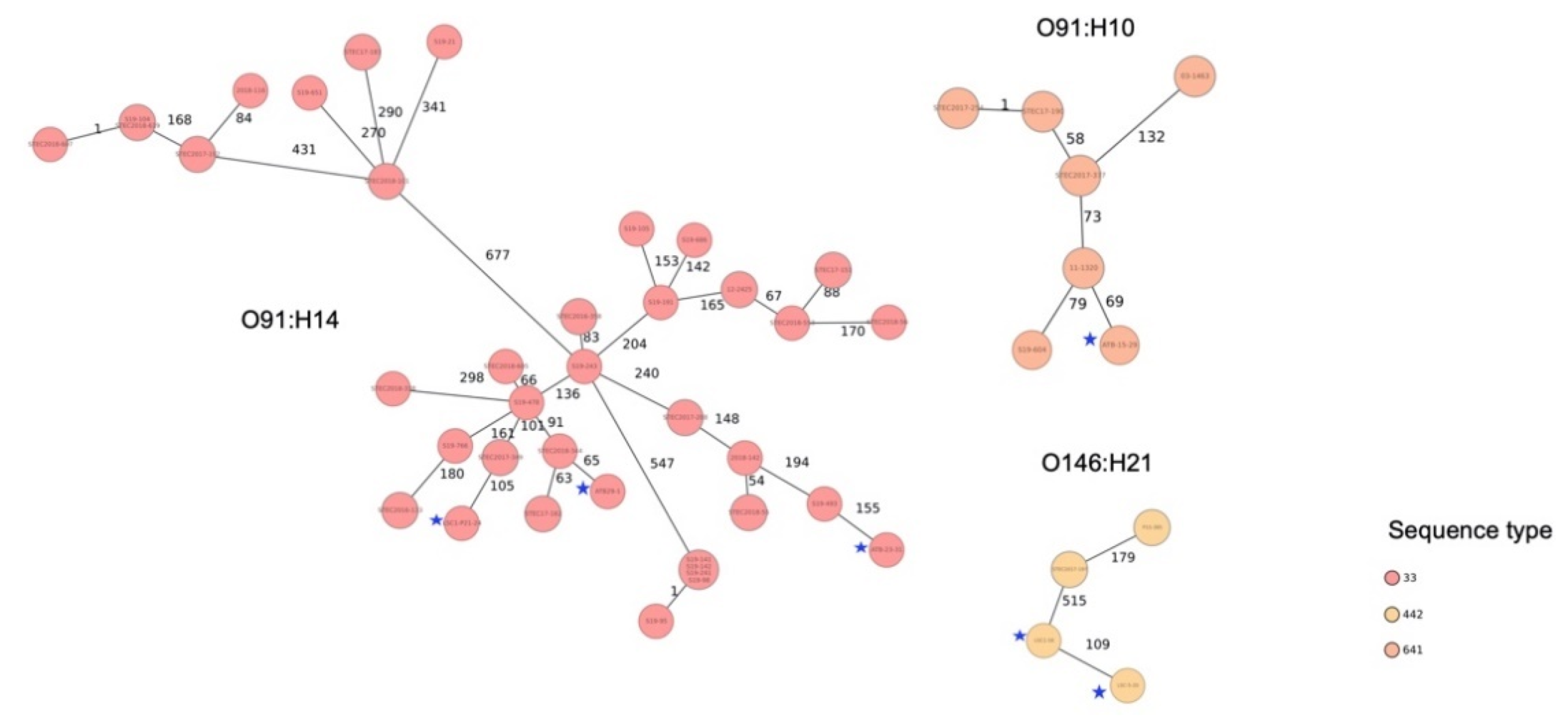
3.4. Sequence Types and Phylogenetic Relationship
3.5. Transmissible Antimicrobial Resistance Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Halloran, C. Raw food diets for companion carnivores: An untapped panacea or a disaster waiting to happen. Companion Anim. 2020, 25, 1–5. [Google Scholar] [CrossRef]
- Davies, R.H.; Lawes, J.R.; Wales, A.D. Raw diets for dogs and cats: A review, with particular reference to microbiological hazards. J. Small Anim. Pract. 2019, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.M.; Chandler, M.L.; Hamper, B.A.; Weeth, L.P. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J. Am. Vet. Med. Assoc. 2013, 243, 1549–1558. [Google Scholar] [CrossRef]
- Dillitzer, N.; Becker, N.; Kienzle, E. Intake of minerals, trace elements and vitamins in bone and raw food rations in adult dogs. Br. J. Nutr. 2011, 106, S53–S56. [Google Scholar] [CrossRef]
- Hellgren, J.; Hästö, L.S.; Wikström, C.; Fernström, L.L.; Hansson, I. Occurrence of Salmonella, Campylobacter, Clostridium and Enterobacteriaceae in raw meat-based diets for dogs. Vet. Rec. 2019, 184, 442. [Google Scholar] [CrossRef]
- van Bree, F.P.J.; Bokken, G.C.A.M.; Mineur, R.; Franssen, F.; Opsteegh, M.; van der Giessen, J.W.B.; Lipman, L.J.A.; Overgaauw, P.A.M. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec 2018, 182, 50. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Rousseau, J.; Arroyo, L. 2005 Bacteriological evaluation of commercial canine and feline raw diets. Can. Vet. J. 2018, 46, 513–516. [Google Scholar]
- Anonymous. Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. Off. J. Eur. Commun. 2011, 54, 1–254. [Google Scholar]
- Finley, R.; Reid-Smith, R.; Weese, J.S. Human health implications of Salmonella-contaminated natural pet treats and raw pet food. Clin. Infect. Dis. 2006, 42, 686–691. [Google Scholar] [CrossRef]
- Nemser, S.M.; Doran, T.; Grabenstein, M.; McConnell, T.; McGrath, T.; Pamboukian, R.; Smith, A.C.; Achen, M.; Danzeisen, G.; Kim, S.; et al. Investigation of Listeria, Salmonella, and toxigenic Escherichia coli in various pet foods. Foodborne Pathog. Dis. 2014, 11, 706–709. [Google Scholar] [CrossRef]
- Byrne, L.; Aird, H.; Jorgensen, F.; Kaindama, L.; Jenkins, C. Investigation into An Outbreak of Shiga Toxin Producing Escherichia coli (STEC) O157 PT 21/28 Stx2 in England, August 2017; Public Health England Publications: London, UK, 2018.
- Karch, H.; Tarr, P.I.; Bielaszewska, M. Enterohaemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 2005, 295, 405–418. [Google Scholar] [CrossRef]
- Yang, X.; Bai, X.; Zhang, J.; Sun, H.; Fu, S.; Fan, R.; He, X.; Scheutz, F.; Matussek, A.; Xiong, Y. Escherichia coli strains producing a novel Shiga toxin 2 subtype circulate in China. Int. J. Med. Microbiol. 2020, 310, 151377. [Google Scholar] [CrossRef]
- Scheutz, F. Taxonomy meets public health: The case of Shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef]
- Fuller, C.A.; Pellino, C.A.; Flagler, M.J.; Strasser, J.E.; Weiss, A.A. Shiga toxin subtypes display dramatic differences in potency. Infect. Immun. 2011, 79, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.W.; Bielaszewska, M.; Zhang, W.L.; Pulz, M.; Kuczius, T.; Ammon, A.; Karch, H. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. J. Infect. Dis. 2002, 185, 74–84. [Google Scholar] [CrossRef]
- Stephan, R.; Hoelzle, L.E. Characterization of Shiga toxin type 2 variant B-subunit in Escherichia coli strains from asymptomatic human carriers by PCR-RFLP. Lett. Appl. Microbiol. 2000, 31, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F. Pathogenicity assessment of Shiga toxin-producing Escherichia coli (STEC) and the public health risk posed by contamination of food with STEC. EFSA J. 2020, 18, e05967. [Google Scholar]
- Paton, A.W.; Srimanote, P.; Talbot, U.M.; Wang, H.; Paton, J.C. A new family of potent AB(5) cytotoxins produced by Shiga toxigenic Escherichia coli. J. Exp. Med. 2004, 200, 35–46. [Google Scholar] [CrossRef]
- Nüesch-Inderbinen, M.; Morach, M.; Cernela, N.; Althaus, D.; Jost, M.; Mäusezahl, M.; Bloomberg, G.; Stephan, R. Serotypes and virulence profiles of Shiga toxin-producing Escherichia coli strains isolated during 2017 from human infections in Switzerland. Int. J. Med. Microbiol. 2018, 308, 933–939. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Scallan, E.; Jones-Bitton, A.; Sargeant, J.M.; Stapleton, J.; Angulo, F.J.; Yeung, D.H.; Kirk, M.D. Global incidence of human Shiga toxin–producing Escherichia coli infections and deaths: A systematic review and knowledge synthesis. Foodborne Path. Dis. 2014, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Cointe, A.; Mariani Kurkdjian, P.; Rafat, C.; Hertig, A. Shiga toxin-associated hemolytic uremic syndrome: A narrative review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Kintz, E.; Brainard, J.; Hooper, L.; Hunter, P. Transmission pathways for sporadic Shiga-toxin producing E. coli infections: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 57–67. [Google Scholar] [CrossRef]
- EURL (European Union Reference Laboratory). Identification and Characterization of Verocytotoxin-Producing Escherichia coli (VTEC) by PCR Amplification of the Main Virulence Genes. 2013. Available online: http://old.iss.it/binary/vtec/cont/EU_RL_VTEC_Method_01_Rev_0.pdf (accessed on 1 June 2020).
- Seemann, T. 2019 Shovill. Available online: https://github.com/tseemann/shovill (accessed on 20 January 2021).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- EURL (European Union Reference Laboratory). Identification of the Subtypes of Verocytotoxin Encoding Genes (vtx) of Escherichia coli by Conventional PCR; EU-RL VTEC_Method_006_Rev 1; EU Reference Laboratory for E. coli. 2013. Available online: https://www.iss.it/documents/20126/1049000/EU_RL_VTEC_Method_06_Rev_1.pdf/1ad8f0c6-8a1b-21c7-dddb-4af28f440822?t=1576447091624 (accessed on 1 June 2020).
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and easy in silico serotyping of Escherichia coli isolates by use of whole-genome sequencing data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Jolley, K.A.; Maiden, M.C. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Gannon, V.; Sargeant, J.M. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 2010, 140, 360–370. [Google Scholar] [CrossRef]
- Fierz, L.; Cernela, N.; Hauser, E.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of Shigatoxin-producing Escherichia coli strains isolated during 2010-2014 from human infections in Switzerland. Front. Microbiol. 2017, 8, 1471. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.C.H.; Delannoy, S.; Lacher, D.W.; Bosilevac, J.M.; Fach, P.; Beutin, L. Shiga toxin-producing serogroup O91 Escherichia coli strains isolated from food and environmental samples. Appl. Environ. Microbiol. 2017, 83, e01231-17. [Google Scholar] [CrossRef]
- Maeda, E.; Murakami, K.; Etoh, Y.; Onozuka, D.; Sera, N.; Asoshima, N.; Honda, M.; Narimatsu, H.; Iyoda, S.; Watahiki, M.; et al. Does sequence type 33 of Shiga toxin-producing Escherichia coli O91 cause only mild symptoms? J. Clin. Microbiol. 2015, 53, 362–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mellmann, A.; Fruth, A.; Friedrich, A.W.; Wieler, L.H.; Harmsen, D.; Werber, D.; Middendorf, B.; Bielaszewska, M.; Karch, H. Phylogeny and disease association of Shiga toxin-producing Escherichia coli O91. Emerg. Infect. Dis. 2009, 15, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Stephan, R.; Untermann, F. Virulence factors and phenotypical traits of verotoxin-producing Escherichia coli strains isolated from asymptomatic human carriers. J. Clin. Microbiol. 1999, 37, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Tarr, P.I.; Bilge, S.S.; Vary, J.C.; Jelacic, S.; Habeeb, R.L.; Ward, T.R.; Baylor, M.R.; Besser, T.E. Iha: A novel Escherichia coli O157: H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 2000, 68, 1400–1407. [Google Scholar] [CrossRef]
- Beutin, L.; Geier, D.; Steinrück, H.; Zimmermann, S.; Scheutz, F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J. Cin. Microbiol. 1993, 31, 2483–2488. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, M.S.; Kim, J.H. Recent updates on outbreaks of Shiga toxin-producing Escherichia coli and its potential reservoirs. Front. Cell Infect. Microbiol. 2020, 10, 273. [Google Scholar] [CrossRef]
- Galarce, N.; Escobar, B.; Sánchez, F.; Paredes-Osses, E.; Alegría-Morán, R.; Borie, C. Virulence genes, Shiga toxin subtypes, serogroups, and clonal relationship of Shiga toxin-producing Escherichia coli strains isolated from livestock and companion animals. Animals 2019, 9, 733. [Google Scholar] [CrossRef]
- Bentancor, A.; Rumi, M.V.; Carbonari, C.; Gerhardt, E.; Larzábal, M.; Vilte, D.A.; Pistone-Creydt, V.; Chinen, I.; Ibarra, C.; Cataldi, A.; et al. Profile of Shiga toxin-producing Escherichia coli strains isolated from dogs and cats and genetic relationships with isolates from cattle, meat and humans. Vet. Microbiol. 2012, 156, 336–342. [Google Scholar] [CrossRef]
- Bentancor, A.; Rumi, M.V.; Gentilini, M.V.; Sardoy, C.; Irino, K.; Agostini, A.; Cataldi, A. Shiga toxin-producing and attaching and effacing Escherichia coli in cats and dogs in a high hemolytic uremic syndrome incidence region in Argentina. FEMS Microbiol. Lett. 2007, 267, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Spurbeck, R.R.; Dinh, P.C.; Walk, S.T.; Stapleton, A.E.; Hooton, T.M.; Nolan, L.K.; Kim, K.S.; Johnson, J.R.; Mobley, H.L.T. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 2012, 80, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In silico genotyping of Escherichia coli isolates for extraintestinal virulence genes by use of whole-genome sequencing data. J. Clin. Microbiol. 2020, 58, e01269–20. [Google Scholar] [CrossRef]
- Santos, A.C.M.; Santos, F.F.; Silva, R.M.; Gomes, T.A.T. Diversity of hybrid- and hetero-pathogenic Escherichia coli and their potential implication in more severe diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Zogg, A.L.; Zurfluh, K.; Schmitt, S.; Nüesch-Inderbinen, M.; Stephan, R. Antimicrobial resistance, multilocus sequence types and virulence profiles of ESBL producing and non-ESBL producing uropathogenic Escherichia coli isolated from cats and dogs in Switzerland. Vet. Microbiol. 2018, 216, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, A.K.; Ribeiro, M.G.; Leite, D.S.; Tiba, M.R.; Moura, C.; Lopes, M.D.; Prestes, N.C.; Salerno, T.; Silva, A.V. Virulence factors in Escherichia coli strains isolated from urinary tract infection and pyometra cases and from feces of healthy dogs. Res. Vet. Sci. 2009, 86, 206–210. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine 6th Revision; World Health Organization: Geneva, Switzerland, 2019. Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 1 March 2021).
- Mir, R.A.; Kudva, I.T. Antibiotic-resistant Shiga toxin-producing Escherichia coli: An overview of prevalence and intervention strategies. Zoonoses Public Health. 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
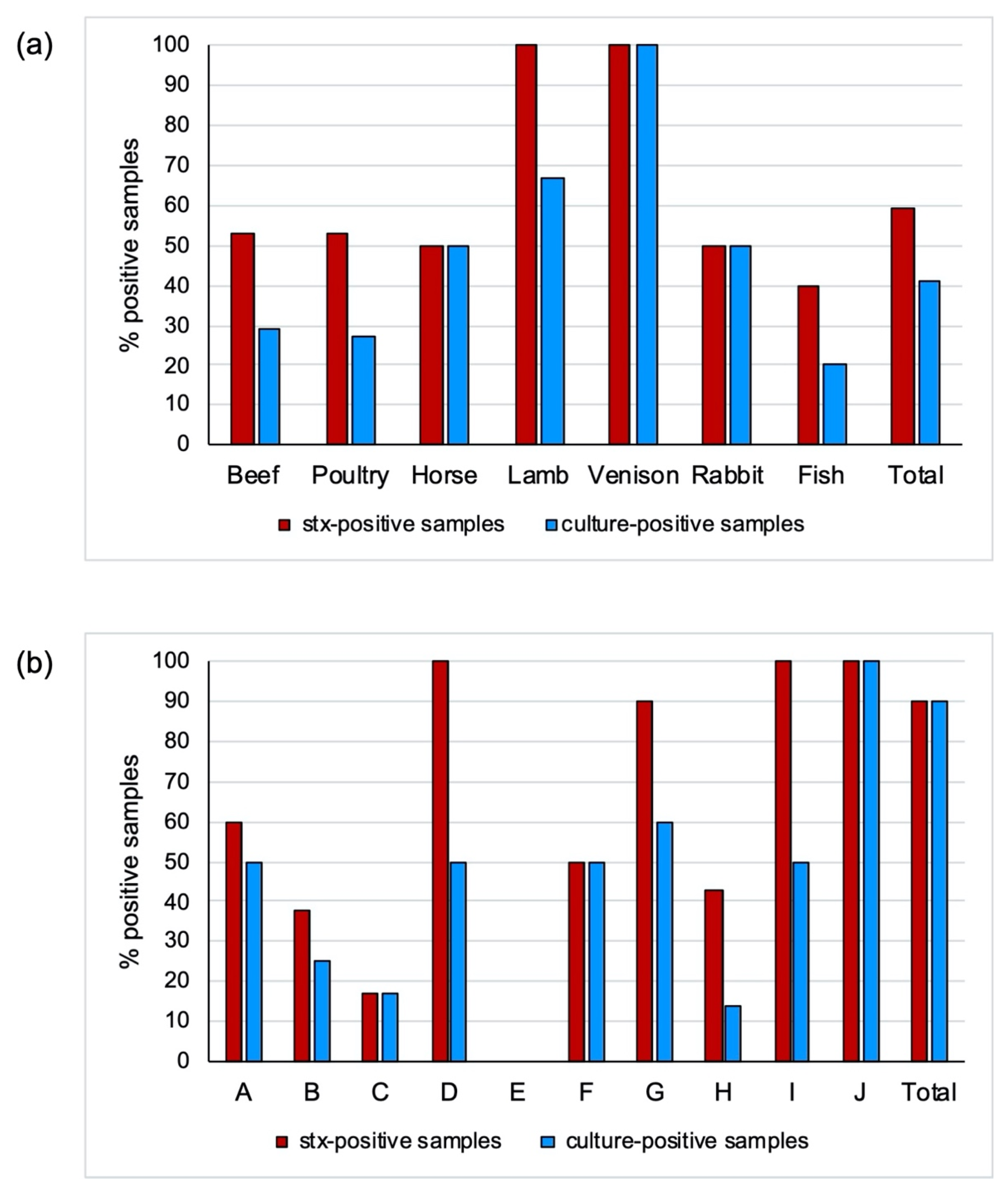
| Molecular Detection of stx Genes | Isolation of STEC Strains a | ||||||
|---|---|---|---|---|---|---|---|
| No. of Samples (%) Positive for | |||||||
| Type of Meat | No. of Samples | No. of stx-Positive Samples (%) | stx1 | stx2 | stx1 and stx2 | No. of STEC-Positive Samples (%) | No. of STEC Isolated |
| Beef | 17 | 9 (53) | 0 (0) | 0 (0) | 9 (53) | 5 (29) | 8 |
| Chicken | 7 | 3 (43) | 1 (14) | 1 (14) | 1 (14) | 1 (14) | 1 |
| Duck | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 1 |
| Horse | 8 | 4 (50) | 0 (0) | 2 (25) | 2 (25) | 4 (50) | 4 |
| Lamb | 6 | 6 (100) | 1 (17) | 0 (0) | 5 (83) | 4 (67) | 4 |
| Moose | 1 | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 1 |
| Ostrich | 1 | 1 (100) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 1 |
| Perch | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 |
| Quail | 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 |
| Rabbit | 4 | 2 (50) | 0 (0) | 0 (0) | 2 (50) | 2 (50) | 2 |
| Reindeer | 1 | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 1 |
| Salmon | 4 | 2 (50) | 1 (25) | 0 (0) | 1 (25) | 1 (25) | 1 |
| Turkey | 5 | 3 (60) | 0 (0) | 0 (0) | 3 (60) | 1 (20) | 1 |
| Venison | 2 | 2 (100) | 0 (0) | 1 (50) | 1 (50) | 2 (100) | 3 |
| Total | 59 | 35 (59) | 3 (5) | 6 (10) | 26 (44) | 24 (41) | 28 |
| Shiga Toxin Genes | Intimin | Transmissible Antimicrobial Resistance Genes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Strain ID | Type of Meat | Serotype | ST | stx1 | stx2 | eae | Other Virulence Factor Genes | Accession NO. | |
| AT 41 | ATC 41-3 | Ostrich | O9:H30 | 1294 | - | stx2g | - | capU, gad, ompT, papA_F12, papC, terC, traT | - | JAETYL000000000 |
| AT 45 | ATC 45-11 | Turkey | O26:H11 | 21 | stx1a | stx2a | + | astA, cif, efa1, espA, espB, espF, espJ, espP, fyuA, ehxA, gad, iha, irp2, iss, iucC, iutA, katP, lpfA, nleA, nleB, nleC, ompT, terC, tir, toxB, traT | aac(3)-IIe, blaTEM-1 | JAETYS000000000 |
| AT 11 | ATB 11-12 | Venison | O27:H30 | 753 | - | stx2b | - | air, chuA, eilA, gad, iha, ireA, iss, ompT, subA, terC, traT | - | JAETXZ000000000 |
| AT 11 | ATC 11-10 | Venison | O54:H45 | 491 | - | stx2b | - | astA, chuA, fyuA, ehxA, gad, iha, ireA, irp2, iss, ompT, papC, pic, senB, sitA, subA, terC, traT, vat, yfcV | - | JAETYH000000000 |
| AT 10 | ATB 10-31 | Lamb | O76:H19 | nd | stx1c | stx2b | - | ehxA, gad, iha, ireA, kpsE, lpfA, pic, senB, sitA, subA, terC, traT | - | JAETXY000000000 |
| AT 14 | ATB 14-66 | Lamb | O76:H19 | nd | stx1c | - | - | ehxA, gad, ireA, kpsE, lpfA, pic, senB, subA, terC, traT | - | JAETYA000000000 |
| AT 15 | ATB 15-29 | Beef | O91:H10 | 641 | - | stx2d | - | espI, gad, iha, ireA, iss, lpfA, ompT, papC, terC | - | JAETYB000000000 |
| AT 23 | ATB 23-31 | Lamb | O91:H14 | 33 | stx1a | stx2b | - | espI, ehxA, gad, iha, ireA, iss, iucC, iutA, lpfA, ompT, sitA, subA, terC, traT, | - | JAETYD000000000 |
| AT 29 | ATB 29-1 | Lamb | O91:H14 | 33 | stx1a | stx2b | - | espI, ehxA, gad, iha, ireA, iss, iucC, iutA, katP, lpfA, ompT, senB, sitA, subA, terC, traT | - | JAETYF000000000 |
| LS 01 | LSB1 P21-24 | Beef | O91:H14 | 33 | stx1a | stx2b | - | espI, ehxA, gad, iha, ireA, iss, iucC, iutA, katP, lpfA, ompT, senB, sitA, subA, terC, traT | - | JAETZB000000000 |
| LS 02 | LSB 2-27b | Rabbit | O100:H30 | 993 | - | stx2e | - | astA, gad, iss, terC, traT | - | JAETYV000000000 |
| AT 47 | ATB 47-1 | Salmon | O113:H4 | 10 | - | stx2d | - | astA, espI, gad, iha, iss, terC | aph(3″)-Ib, aph(6)-Id, sul2, tet(B) | JAETYG000000000 |
| AT 44 | ATC 44-40 | Moose | O113:H21 | 56 | - | stx2d | - | espP, gad, iha, hra, iss, lpfA, ompT, terC, traT | - | JAETYM000000000 |
| AT 46 | ATC 46-2 | Rabbit | O113:H21 | 223 | - | stx2a | - | epeA, espP, ehxA, gad, iha, iss, lpfA, ompT, subA, terC, traT | - | JAETYT000000000 |
| AT 04 | ATB 4-67 | Beef | O123:H16 | nd | stx1a | - | - | afaA, afaB, afaC, afaD, afaE8, cdtB, espP, gad, hra, iha, iss, iucC, iutA, ompT, terC, traT | sul1, aadA, aph(3′)-Ia, aph(3″)-Ib, aph(6)-Id, blaTEM-1, dfrA1, sul2, tet(A) | JAETYK000000000 |
| AT 15 | ATC 15-17 | Beef | O145:H28 | 32 | stx1a | - | + | astA, chuA, cif, espA, espB, espF, espJ, espP, ehxA, gad, iha, iss, neuC, nleA, nleB, nleC, ompT, terC, tir, toxB, traT | - | JAETYI000000000 |
| LS 01 | LSC 1-58 | Beef | O146:H21 | 442 | stx1c | stx2b | - | espI, ehxA, gad, iha, ireA, iss, iucC, iutA, kpsE, lpfA, ompT, senB, subA, terC, traT | - | JAETYZ000000000 |
| LS 05 | LSC 5-20 | Horse | O146:H21 | 442 | stx1c | - | - | ehxA, gad, iha, ireA, iss, iucC, iutA, kpsE, lpfA, ompT, senB, subA, terC, traT | - | JAETYY000000000 |
| LS 01 | LSC 1-7 | Beef | O146:H21 | 4687 | stx1c | - | - | ehxA, gad, iha, ireA, iss, iucC, iutA, kpsE, lpfA, ompT, senB, subA, terC, traT | - | JAETZA000000000 |
| AT 21 | ATC 21-17 | Venison | O146:H28 | 738 | - | stx2b | - | astA, chuA, hra, iha, ireA, iss, lpfA, ompT, subA, terC, traT, usp | - | JAETYJ000000000 |
| AT 20 | ATC 20-47 | Horse | O146:H28 | 738 | - | stx2b | - | astA, chuA, hra, iha, ireA, iss, lpfA, ompT, subA, terC, traT, usp | - | JAETYP000000000 |
| AT 39 | ATC 39-3 | Horse | O155:H21 | 683 | - | stx2e | - | astA, gad, iha, iss, lpfA, ompT, sepA, terC, traT | - | JAETYR000000000 |
| AT 49 | ATC 49-13 | Reindeer | O162:H7 | 316 | - | stx2b | - | ehxA, gad, iha, ireA, iss, lpfA, ompT, subA, terC, traT | - | JAETYN000000000 |
| LS 06 | LSC 6-3 | Beef | O168:H8 | 718 | - | stx2b/2d | - | gad, iha, hra, lpfA, terC, traT | aph(6)-Id, aph(3″)-Ib, sul2, tet(B) | JAETZC000000000 |
| AT 09 | ATC 9-6 | Duck | O168:H8 | 718 | - | stx2d | - | gad, hra, iha, lpfA, terC, traT | aph(3″)-Ib, aph(6)-Id, sul2, tet(B) | JAETYO000000000 |
| AT 07 | ATC 7-7 | Horse | O166:H28 | 1819 | stx1c | stx2b | - | air, chuA, eilA, ehxA, gad, iha, hra, ireA, iss, iucC, iutA, kpsE, ompT, senB, sitA, subA, terC, traT | - | JAETYU000000000 |
| AT 36 | ATC 36-6 | Chicken | O176:H4 | 57 | stx1c | - | - | chuA, espI, fyuA, ehxA, gad, iha, ireA, irp2, iss, kpsE, subA, terC | - | JAETYQ000000000 |
| AT 06 | ATB 6-118 | Beef | O183:H18 | 657 | stx1a | stx2a | - | chuA, cvaC, epeA, espP, ehxA, iha, iss, lpfA, ompT, subA, terC, traT | aph(3″)-Ib, aph(6)-Id, blaTEM-1 | JAEUYO000000000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Treier, A.; Stephan, R.; Stevens, M.J.A.; Cernela, N.; Nüesch-Inderbinen, M. High Occurrence of Shiga Toxin-Producing Escherichia coli in Raw Meat-Based Diets for Companion Animals—A Public Health Issue. Microorganisms 2021, 9, 1556. https://doi.org/10.3390/microorganisms9081556
Treier A, Stephan R, Stevens MJA, Cernela N, Nüesch-Inderbinen M. High Occurrence of Shiga Toxin-Producing Escherichia coli in Raw Meat-Based Diets for Companion Animals—A Public Health Issue. Microorganisms. 2021; 9(8):1556. https://doi.org/10.3390/microorganisms9081556
Chicago/Turabian StyleTreier, Andrea, Roger Stephan, Marc J. A. Stevens, Nicole Cernela, and Magdalena Nüesch-Inderbinen. 2021. "High Occurrence of Shiga Toxin-Producing Escherichia coli in Raw Meat-Based Diets for Companion Animals—A Public Health Issue" Microorganisms 9, no. 8: 1556. https://doi.org/10.3390/microorganisms9081556
APA StyleTreier, A., Stephan, R., Stevens, M. J. A., Cernela, N., & Nüesch-Inderbinen, M. (2021). High Occurrence of Shiga Toxin-Producing Escherichia coli in Raw Meat-Based Diets for Companion Animals—A Public Health Issue. Microorganisms, 9(8), 1556. https://doi.org/10.3390/microorganisms9081556






