Contribution of the Locus of Heat Resistance to Growth and Survival of Escherichia coli at Alkaline pH and at Alkaline pH in the Presence of Chlorine
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Growth Conditions
2.2. Construction of Derivatives of E. coli MG1655
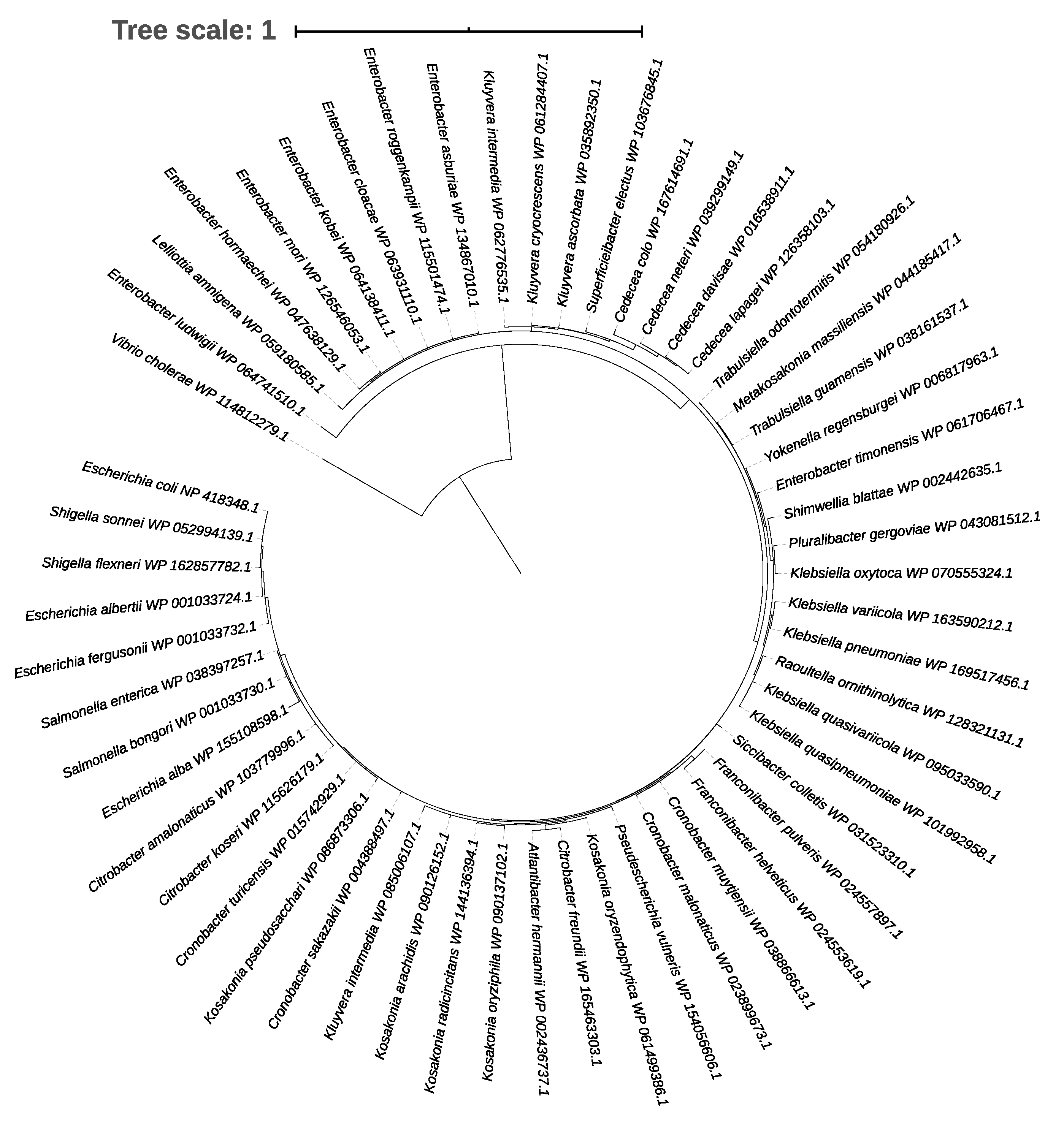
2.3. Phylogenetic Tree of the CpxR Response Regulator
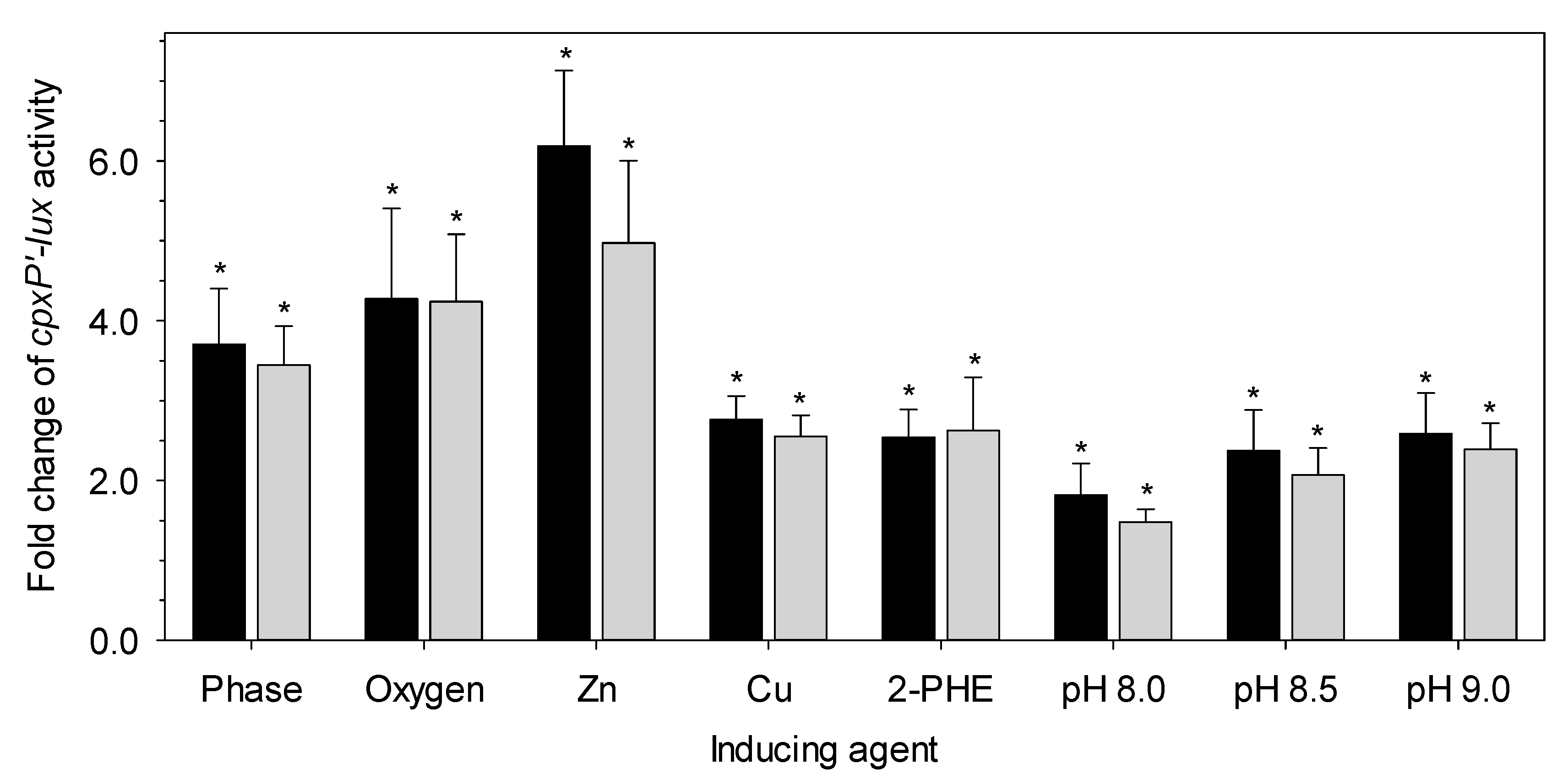
2.4. Determination of the Cpx Pathway by Bioluminescence Assay
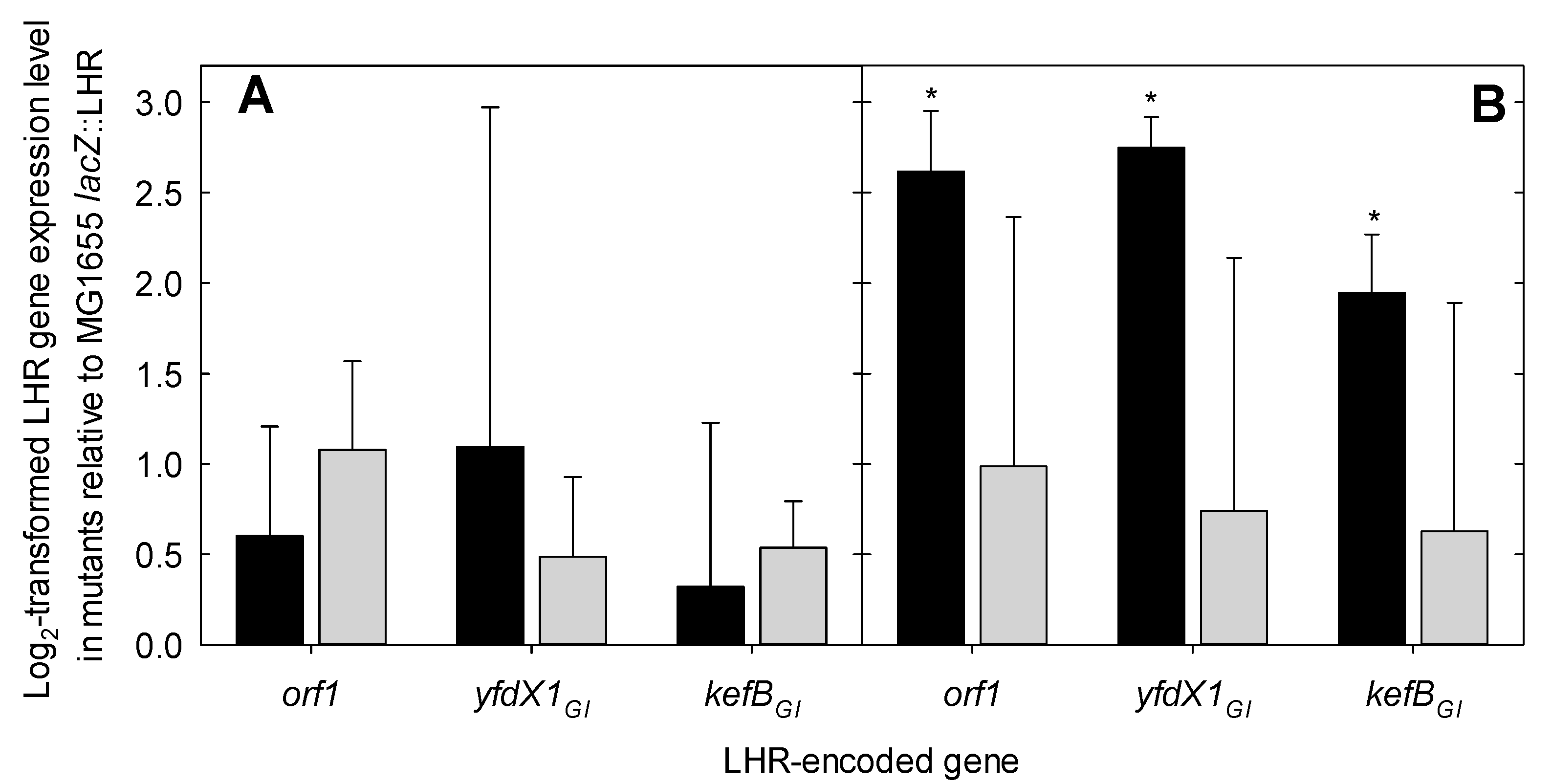
2.5. Measurement of LHR Gene Expression by RT-qPCR
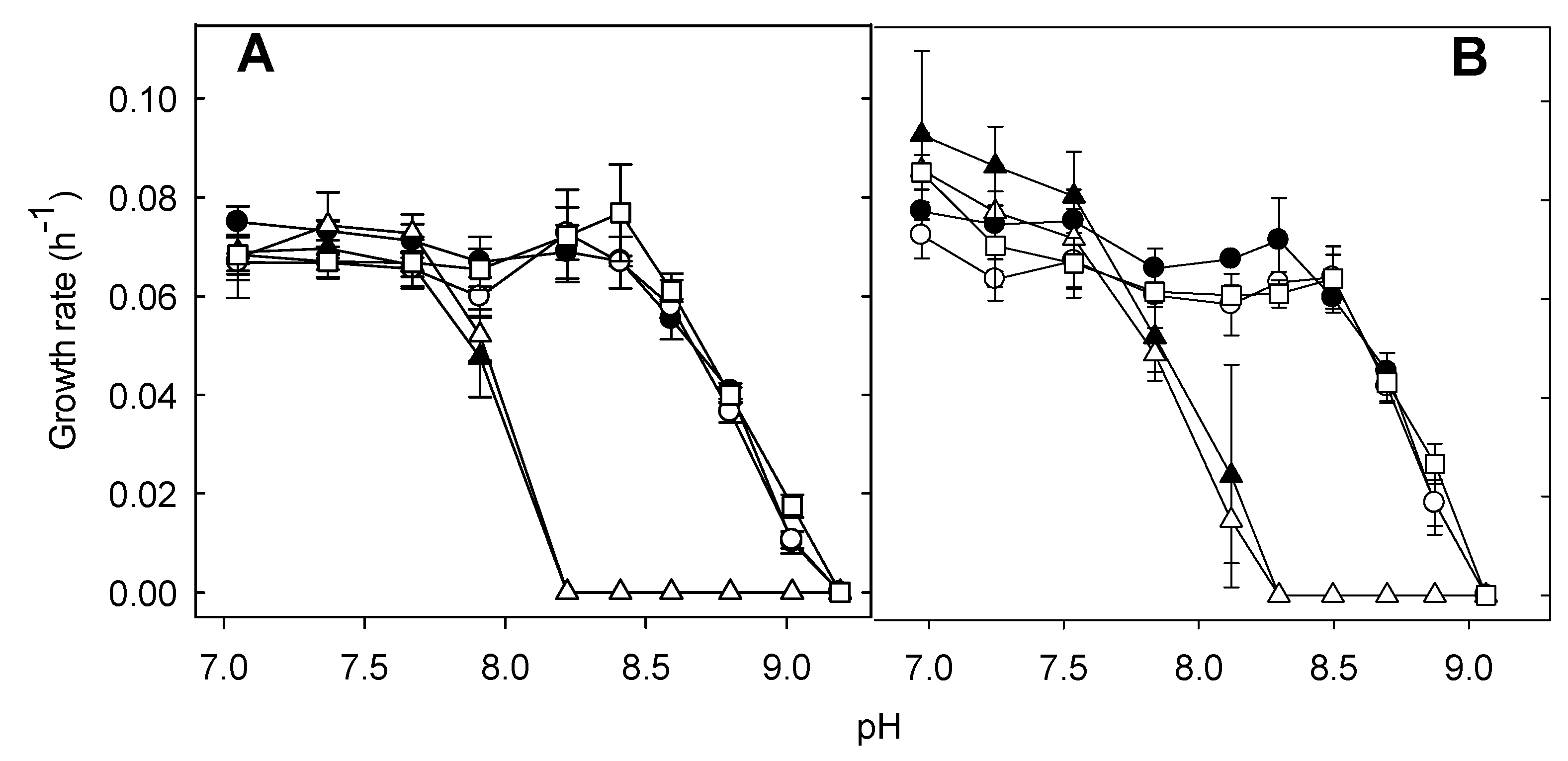
2.6. Determination of the Growth Rates
2.7. Determination of the Tolerance to Extreme Alkaline pH with or without Chlorine Treatment
2.8. Statistical Analysis
3. Results
3.1. Coexistence of the CpxR Response Regulator and the LHR
3.2. The Presence of the LHR Does Not Alter Cpx Pathway Activity
3.3. LHR Transcriptional Level Is Affected by CpxR but Not by the EvgA Response Regulator at Alkaline pH
3.4. Cpx but Not the LHR Is Necessary for Growth in Alkaline pH
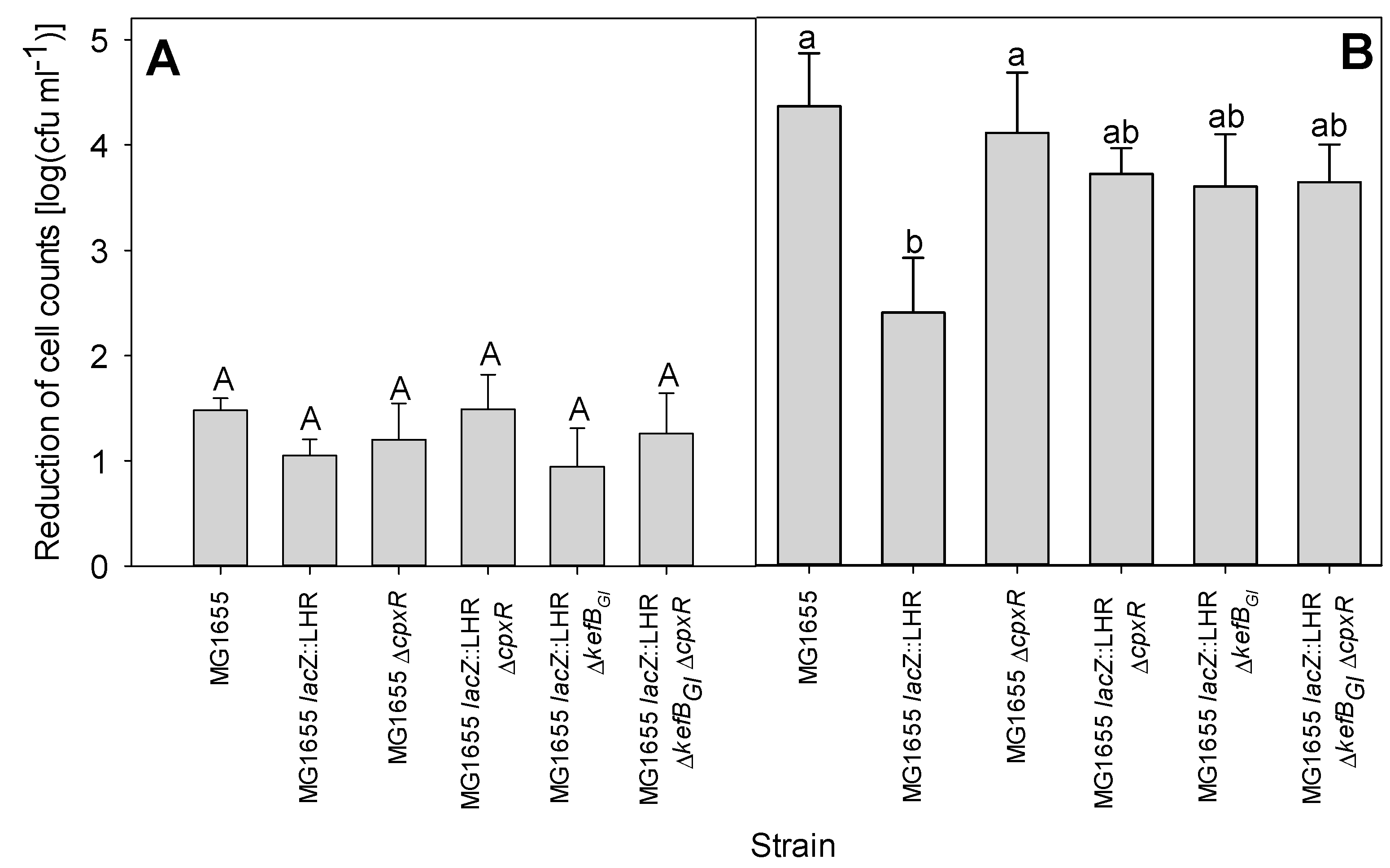
3.5. LHR Improves Bacterial Survival to Extreme Alkaline pH in the Presence of Chlorine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, J.R.; Russo, T.A. Extraintestinal pathogenic Escherichia coli: “The other bad E coli”. J. Lab. Clin. Med. 2002, 139, 155–162. [Google Scholar] [CrossRef]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent Advances in Understanding Enteric Pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.A.; Rubinelli, P.M.; Park, S.H.; Carbonero, F.; Ricke, S.C. Shiga toxin-producing Escherichia coli in food: Incidence, ecology, and detection strategies. Food Control 2016, 59, 407–419. [Google Scholar] [CrossRef]
- Sharma, M.; Beuchat, L.R. Sensitivity of Escherichia coli O157:H7 to Commercially Available Alkaline Cleaners and Subsequent Resistance to Heat and Sanitizers. Appl. Environ. Microbiol. 2004, 70, 1795–1803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hurst, J.K.; Barrette, W.C.; Michel, B.R.; Rosen, H. Hypochlorous acid and myeloperoxidase-catalyzed oxidation of iron-slfur clusters in bacterial respiratory dehydrogenases. JBIC J. Biol. Inorg. Chem. 1991, 202, 1275–1282. [Google Scholar] [CrossRef]
- Dukan, S.; Touati, D. Hypochlorous acid stress in Escherichia coli: Resistance, DNA damage, and comparison with hydrogen peroxide stress. J. Bacteriol. 1996, 178, 6145–6150. [Google Scholar] [CrossRef]
- Venkobachar, C.; Iyengar, L.; Rao, A.P. Mechanism of disinfection: Effect of chlorine on cell membrane functions. Water Res. 1977, 11, 727–729. [Google Scholar] [CrossRef]
- Mendonca, A.F.; Amoroso, T.L.; Knabel, S.J. Destruction of gram-negative food-borne pathogens by high pH involves disruption of the cytoplasmic membrane. Appl. Environ. Microbiol. 1994, 60, 4009–4014. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, Y.; Zhi, S.; Simpson, D.J.; Gill, A.; McMullen, L.M.; Neumann, N.F.; Gänzle, M.G. The Locus of Heat Resistance Confers Resistance to Chlorine and Other Oxidizing Chemicals in Escherichia coli. Appl. Environ. Microbiol. 2019, 86, e02123-19. [Google Scholar] [CrossRef]
- Zhi, S.; Banting, G.; Li, Q.; Edge, T.A.; Topp, E.; Sokurenko, M.; Scott, C.; Braithwaite, S.; Ruecker, N.J.; Yasui, Y.; et al. Evidence of Naturalized Stress-Tolerant Strains of Escherichia coli in Municipal Wastewater Treatment Plants. Appl. Environ. Microbiol. 2016, 82, 5505–5518. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.G.; Zheng, J.; Garcia-Hernandez, R.; Ruan, L.; Gänzle, M.G.; McMullen, L.M. Genetic determinants of heat resistance in Escherichia coli. Front. Microbiol. 2015, 6, 932. [Google Scholar] [CrossRef] [PubMed]
- Boll, E.J.; Marti, R.; Hasman, H.; Overballe-Petersen, S.; Stegger, M.; Ng, K.; Knøchel, S.; Krogfelt, K.A.; Hummerjohann, J.; Struve, C. Turn Up the Heat—Food and Clinical Escherichia coli Isolates Feature Two Transferrable Loci of Heat Resistance. Front. Microbiol. 2017, 8, 579. [Google Scholar] [CrossRef]
- Mercer, R.; Nguyen, O.; Ou, Q.; McMullen, L.; Gänzle, M.G. Functional Analysis of Genes Comprising the Locus of Heat Resistance in Escherichia coli. Appl. Environ. Microbiol. 2017, 83, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Franke, K.B.; Kamal, S.M.; Kim, H.; Lünsdorf, H.; Jäger, J.; Nimtz, M.; Trček, J.; Jänsch, L.; Bukau, B.; et al. Stand-alone ClpG disaggregase confers superior heat tolerance to bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, E273–E282. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mercer, R.; Behr, J.; Heinzlmeir, S.; McMullen, L.M.; Vogel, R.F.; Gänzle, M.G. Heat and Pressure Resistance in Escherichia coli Relates to Protein Folding and Aggregation. Front. Microbiol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Haslbeck, M. Small Heat Shock Proteins in Bacteria, 1st ed.; de Bruijn, F.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 2, ISBN 9781119004813. [Google Scholar]
- Lee, C.; Wigren, E.; Trček, J.; Peters, V.; Kim, J.; Hasni, M.S.; Nimtz, M.; Lindqvist, Y.; Park, C.; Curth, U.; et al. A novel protein quality control mechanism contributes to heat shock resistance of worldwide-distributed Pseudomonas aeruginosa clone C strains. Environ. Microbiol. 2015, 17, 4511–4526. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Manna, C.; Chakrabarti, J.; Ghosh, M. Reversible thermal unfolding of a yfdX protein with chaperone-like activity. Sci. Rep. 2016, 6, 29541. [Google Scholar] [CrossRef]
- Nishino, K.; Yamaguchi, A. Overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J. Bacteriol. 2001, 183, 1455–1458. [Google Scholar] [CrossRef]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta Biomembr. 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Riet, K.V. T Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef]
- Ferguson, G.P.; McLaggan, D.; Booth, I.R. Potassium channel activation by glutathione-S-conjugates in Escherichia coli: Protection against methylglyoxal is mediated by cytoplasmic acidification. Mol. Microbiol. 1995, 17, 1025–1033. [Google Scholar] [CrossRef]
- Jianming, D.; Shiro, L.; Hoi-Shan, K.; Zhe, L.; Lin, E. The deduced amino-acid sequence of the cloned cpxR gene suggests the protein is the cognate regulator for the membrane sensor, CpxA, in a two-component signal transduction system of Escherichia coli. Gene 1993, 136, 227–230. [Google Scholar] [CrossRef]
- Weber, R.F.; Silverman, P.M. The Cpx proteins of Escherichia coli K12. J. Mol. Biol. 1988, 203, 467–478. [Google Scholar] [CrossRef]
- Raivio, T.L.; Silhavy, T.J. The σE and Cpx regulatory pathways: Overlapping but distinct envelope stress responses. Curr. Opin. Microbiol. 1999, 2, 159–165. [Google Scholar] [CrossRef]
- Raivio, T.L.; Leblanc, S.K.D.; Price, N.L. The Escherichia coli Cpx Envelope Stress Response Regulates Genes of Diverse Function that Impact Antibiotic Resistance and Membrane Integrity. J. Bacteriol. 2013, 195, 2755–2767. [Google Scholar] [CrossRef]
- MacRitchie, D.M.; Ward, J.D.; Nevesinjac, A.Z.; Raivio, T.L. Activation of the Cpx Envelope Stress Response Down-Regulates Expression of Several Locus of Enterocyte Effacement-Encoded Genes in Enteropathogenic Escherichia coli. Infect. Immun. 2008, 76, 1465–1475. [Google Scholar] [CrossRef][Green Version]
- Reisch, C.R.; Prather, K.L.J. The no-SCAR (Scarless Cas9 Assisted Recombineering) system for genome editing in Escherichia coli. Sci. Rep. 2015, 5, 15096. [Google Scholar] [CrossRef]
- Cherepanov, P.P.; Wackernagel, W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 1995, 158, 9–14. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Thomason, L.C.; Costantino, N.; Court, D.L. E. coli Genome Manipulation by P1 Transduction. Curr. Protoc. Mol. Biol. 2007. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2. [Google Scholar] [CrossRef]
- Ma, A.; Chui, L. Identification of heat resistant Escherichia coli by qPCR for the locus of heat resistance. J. Microbiol. Methods 2017, 133, 87–89. [Google Scholar] [CrossRef]
- Fang, Y.; Mercer, R.G.; McMullen, L.M.; Gänzle, M.G. Induction of Shiga Toxin-Encoding Prophage by Abiotic Environmental Stress in Food. Appl. Environ. Microbiol. 2017, 83, e01378-17. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef]
- Price, N.L.; Raivio, T.L. Characterization of the Cpx Regulon in Escherichia coli Strain MC4100. J. Bacteriol. 2008, 191, 1798–1815. [Google Scholar] [CrossRef]
- De Wulf, P.; Kwon, O.; Lin, E.C.C. The CpxRA Signal Transduction System of Escherichia coli: Growth-Related Autoactivation and Control of Unanticipated Target Operons. J. Bacteriol. 1999, 181, 6772–6778. [Google Scholar] [CrossRef]
- Nakayama, S.; Watanabe, H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 1995, 177, 5062–5069. [Google Scholar] [CrossRef] [PubMed]
- Danese, P.N.; Silhavy, T.J. CpxP, a Stress-Combative Member of the Cpx Regulon. J. Bacteriol. 1998, 180, 831–839. [Google Scholar] [CrossRef]
- Lee, L.J.; Barrett, J.A.; Poole, R.K. Genome-Wide Transcriptional Response of Chemostat-Cultured Escherichia coli to Zinc. J. Bacteriol. 2005, 187, 1124–1134. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishihama, A. Characterization of Copper-Inducible Promoters Regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 2006, 70, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.J.; Voigt, C.A. Characterization of combinatorial patterns generated by multiple two-component sensors in E. coli that respond to many stimuli. Biotechnol. Bioeng. 2011, 108, 666–675. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Munro, A.W.; Douglas, R.M.; McLaggan, D.; Booth, I.R. Activation of potassium channels during metabolite detoxification in Escherichia coli. Mol. Microbiol. 1993, 9, 1297–1303. [Google Scholar] [CrossRef]
- Masuda, N.; Church, G.M. Escherichia coli Gene Expression Responsive to Levels of the Response Regulator EvgA. J. Bacteriol. 2002, 184, 6225–6234. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, S.; Kim, J.-S.; Lee, H.-R.; Shin, H.-C.; Lee, M.-S.; Jin, K.S.; Kim, C.-H.; Ku, B.; Ryu, C.-M.; et al. Structural and Physiological Exploration of Salmonella Typhi YfdX Uncovers Its Dual Function in Bacterial Antibiotic Stress and Virulence. Front. Microbiol. 2019, 9, 3329. [Google Scholar] [CrossRef]
- Liu, C.-J.; Lin, C.-T.; Chiang, J.-D.; Lin, C.-Y.; Tay, Y.-X.; Fan, L.-C.; Peng, K.-N.; Lin, C.-H.; Peng, H.-L. RcsB regulation of the YfdX-mediated acid stress response in Klebsiella pneumoniae CG43S3. PLoS ONE 2019, 14, e0212909. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.M.; Yohannes, E.; Bondurant, S.S.; Radmacher, M.; Slonczewski, J.L. pH Regulates Genes for Flagellar Motility, Catabolism, and Oxidative Stress in Escherichia coli K-12. J. Bacteriol. 2005, 187, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, M.J.; Glenn, A.R. Problems of Adverse pH and Bacterial Strategies to Combat It; Wiley: Chichester, UK, 1999. [Google Scholar]
- Hicks, D.B.; Liu, J.; Fujisawa, M.; Krulwich, T.A. F1F0-ATP synthases of alkaliphilic bacteria: Lessons from their adaptations. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1362–1377. [Google Scholar] [CrossRef]
- Mayer, C.; Uslar, M.; Suding, T.; Weidelt, T. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 2010, 184, 4246–4258. [Google Scholar] [CrossRef]
- Enomoto, K.; Koyama, N. Effect of Growth pH on the Phospholipid Contents of the Membranes from Alkaliphilic Bacteria. Curr. Microbiol. 1999, 39, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kadim, M.I.; Khoo, W.J.; Zheng, Q.; Setyawati, M.I.; Shin, Y.-J.; Lee, S.-C.; Yuk, H.-G. Membrane lipid composition and stress/virulence related gene expression of Salmonella Enteritidis cells adapted to lactic acid and trisodium phosphate and their resistance to lethal heat and acid stress. Int. J. Food Microbiol. 2014, 191, 24–31. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Ito, M.; Hicks, D.B.; Gilmour, R.; Guffanti, A.A. pH homeostasis and ATP synthesis: Studies of two processes that necessitate inward proton translocation in extremely alkaliphilic Bacillus species. Extremophiles 1998, 2, 217–222. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH Measurement and Homeostasis in Bacteria and Archaea; Elsevier: Amsterdam, The Netherlands, 2009; Volume 55, ISBN 9780123747907. [Google Scholar]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Genet. 2011, 9, 330–343. [Google Scholar] [CrossRef]
- Krulwich, T.A.; Hicks, D.B.; Ito, M. Cation/proton antiporter complements of bacteria: Why so large and diverse? Mol. Microbiol. 2009, 74, 257–260. [Google Scholar] [CrossRef]
- Nakamura, T.; Tokuda, H.; Unemoto, T. K+/H+ antiporter functions as a regulator of cytoplasmic pH in a marine bacterium, Vibrio alginolyticus. Biochim. Biophys. Acta Biomembr. 1984, 776, 330–336. [Google Scholar] [CrossRef]
- Padan, E.; Schuldiner, S. Molecular physiology of the Na+/H+ antiporter in Escherichia coli. J. Exp. Biol. 1994, 196, 443–456. [Google Scholar]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nat. Cell Biol. 2005, 435, 1197–1202. [Google Scholar] [CrossRef]
- Saito, H.; Kobayashi, H. Bacterial Responses to Alkaline Stress. Sci. Prog. 2003, 86, 271–282. [Google Scholar] [CrossRef]
- Dartigalongue, C.; Raina, S. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 1998, 17, 3968–3980. [Google Scholar] [CrossRef]
- López, C.; Checa, S.K.; Soncinia, F.C. CpxR/CpxA controls scsABCD transcription to counteract copper and oxidative stress in Salmonella enterica Serovar Typhimurium. J. Bacteriol. 2018, 200, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.P.; Booth, I.R. Importance of Glutathione for Growth and Survival of Escherichia coli Cells: Detoxification of Methylglyoxal and Maintenance of Intracellular K+. J. Bacteriol. 1998, 180, 4314–4318. [Google Scholar] [CrossRef]
- Elmore, M.J.; Lamb, A.J.; Ritchie, G.Y.; Douglas, R.M.; Munro, A.; Gajewska, A.; Booth, I.R. Activation potassium efflux from Escherichia coli by glutathione metabolites. Mol. Microbiol. 1990, 4, 405–412. [Google Scholar] [CrossRef]
- Roosild, T.P.; Castronovo, S.; Healy, J.; Miller, S.; Pliotas, C.; Rasmussen, T.; Bartlett, W.; Conway, S.J.; Booth, I.R. Mechanism of ligand-gated potassium efflux in bacterial pathogens. Proc. Natl. Acad. Sci. USA 2010, 107, 19784–19789. [Google Scholar] [CrossRef]
- Harwood, D.T.; Kettle, A.J.; Winterbourn, C.C. Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC–MS/MS method. Biochem. J. 2006, 399, 161–168. [Google Scholar] [CrossRef]
- Chesney, J.A.; Eaton, J.W.; Mahoney, J.R. Bacterial glutathione: A sacrificial defense against chlorine compounds. J. Bacteriol. 1996, 178, 2131–2135. [Google Scholar] [CrossRef]
- Smirnova, G. Effects of menadione and hydrogen peroxide on glutathione status in growing Escherichia coli. Free. Radic. Biol. Med. 2000, 28, 1009–1016. [Google Scholar] [CrossRef]
- Bhat, K.G.; Alex, K. Acid resistance in enteric bacteria. Natl. Med. J. India 1998, 11, 151–152. [Google Scholar]
- De Biase, D.; Lund, P.A. The Escherichia coli Acid Stress Response and Its Significance for Pathogenesis; Elsevier: Amsterdam, The Netherlands, 2015; Volume 92, ISBN 978-0-12-802249-8. [Google Scholar]
- Lund, P.; Tramonti, A.; De Biase, D. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef]
- Richard, H.; Foster, J.W. Escherichia coli Glutamate and Arginine-Dependent Acid Resistance Systems Increase Internal pH and Reverse Transmembrane Potential. J. Bacteriol. 2004, 186, 6032–6041. [Google Scholar] [CrossRef]
- Castanie-Cornet, M.-P.; Penfound, T.A.; Smith, D.; Elliott, J.F.; Foster, J.W. Control of Acid Resistance in Escherichia coli. J. Bacteriol. 1999, 181, 3525–3535. [Google Scholar] [CrossRef]
- Iyer, R.; Williams, C.; Miller, C. Arginine-Agmatine Antiporter in Extreme Acid Resistance in Escherichia coli. J. Bacteriol. 2003, 185, 6556–6561. [Google Scholar] [CrossRef]
- Meng, S.Y.; Bennett, G.N. Nucleotide sequence of the Escherichia coli cad operon: A system for neutralization of low extracellular pH. J. Bacteriol. 1992, 174, 2659–2669. [Google Scholar] [CrossRef][Green Version]
- Kern, R.; Malki, A.; Abdallah, J.; Tagourti, J.; Richarme, G. Escherichia coli HdeB Is an Acid Stress Chaperone. J. Bacteriol. 2006, 189, 603–610. [Google Scholar] [CrossRef]
| Strain/Plasmid | Description | Reference |
|---|---|---|
| E. coli MG1655 | E. coli K-12 derivatives | |
| E. coli MG1655 lacZ::LHR | Full-length LHR with its promoter inserted into MG1655 lacZ | This study |
| E. coli MG1655 ΔcpxR::Kan | E. coli MG1655 with chromosomal cpxR replaced by the kanamycin resistance cassette | This study |
| E. coli MG1655 lacZ::LHR ΔcpxR::Kan | E. coli MG1655 lacZ::LHR with chromosomal cpxR replaced by the kanamycin resistance cassette | This study |
| E. coli MG1655 lacZ::LHR ΔkefBGI::FRT | E. coli MG1655 lacZ::LHR with LHR kefBGI replaced by the FRT scar site | This study |
| E. coli MG1655 lacZ::LHR ΔkefBGI::FRT ΔcpxR::Kan | E. coli MG1655 lacZ::LHR with LHR kefBGI replaced by the FRT scar site and chromosomal cpxR replaced by the kanamycin resistance cassette | This study |
| E. coli MG1655 lacZ::LHR ΔevgA::Kan | E. coli MG1655 with chromosomal evgA replaced by the kanamycin resistance cassette | This study |
| pJW15 | Promoterless luminescence reporter plasmid containing luxCDABE operon, orip15A; Kanr | [28] |
| pJW25 | pJW15 plasmid containing cpxP promoter; Kanr | [28] |
| pLHR | Low-copy plasmid containing the LHR | [12] |
| pKDsg-lacZ | Plasmid containing crispr-targeting sequences for lacZ | This Study |
| pCas9cr4 | Plasmid with cas9 expressed under control of the PTET promoter | [29] |
| pCP20 | Plasmid enabling Flp-catalyzed excision of the antibiotic resistance gene | [30] |
| Primer | Sequence (5′-3′) | Ref. a) |
|---|---|---|
| sgRNA-lacZ-F | GGCCAGTGAATCCGTAATCAGTTTTAGAGCTAGAAATAGCAAG | |
| sgRNA-lacZ-R | TGATTACGGATTCACTGGCCGTGCTCAGTATCTCTATCACTGA | |
| Targeting sequence | GGCCAGTGAATCCGTAATCA | |
| LHR-16-F | CGGTATCGCCGTCGACGACG | |
| lacZ-upstream | GCTGTTGCCCGTCTCACTGG | |
| LHR-2-R | GCCGGAATTTCCCCGTGTGC | |
| lacZ-downstream | GGACGACGACAGTATCGGCC | |
| yfdX1-check-F | TCGGTAAAGAAAGCGGTCAAG | |
| yfdX1-check-R | CATCGGAAGGTTGTCGGTTT | |
| kefB-P2 | CATCGTGCGCTGGACGTCGACGCAAGTGGGACGCTGACCGATGGGAATTAGCCATGGTCC | |
| kefB-P1 | TGGTCACGTAAGACCTGAAATGGGTTAAGGCGTGTTGATTGTGTAGGCTGGAGCTGCTTC | |
| kefB-check-F | TTGCTGGGGTATCTCTCTGT | |
| kefB-check-R | CAGCCACATCAATAGCAGGA | |
| cpxR F | CTATGCGCATCATTTGCTCC | |
| cpxR R | CATGCTGCTCAATCATCAGC | |
| k1 | CAGTCATAGCCGAATAGCCT | [30] |
| evgA F | GACGCCTTATGTCTGTATTAC | |
| evgA R | GTTGCTGCGAATCGGTATG | |
| Orf1-F | GGTGATTTTCACGCTCGATG | |
| Orf1-R | TCGGATGACTTCTGCTGTTC | |
| ORF8-F | TCGGTAAAGAAAGCGGTCAAG | [34] |
| ORF8-R | CATCGGAAGGTTGTCGGTTT | [34] |
| Orf13-F | TTGCTGGGGTATCTCTCTGT | |
| Orf13-R | CAGCCACATCAATAGCAGGA | |
| gapA-F | GTTGACCTGACCGTTCGTCT | [35] |
| gapA-R | ACGTCATCTTCGGTGTAGCC | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Wang, Z.; McMullen, L.M.; Raivio, T.; Simpson, D.J.; Gänzle, M.G. Contribution of the Locus of Heat Resistance to Growth and Survival of Escherichia coli at Alkaline pH and at Alkaline pH in the Presence of Chlorine. Microorganisms 2021, 9, 701. https://doi.org/10.3390/microorganisms9040701
Zhu T, Wang Z, McMullen LM, Raivio T, Simpson DJ, Gänzle MG. Contribution of the Locus of Heat Resistance to Growth and Survival of Escherichia coli at Alkaline pH and at Alkaline pH in the Presence of Chlorine. Microorganisms. 2021; 9(4):701. https://doi.org/10.3390/microorganisms9040701
Chicago/Turabian StyleZhu, Tongbo, Zhiying Wang, Lynn M. McMullen, Tracy Raivio, David J. Simpson, and Michael G. Gänzle. 2021. "Contribution of the Locus of Heat Resistance to Growth and Survival of Escherichia coli at Alkaline pH and at Alkaline pH in the Presence of Chlorine" Microorganisms 9, no. 4: 701. https://doi.org/10.3390/microorganisms9040701
APA StyleZhu, T., Wang, Z., McMullen, L. M., Raivio, T., Simpson, D. J., & Gänzle, M. G. (2021). Contribution of the Locus of Heat Resistance to Growth and Survival of Escherichia coli at Alkaline pH and at Alkaline pH in the Presence of Chlorine. Microorganisms, 9(4), 701. https://doi.org/10.3390/microorganisms9040701







