Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Physicochemical Properties of Lactobacillus Strains
2.2.1. Cell Surface Hydrophobicity
2.2.2. Auto-Aggregation
2.3. NaCl Tolerance Assay
2.4. Caco-2 Cells Assay
2.5. Quantification of Adherence of Lactobacillus Strains
2.6. Adhesion Inhibition of Pathogen Bacteria by Lactobacillus Strains
2.7. Antimicrobial Activity of Cell-Free Supernatants (CFSs)
Preparation CFSs from Lactobacillus Strains
2.8. Immunomodulatory Activitie of Lactobacillus Strains on the Secretion of IL-8 and NO-Induced by LPS on Caco-2 Cells
2.9. Inflammatory Stress Protection Induced by Lactobacillus Strains
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Properties of Lactobacillus Strains
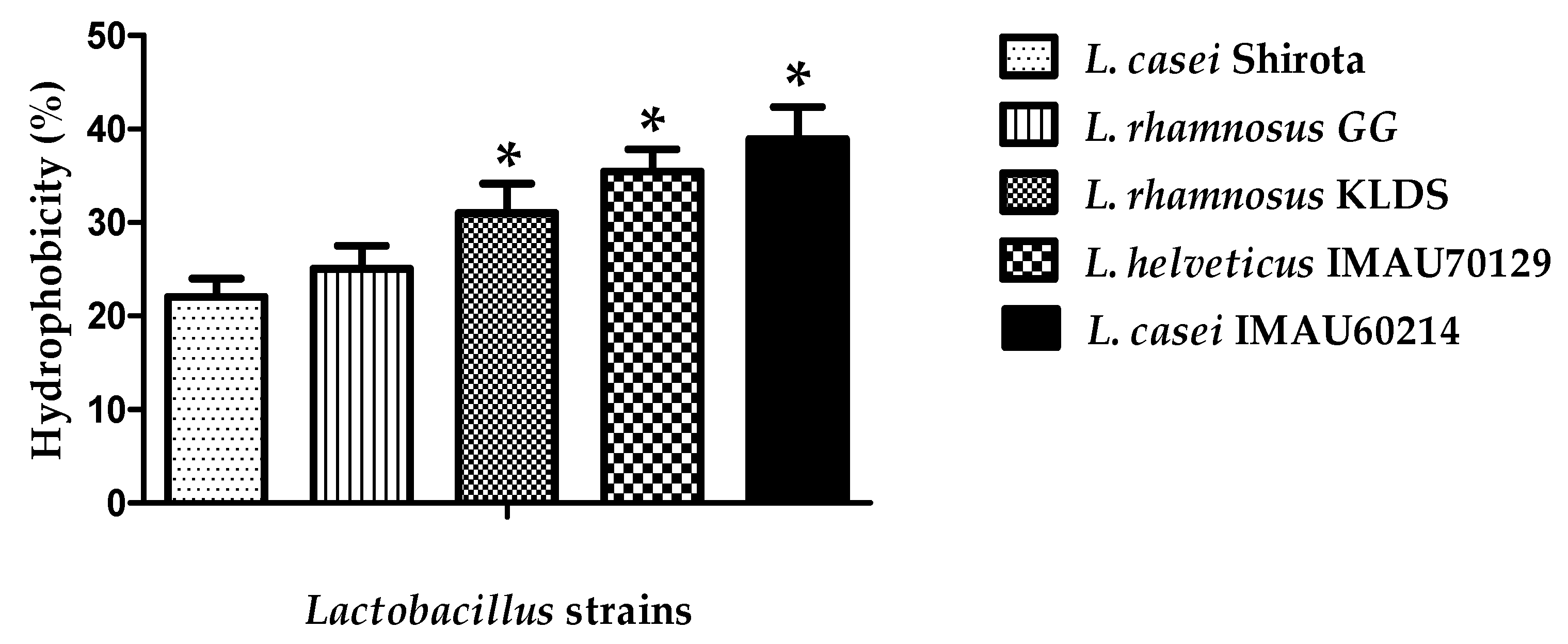
3.1.1. Cell Surface Hydrophobicity
3.1.2. Auto-Aggregation
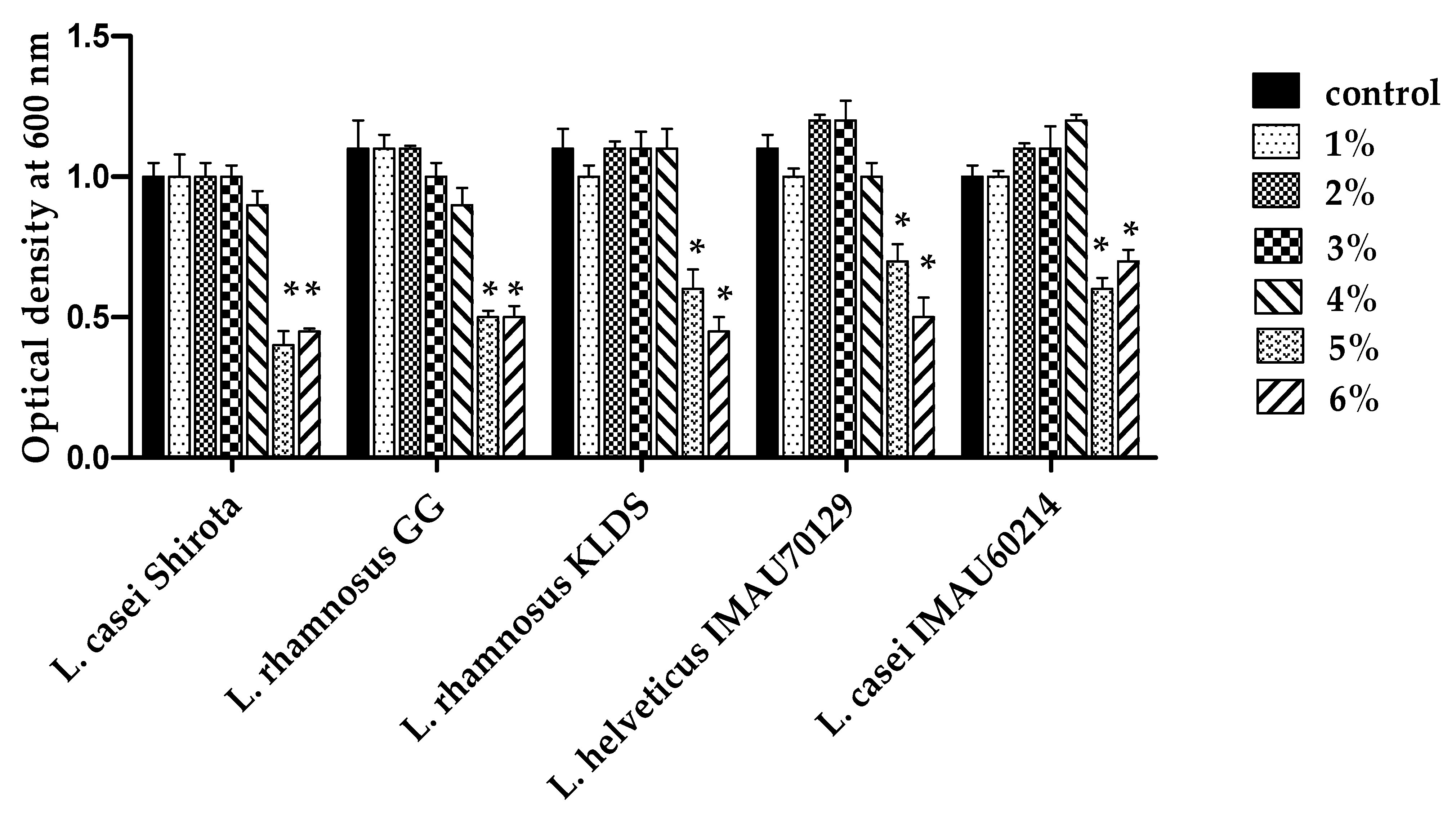
3.2. NaCL Tolerance
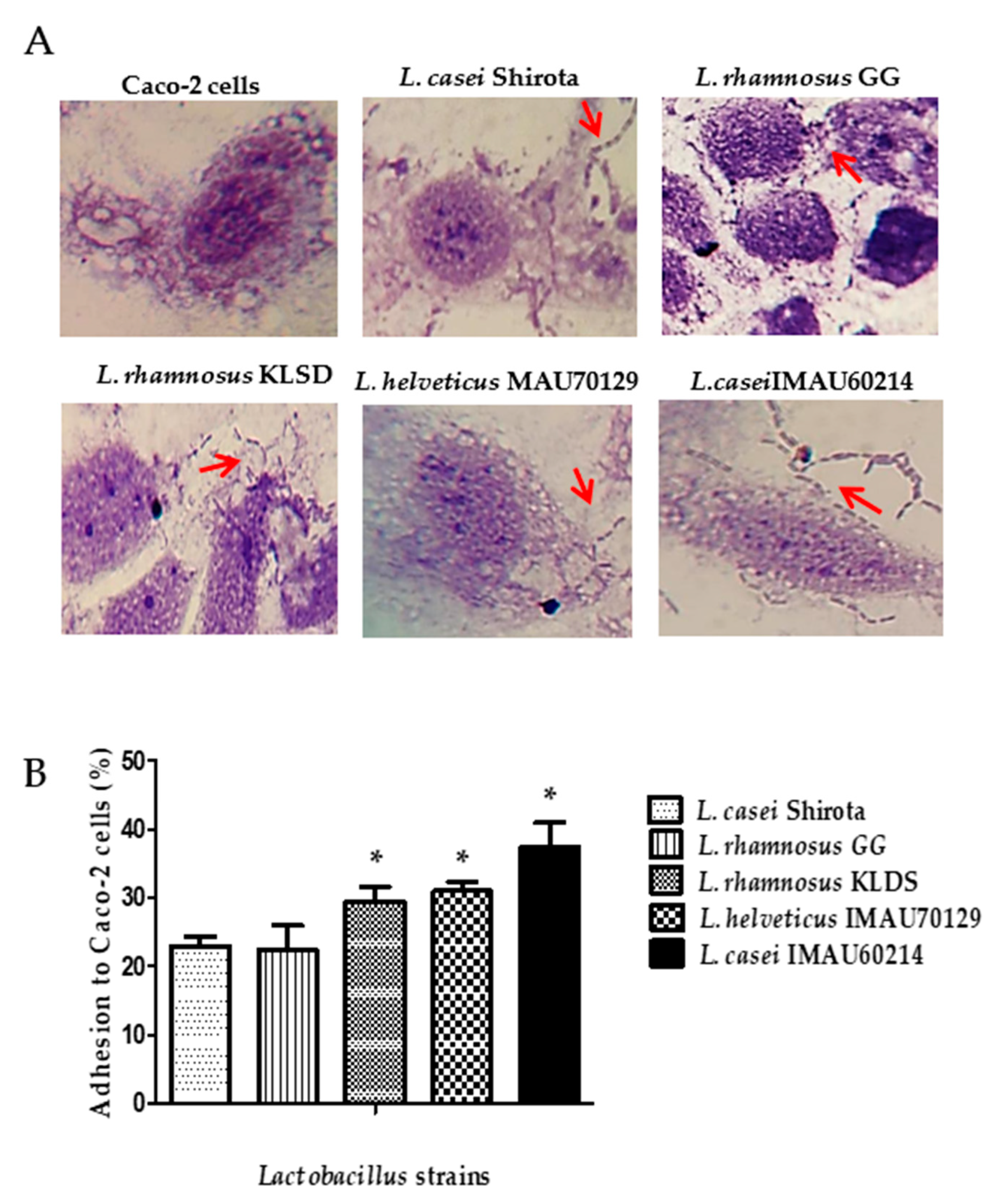
3.3. Caco-2 Cells Adherence Assay
3.4. Adherence Inhibition of Pathogen Bacteria by Lactobacillus Strains
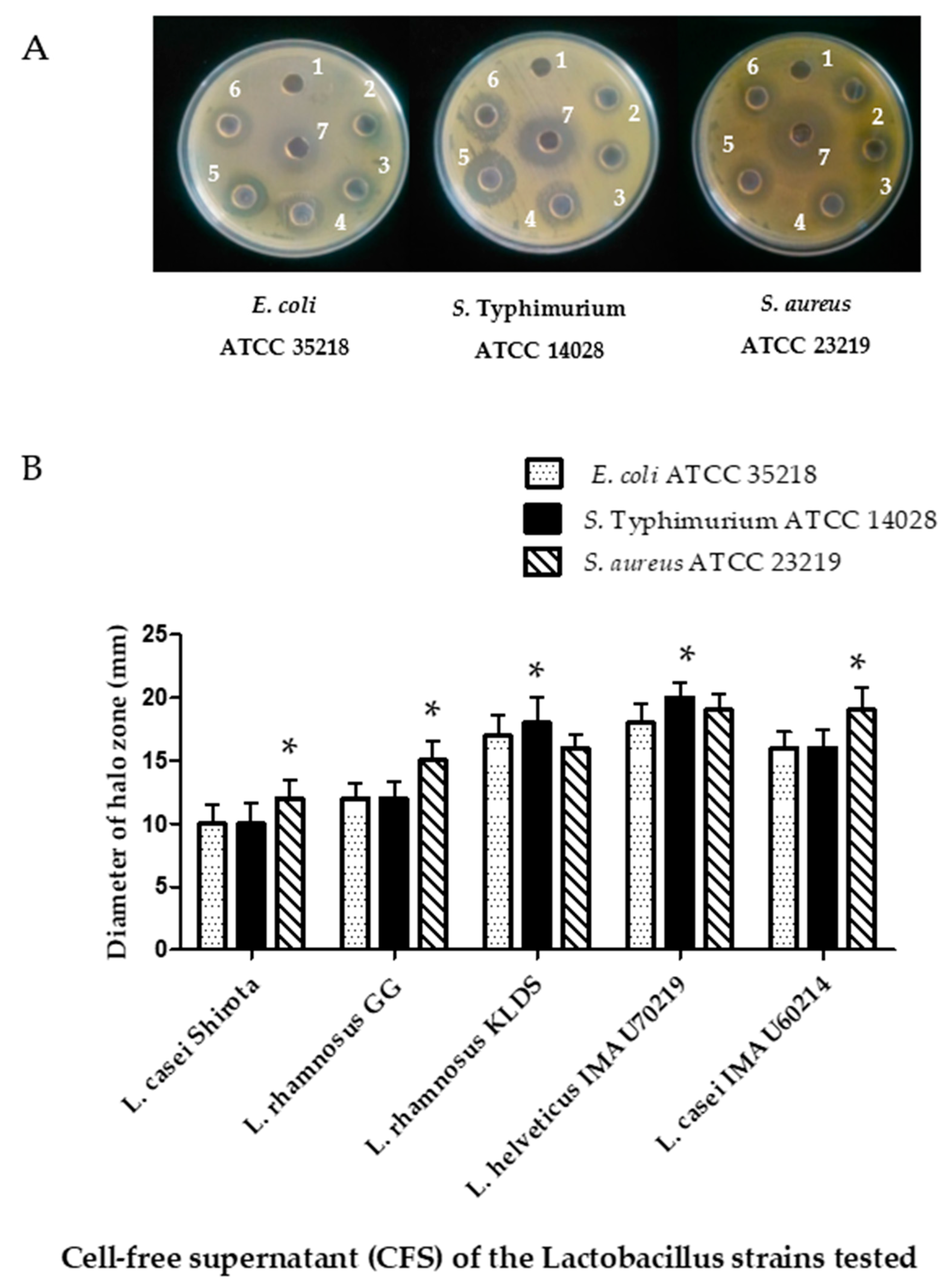
3.5. Antimicrobial Activity of Cell-Free Supernatants (CFSs) from Lactobacillus Strains
3.6. Immunomodulatory Activity of Lactobacillus Strains on the Secretion of IL-8 and NO-Induced by LPS on Caco-2 Cells
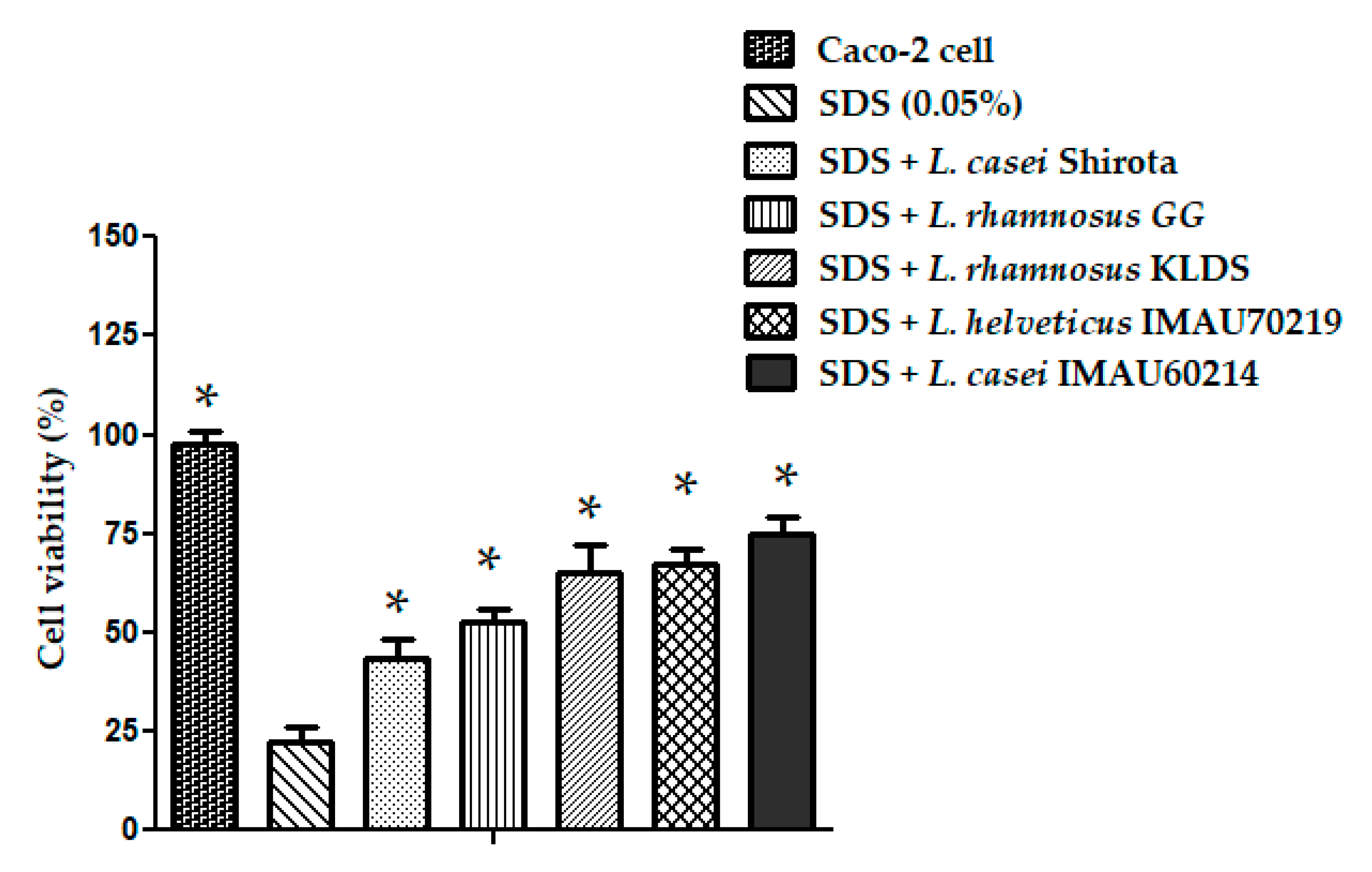
3.7. Inflammatory Stress Protection Induced by Lactobacillus Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Liévin, L.; Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious agents. Clin. Microbiol. Rev. 2014, 2, 167–199. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 16, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Lawley, T.D. Therapeutic modulation of intestinal dysbiosis. Pharmacol. Res. 2013, 1, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of thegenus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef]
- Duary, R.K.; Batish, V.K.; Grover, S. Immunomodulatory activity of two potential probiotic strains in LPS-stimulated HT-29 cells. Genes Nutr. 2014, 9, 398. [Google Scholar] [CrossRef]
- Reid, G. The growth potential for dairy probiotics. Int. Dairy J. 2015, 49, 16–22. [Google Scholar] [CrossRef]
- Goto, Y. Epithelial Cells as a Transmitter of Signals from Commensal Bacteria and Host Immune Cells. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 2003, 4, 658–672. [Google Scholar] [CrossRef]
- Chung, Y.W.; Choi, J.H.; Oh, T.Y.; Eun, C.S.; Han, D.S. Lactobacillus casei prevents the development of dextran sulphate sodium-induced colitis in Toll-like receptor 4 mutant mice. Clin. Exp. Immunol. 2008, 1, 182–189. [Google Scholar] [CrossRef]
- Tien, M.T.; Girardin, S.E.; Regnault, B.; Le Bourhis, L.; Dillies, M.A.; Coppée, J.Y.; Bourdet-Sicard, R.; Sansonetti, P.J.; Pédron, T. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 2006, 2, 1228–1237. [Google Scholar] [CrossRef]
- Kaji, R.; Kiyoshima-Shibata, J.; Tsujibe, S.; Nanno, M.; Shida, K. Short communication: Probiotic induction of interleukin-10 and interleukin-12 production by macrophages is modulated by co-stimulation with microbial components. J. Dairy Sci. 2018, 4, 2838–2841. [Google Scholar] [CrossRef]
- Lai, H.H.; Chiu, C.H.; Kong, M.S.; Chang, C.J.; Chen, C.C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019, 5, 1150. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Faseleh, J.M.; Ho, Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.Y.; Li, C.; Qin, Y.Q.; Yin, R.L.; Du, S.W.; Ye, F.; Liu, H.F.; Wang, M.P.; Sun, Y.; Li, X.; et al. Lactobacilli reduce chemokine IL-8 production in response to TNF-α and Salmonella challenge of Caco-2 cells. BioMed Res. Int. 2013, 2013, 925219. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Laureano, R.; Fani, L.; Tascini, C.; Galano, A.; Antonelli, A.; Rossolini, G.M. Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: Case report and review of the literature. Infection 2015, 6, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Ramírez, L.M.; Pérez-Solano, R.A.; Castañón-Alonso, S.L.; Moreno Guerrero, S.S.; Ramírez-Pacheco, A.; García Garibay, M.; Eslava, C. Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages. J. Immunol. Res. 2017, 2017, 4607491. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guerrero, A.; Hernández-Sánchez, H.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M.; Figueroa-González, I. Commercial probiotic bacteria and prebiotic carbohydrates: A fundamental study on prebiotics uptake, antimicrobials production and inhibition of pathogens. J. Sci. Food. Agric. 2014, 94, 2246–2252. [Google Scholar] [CrossRef]
- Kotzamanidis, C.; Kourelis, A.; Litopoulou-Tzanetaki, E.; Tzanetakis, N.; Yiangou, M. Evaluation of adhesion capacity, cell surface traits and immunomodulatory activity of presumptive probiotic Lactobacillus strains. Int. J. Food. Microbiol. 2010, 140, 154–163. [Google Scholar] [CrossRef]
- Archer, A.C.; Halami, P.M. Probiotic attributes of Lactobacillus fermentum isolated from human feces and dairy products. Appl. Microbiol. Biotechnol. 2015, 99, 8113–8123. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, F.; Wan, C.; Xiong, Y.; Shah, N.P.; Wei, H.; Tao, X. Evaluation of probiotic properties of Lactobacillus plantarum WLPL04 isolated from human breast milk. J. Dairy Sci. 2016, 99, 1736–1746. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Q.; Zhang, Y.; Ma, W.; Ning, K.; Xiang, J.Y.; Cui, J.; Xiang, H. Combination of probiotics with different functions alleviate DSS-induced colitis by regulating intestinal microbiota, IL-10, and barrier function. Appl. Microbiol. Biotechnol. 2020, 104, 335–349. [Google Scholar] [CrossRef]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, L.; Jiang, Z. Dietary Additive Probiotics Modulation of the Intestinal Microbiota. Protein. Pept. Lett. 2017, 24, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Kich, D.M.; Vincenzi, A.; Majolo, F.; Volken de Souza, C.F.; Goettert, M.I. Probiotic: Effectiveness nutrition in cancer treatment and prevention. Nutr. Hosp. 2016, 33, 1430–1437. [Google Scholar] [CrossRef]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef]
- Casellas, F.; Burgos, R.; Marcos, A.; Santos, J.; Ciriza-de-Los-Ríos, C.; García-Manzanares, Á.; Polanco, I.; Puy-Portillo, M.; Villarino, A.; Lema-Marqués, B.; et al. Consensus document on exclusion diets in irritable bowel syndrome (IBS). Nutr. Hosp. 2018, 35, 1450–1466. [Google Scholar] [CrossRef]
- Liu, J.; Hu, D.; Chen, Y.; Huang, H.; Zhang, H.; Zhao, J.; Gu, Z.; Chen, W. Strain-specific properties of Lactobacillus plantarum for prevention of Salmonella infection. Food Funct. 2018, 9, 3673–3682. [Google Scholar] [CrossRef]
- Archer, A.C.; Kurrey, N.K.; Halami, P.M. In vitro adhesion and anti-inflammatory properties of native Lactobacillus fermentum and Lactobacillus delbrueckii spp. J. Appl. Microbiol. 2018, 125, 243–256. [Google Scholar] [CrossRef]
- Castellanos, T.; Ascencio, F.; Bashan, Y. Cell-surface hydrophobicity and cell-surface charge of Azospirillum spp. FEMS Microbiol. Ecol. 1997, 24, 159–172. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jeong, H.S.; Lee, H.Y.; Ahn, J. Assessment of cell surface properties and adhesion potential of selected probiotic strains. Lett. Appl. Microbiol. 2009, 49, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Rokana, N.; Singh, B.P.; Thakur, N.; Sharma, C.; Gulhane, R.D.; Panwar, H. Screening of cell surface properties of potential probiotic lactobacilli isolated from human milk. J. Dairy Res. 2018, 85, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef] [PubMed]
- Panicker, A.S.; Ali, S.A.; Anand, S.; Panjagari, N.R.; Kumar, S.; Mohanty, A.K.; Behare, P.V. Evaluation of some in vitro probiotic properties of Lactobacillus fermentum Strains. J. Food Sci. Technol. 2018, 55, 2801–2807. [Google Scholar] [CrossRef]
- Dos Santos, A.S.; de Albuquerque, T.M.R.; de Brito Alves, J.L.; de Souza, E.L. Effects of Quercetin and Resveratrol on in vitro Properties Related to the Functionality of Potentially Probiotic Lactobacillus Strains. Front. Microbiol. 2019, 24, 2229. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.K.; Hasslöf, P.; Stecksén-Blicks, C.; Twetman, S. Co-aggregation and growth inhibition of probiotic lactobacilli and clinical isolates of mutans streptococci: An in vitro study. Acta Odontol. Scand. 2011, 69, 263–268. [Google Scholar] [CrossRef]
- Matsuzaki, T. Immunomodulation by treatment with Lactobacillus casei strain Shirota. Int. J. Food Microbiol. 1998, 26, 133–140. [Google Scholar] [CrossRef]
- Kanmani, P.; Kim, H. Functional capabilities of probiotic strains on attenuation of intestinal epithelial cell inflammatory response induced by TLR4 stimuli. Biofactors 2019, 45, 223–235. [Google Scholar] [CrossRef]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy. Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Li, P.L.; Liu, Z. The correlation between surface hydrophobicity and adherence of Bifidobacterium strains from centenarians’ faeces. Anaerobe 2006, 3, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ismail, M.B.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 9, fnv047. [Google Scholar] [CrossRef] [PubMed]
- Vlková, E.; Rada, V.; Smehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in bifidobacteria and clostridia. Folia Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Malago, J.J.; Tooten, P.C.; Koninkx, J.F. Anti-inflammatory properties of probiotic bacteria on Salmonella-induced IL-8 synthesis in enterocyte-like Caco-2 cells. Benef. Microbes 2010, 1, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, H.C.; de Sousa, M.D.; Ramos, C.L.; Días, D.R.; Schwan, R.F. Probiotic Properties of Lactobacilli and Their Ability to Inhibit the Adhesion of Enteropathogenic Bacteria to Caco-2 and HT-29 Cells. Probiotics Antimicrob. Proteins 2021, 13, 102–112. [Google Scholar] [CrossRef]
- Alizadeh, B.B.; Noshad, M.; Falah, F. Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb. Pathog. 2019, 136, 103677. [Google Scholar] [CrossRef]
- Sunanliganon, C.; Thong-Ngam, D.; Tumwasorn, S.; Klaikeaw, N. Lactobacillus plantarum B7 inhibits Helicobacter pylori growth and attenuates gastric inflammation. World J. Gastroenterol. 2012, 18, 2472–2480. [Google Scholar] [CrossRef]
- Jankowska, A.; Laubitz, D.; Antushevich, H.; Zabielski, R.; Grzesiuk, E. Competition of Lactobacillus paracasei with Salmonella enterica for adhesion to Caco-2 cells. J. Biomed. Biotechnol. 2008, 2008, 357964. [Google Scholar] [CrossRef] [PubMed]
- Tabashsum, Z.; Peng, M.; Alvarado-Martinez, Z.; Aditya, A.; Bhatti, J.; Romo, P.B.; Young, A.; Biswas, D. Competitive reduction of poultry-borne enteric bacterial pathogens in chicken gut with bioactive Lactobacillus casei. Sci. Rep. 2020, 10, 16259. [Google Scholar] [CrossRef] [PubMed]
- Boonma, P.; Spinler, J.K.; Venable, S.F.; Versalovic, J.; Tumwasorn, S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Perdigon, G.; Alvarez, S.; Rachid, M.; Agüero, G.; Gobbato, N. Immune system stimulation by probiotics. J. Dairy Sci. 1995, 78, 1597–1606. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Ramírez, L.M.; Hernández-Chiñas, U.; Moreno-Guerrero, S.S.; Ramírez-Pacheco, A.; Eslava, C.A. Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products. Microorganisms 2021, 9, 825. https://doi.org/10.3390/microorganisms9040825
Rocha-Ramírez LM, Hernández-Chiñas U, Moreno-Guerrero SS, Ramírez-Pacheco A, Eslava CA. Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products. Microorganisms. 2021; 9(4):825. https://doi.org/10.3390/microorganisms9040825
Chicago/Turabian StyleRocha-Ramírez, Luz María, Ulises Hernández-Chiñas, Silvia Selene Moreno-Guerrero, Arturo Ramírez-Pacheco, and Carlos A. Eslava. 2021. "Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products" Microorganisms 9, no. 4: 825. https://doi.org/10.3390/microorganisms9040825
APA StyleRocha-Ramírez, L. M., Hernández-Chiñas, U., Moreno-Guerrero, S. S., Ramírez-Pacheco, A., & Eslava, C. A. (2021). Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products. Microorganisms, 9(4), 825. https://doi.org/10.3390/microorganisms9040825





