Abstract
Cupriavidus metallidurans CH34 is a well-studied metal-resistant β-proteobacterium and contains a battery of genes participating in metal metabolism and resistance. Here, we generated a mutant (CH34ZnR) adapted to high zinc concentrations in order to study how CH34 could adaptively further increase its resistance against this metal. Characterization of CH34ZnR revealed that it was also more resistant to cadmium, and that it incurred seven insertion sequence-mediated mutations. Among these, an IS1088 disruption of the glpR gene (encoding a DeoR-type transcriptional repressor) resulted in the constitutive expression of the neighboring ATP-binding cassette (ABC)-type transporter. GlpR and the adjacent ABC transporter are highly similar to the glycerol operon regulator and ATP-driven glycerol importer of Rhizobium leguminosarum bv. viciae VF39, respectively. Deletion of glpR or the ABC transporter and complementation of CH34ZnR with the parental glpR gene further demonstrated that loss of GlpR function and concomitant derepression of the adjacent ABC transporter is pivotal for the observed resistance phenotype. Importantly, addition of glycerol, presumably by glycerol-mediated attenuation of GlpR activity, also promoted increased zinc and cadmium resistance in the parental CH34 strain. Upregulation of this ABC-type transporter is therefore proposed as a new adaptation route towards metal resistance.
1. Introduction
Metal homeostasis is important for all bacteria since they have to react swiftly to both the scarcity and excess of either essential or toxic metals [1,2,3]. To defend themselves against high metal toxicity, bacteria depend on multiple resistance mechanisms, including efflux pumps, proteins changing the oxidation state of metals, and intra- or extracellular sequestration of metals [4,5,6,7]. In addition, extracytoplasmic function (ECF) sigma factors play an important role in the response to environmental stressors as well as in metal homeostasis [8].
One of the essential metals is zinc, which occurs naturally in air, water, rocks, and soil. The average natural zinc level in the Earth’s crust is 70 mg/kg (dry weight), generally ranging between 10 and 300 mg/kg [9]. However, zinc has been concentrated to much higher levels at some locations, either by natural geological and chemical processes or through anthropogenic interventions because of the wide use of zinc compounds in industry, agriculture, and medicine [10,11,12,13,14,15,16]. Despite its essential role as a trace element in various biological processes, including proper functioning of specific enzymes, stabilization of DNA, and expression of genes [17], excess zinc has significant toxicity and acts as a potent disrupter of biological systems [18]. This duality of zinc properties requires a tight regulation of its intracellular homeostasis.
Cupriavidus metallidurans CH34 was one of the first bacteria to be isolated from industrial sites characterized by an extremely high metal content [19] and contains an unprecedented number of genes involved in the resistance and processing of metals [7]. C. metallidurans strains do not contain a high-affinity zinc uptake system like the ATP-binding cassette (ABC) uptake system ZnuABC from Escherichia coli [20]. Instead, uptake of zinc is accomplished by a set of highly redundant metal cation uptake systems with only minimal selectivity. In strain CH34, the only known import system with some specificity for zinc is ZupT [21,22], which is needed to deliver zinc under conditions of low availability [23]. The expression of zupT is upregulated under conditions of zinc starvation and repressed by FurC when sufficient zinc is present [24]. Deletion of zupT results in numerous defects caused by disturbed zinc homeostasis at lower and higher zinc concentrations [22].
C. metallidurans CH34 accomplishes metal detoxification by the concerted action of efflux systems, which may be followed by metal sequestration or complexation [25,26,27,28,29]. Various transporters remove excess zinc either from the cytoplasm or the periplasm. The most important zinc resistance operon is the czc cluster on megaplasmid pMOL30 [7,19,26,30,31]. The high-level metal resistance system Czc mediates the efflux of Co2+, Zn2+ and Cd2+, and loss of pMOL30 results in a drastically reduced zinc resistance [19]. The czc determinant is organized into two divergently transcribed gene clusters, i.e., czcNICBADRSE and czcP. The first cluster encodes CzcCBA belonging to the heavy metal efflux (HME)-Resistance-nodulation-division (RND)-driven efflux systems [32] and is comprised of three components spanning both outer and cytoplasmic membrane with an outer membrane protein (CzcC), a membrane fusion protein (CzcB), and a substrate-binding inner membrane transporter (CzcA) [33,34]. RND-driven efflux systems are responsible for the export of their substrates from the periplasm to the outside of the cell [35,36,37,38]. The czcD gene codes for a secondary transport system belonging to the Cation Diffusion Facilitator (CDF) family [27]. The second cluster codes for CzcP, a PIB4-type ATPase, which functions as a resistance enhancer exporting Zn2+ much more rapidly than PIB2-type ATPases, but it relies on the action of the latter to provide a basic resistance level [38]. The czc operon is zinc-inducible and under the control of the two-component regulatory system of CzcS (a histidine sensor kinase) and CzcR (a response regulator) [30,39]. However, a complex interplay exists between the plasmid-borne regions and chromosomal metal resistance clusters in zinc resistance. Two other efflux systems are inducible by zinc, i.e., the PIB2-type ATPases ZntA and CadA (both located on the chromosome), but their zinc induction is prevented in the presence of the czc operon [40]. Two more proteins of the CDF family, i.e., DmeF and FieF, which are both chromosomally encoded, were described to be mainly involved in cobalt and iron homeostasis, respectively, but have broad substrate spectrum and can probably also transport zinc [41]. In addition, C. metallidurans harbors a second RND transporter involved in zinc efflux, namely the chromosomally encoded Zne transporter [42,43]. Although this transporter is highly specific for zinc, it seems to have a dedicated function in zinc homeostasis and not in resistance to high zinc concentrations as the plasmid-free strain AE104 is zinc-sensitive [44]. The CDF and ATPase efflux systems transport their substrate from the cytoplasm to the periplasm, where RND-driven efflux systems will export these metals to the outside of the cell [8,27].
While the current zinc resistance mechanisms of CH34 have been well established, much less is known about its adaptive potential in the face of zinc stress. In this study we generated a mutant adapted to high zinc concentrations in order to shine a light on possible adaptive changes in CH34′s genome. As such, a novel determinant underlying increased zinc resistance could be identified.
2. Materials and Methods
2.1. Strains, Media, and Culture Conditions
The bacterial strains and plasmids used in this study are listed in Table 1 and Table 2. C. metallidurans was routinely cultured at 30 °C in Tris-buffered mineral medium (6.06 g/L Tris/HCl, 4.68 g/L NaCl, 1.49 g/L KCl, 1.07 g/L NH4Cl; 0.43 g/L Na2SO4, 0.2 g/L MgCl2·6H20, 0.03 g/L CaCl2·2H20, 0.04 g/L Na2HPO4·2H2O, 4.8 mg/L Fe(III)(NH4)citrate; 144 µg/L ZnSO4·7 H2O, 99 µg/L MnCl2·4H2O, 62 µg/L H3BO3, 190 µg/L CoCl2·6H2O, 17 µg/L CuCl2·2H2O, 24 µg/L NiCl2·6H2O, 36 µg/L Na2MoO4·2H2O) supplemented with 0.2% (w/v) sodium gluconate (MM284). Escherichia coli strains were routinely cultured at 37 °C in Lysogeny broth (LB). Liquid cultures were grown in the dark on a rotary shaker at 150 rpm. For culturing on agar plates, 2% (w/v) agar (Thermo Scientific, Oxoid) was added. When appropriate, the following chemicals (Sigma-Aldrich or Thermo Scientific) were added to the growth medium at the indicated final concentrations: kanamycin (50 µg/mL for E. coli (Km50) or 1500 µg/mL for C. metallidurans (Km1500)), tetracycline (20 µg/mL (Tc20)), chloramphenicol (30 µg/mL (Cm30)), Zn2+ (0.3, 12, 24, or 25 mM as zinc sulfate heptahydrate), Ni2+ (1.25, 2.5, or 5 mM as nickel chloride hexahydrate), Cd2+ (1, 1.5, 2, 3, or 4 mM as cadmium chloride hemipentahydrate), Co2+ (1.25, 2.5, or 5 mM as cobalt chloride hexahydrate), 5-bromo-4-chloro-3-indolyl-β-galactopyranoside (X-Gal; 40 µg/mL), and isopropyl β-D-1-thiogalactopyranoside (IPTG; 0.1 mM). Glycerol (Merck Millipore) was added to MM284 at a final concentration of 1% (w/v).

Table 1.
Strains used in this study.

Table 2.
Plasmids used in this study.
2.2. Isolation of Zinc-Resistant Mutants
C. metallidurans CH34 was first cultivated in MM284 at 30 °C until stationary phase and subsequently diluted 1:100 in MM284. Then Zn2+ was added to a final concentration of 12 mM. After four days of growth at 30 °C, 109 cells were pelleted and cell suspensions (100 µL) of a serial tenfold dilution in saline (0.85% NaCl) were spread on MM284 agar plates containing a final concentration of 24 mM Zn2+ and incubated at 30 °C. Colony-forming units (CFU) were counted after day two and survival frequency was calculated as viable cell count on MM284 24 mM Zn2+ agar plates divided by viable cell count on MM284 agar plates.
2.3. Assessment of Zinc-Resistant Phenotype
The susceptibility of CH34 and CH34ZnR to a metal ion were determined by the minimal inhibitory concentration (MIC). The strains were cultivated in biological triplicates by inoculating 2 mL MM284 or MM284 supplemented with different metals with 20 µL of a stationary phase C. metallidurans CH34 or CH34ZnR culture. The MIC is defined as the lowest metal ion concentration that inhibits visible growth of the culture after two days of incubation at 30 °C.
In addition, the resistance of C. metallidurans strains was further assessed with dose–response growth curves, which were conducted in MM284 supplemented with different zinc or cadmium concentrations. Precultures were incubated at 30 °C until the stationary phase, diluted 1:100 in fresh medium with increasing zinc/cadmium concentrations, and incubated at 30 °C for 72 h. Next, the optical density at 600 nm (OD600) was determined in a 24-well cell culture plate (Flat-bottom, Greiner Bio-One, Vilvoorde, Belgium) which was placed into a CLARIOstar® (BMG LABTECH, De Meern, The Netherlands). It is noteworthy that high Zn2+ concentrations resulted in precipitation (of zinc hydroxide) in an abiotic non-inoculated control, but did not impact optical density measurements (Figure S1).
To further analyze the phenotype of CH34ZnR, the cell survival of both CH34 and CH34ZnR at a high zinc concentration (25 mM Zn2+) were determined. Stationary phase cultures were 1:100 diluted in fresh MM284 or MM284 supplemented with 0.3 mM Zn2+ (pre-induction assay) and these subcultures were allowed to grow for two days. Afterwards, 2-mL cell suspensions (biological triplicate) were transferred to a 24-well cell culture plate and Zn2+ was added to a final concentration of 25 mM. Next, 20 µL aliquots were withdrawn at different time points and cell suspensions (100 µL) of a serial dilution were spread on LB agar. The CFU/mL was determined after two days of incubation at 30 °C.
2.4. Construction of Plasmids
The glpR gene (Rmet_2235) from C. metallidurans CH34 and its zinc-resistant derivative CH34ZnR were amplified by PCR (Phusion High-Fidelity DNA polymerase, Thermo Scientific, Aalst, Belgium) with primer pair Rmet_2235_Fw-Rv (Table S1), providing HindIII/EcoRI recognition sites. Afterwards, these PCR products were cloned as a HindIII/EcoRI fragment into pBBR1MCS2. The resulting pBBR-glpR and pBBR-glpRR plasmids from E. coli DG1 transformants selected on LB Km50 were further confirmed by sequencing prior to conjugation (triparental with E. coli HB101 pRK600 as helper) to C. metallidurans CH34 and CH34ZnR.
2.5. Construction of Deletion Mutant Strains
The glpR gene (Rmet_2235) and the genes coding for the ABC-type transporter (Rmet_2229-2234) were amplified from C. metallidurans CH34 by PCR (Phusion High-Fidelity DNA polymerase) with primer pairs Rmet_2235_Fw-Rv and Rmet_2229_Fw-Rmet_2234_Rv (Table S1), respectively, providing HindIII/EcoRI recognition sites. Afterwards, these PCR products were cloned as a HindIII/EcoRI fragment into the mobilizable suicide vector pK18mob. The resulting pglpR and pRmet_2229-34 plasmids from E. coli DG1 transformants selected on LB Km50 were further confirmed by sequencing prior to amplifying of the flanking sequences of the RglpR or Rmet_2229-2234, respectively, by inverse PCR (Phusion High-Fidelity DNA polymerase) with primer pairs Rmet_2235_tet_Fw-Rv or Rmet_2229-2234_tet_Fw-Rv (Table S1), respectively, providing BcuI/XbaI recognition sites. At the same time the tet gene from pACYC184 [46] was amplified by PCR (Phusion High-Fidelity DNA polymerase) with primer pair Tet_Fw-Rv (Table S1), providing BcuI/XbaI recognition sites. Afterwards, this PCR product was cloned as a BcuI/XbaI fragment into the former inverse PCR products. The resulting pglpR::tet and pRmet_2229-34::tet plasmids from E. coli DG1 transformants selected on LB Tc20Km50 were further confirmed by sequencing prior to conjugation (triparental with E. coli HB101 pRK600 as helper) to C. metallidurans CH34 or CH34ZnR. The resulting transformants selected on MM284 Tc20 were replica-plated on MM284 Tc20 and MM284 Km1500. CH34 ∆glpR::tet (CH34 ∆glpR), CH34 ∆Rmet_2229_34::tet (CH34 ∆29-34), and CH34ZnR ∆Rmet_2229_34::tet (CH34ZnR ∆29-34) cells resistant to Tc20 but sensitive to Km1500 were further confirmed by sequencing.
2.6. Whole-Genome Gene Expression Analysis and qRT-PCR
Whole-genome gene expression analysis of CH34 and CH34ZnR was performed to examine which genes played a role in the increased zinc resistance. The strains were cultivated by inoculating 30 mL MM284 in biological triplicates with 300 µL of an exponentially growing C. metallidurans CH34 or CH34ZnR culture at 30 °C. These subcultures were allowed to grow until an OD600 value of around 0.6 was reached. Next, each subculture was immediately subdivided in 15 microcentrifuge tubes of 2 mL and cells were harvested by centrifugation for 2 min at 10,000 rpm. Supernatant was removed and the bacterial pellets were flash frozen by immersion into liquid nitrogen and kept frozen at −80 °C at all times. RNA extraction, labeling and hybridization, microarray spotting, scanning, and data analysis were performed according to the work of [48]. The full description of the microarray data has been deposited at the Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE156826.
In addition, total RNA was extracted from CH34, CH34 ΔglpR, and CH34ZnR (similar conditions as above) and single-stranded complementary DNA (cDNA) was synthesized from 1 μg total RNA using random hexamers as primers and the TaqMan Reverse Transcription Reagents (Thermo Scientific). The quantity of synthesized cDNA was measured with a NanoDrop® 2000 spectrophotometer (Thermo Scientific). The expression of Rmet_2229 was analyzed by qRT-PCR using the QuantiNova SYBR Green RT-PCR kit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s protocol with primers qF_2229 and qR_2229. Expression was compared with that of the 16S rRNA gene (Table S1). RT-qPCRs were performed with a Rotor-Gene Q (Qiagen).
2.7. Genome Sequencing
Whole-genome sequencing was performed to identify mutations responsible for the increased zinc-resistance of CH34ZnR. The strain was cultivated by inoculating 4 mL LB at 30 °C, and total DNA was extracted using the QIAamp DNA Mini Kit (Qiagen). The quantity and quality of extracted DNA was measured using a NanoDropTM 1000 spectrophotometer (Thermo Scientific). Ten micrograms of DNA were sent for Illumina sequencing (Baseclear, Leiden, The Netherlands). Large insertions and deletions (>200 bp) were identified using an in-house developed software able to exploit the paired-end characteristics of the sequencing data, where either an unexpected increase of unpaired sequencing reads was used as an indicator for insertions, and an increase in the distribution of insert sizes interpreted as deletion. The Genome Analysis Toolikt (GATK) was used to identify point mutations, i.e., single-nucleotide polymorphisms (SNPs) and small indels [49,50]. Sequencing data are available within the Sequencing Read Archive (SRA) of NCBI using the accession number PRJNA658861.
3. Results
3.1. Isolation and Characterization of Zinc-Resistant CH34 Derivatives
Direct exposure of C. metallidurans CH34 to a high Zn2+ concentration (24 mM Zn2+) is bactericidal, as no survivors were observed even after prolonged incubation (over two weeks). However, CH34 derivatives could be isolated on MM284 agar plates with 24 mM Zn2+ when it was first cultivated in liquid MM284 medium with 12 mM Zn2+ (i.e., parental MIC). Growth in liquid MM284 medium with 12 mM Zn2+ was observed after four days of incubation (no visible growth after two days of incubation) and subsequent plating of this culture on MM284 agar plates with 24 mM Zn2+ yielded zinc-resistant CH34 mutants at a frequency of 2.37 ± 0.30 × 10−7 (calculated after two days of incubation at 30 °C). One mutant was further purified on MM284 and retested for its resistance. The latter, designated as CH34ZnR, exhibited an inheritable resistance phenotype and was further characterized phenotypically. Compared to its parental strain, CH34ZnR exhibited a two-fold increased resistance to both Zn2+ and Cd2+. No increased resistance to Ni2+ and Co2+ was observed (Table 3), indicating that the involved resistance mechanism(s) can detoxify both Zn2+ and Cd2+.

Table 3.
Minimal inhibitory concentration (MIC, mM) of different metals determined in liquid MM284 medium for different C. metallidurans strains.
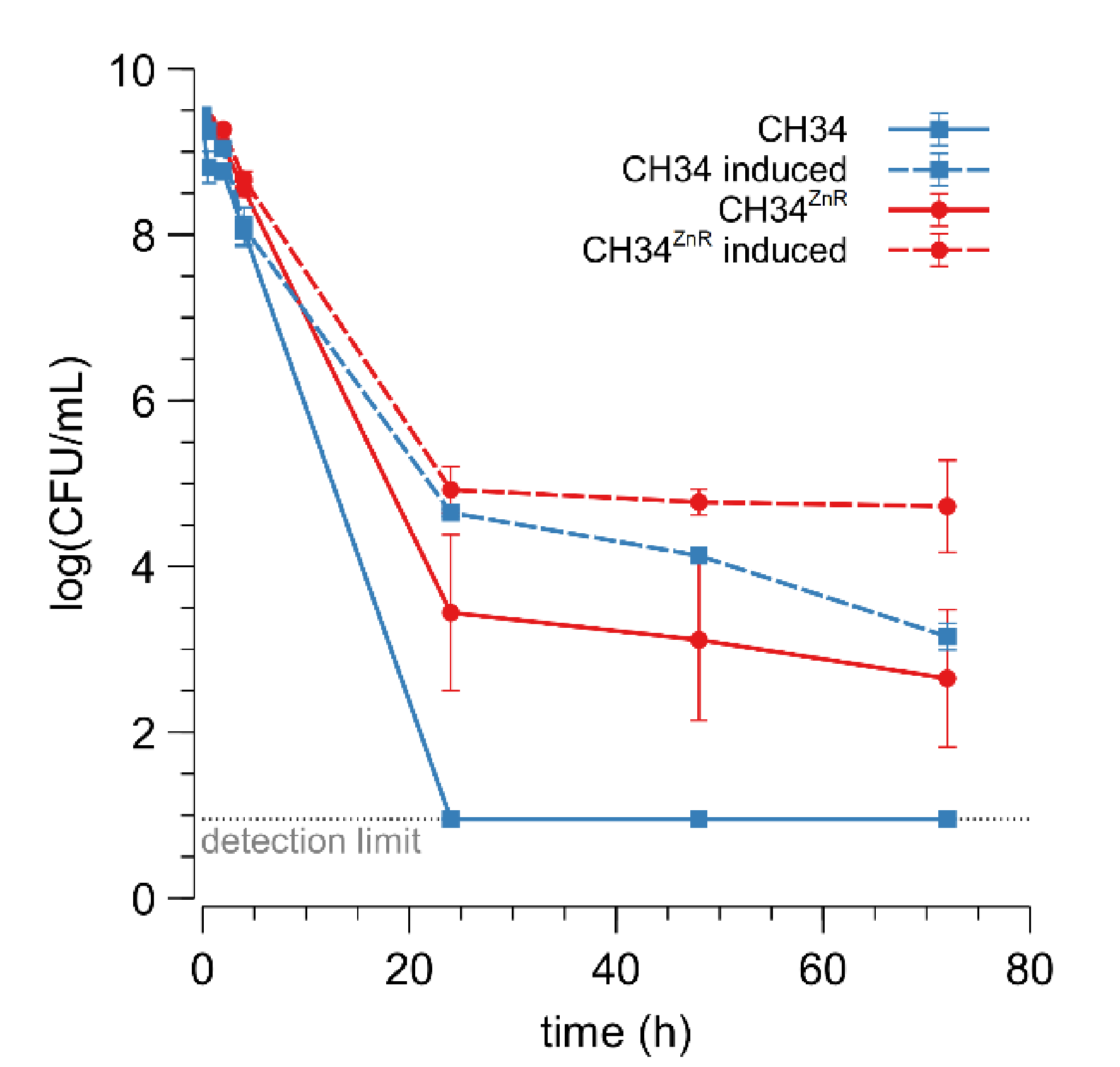
In addition to MIC determination, inactivation dynamics in the presence of a high zinc concentration (25 mM) were examined for the parent and its CH34ZnR derivative (Figure 1). The fraction of surviving CH34 cells drastically decreased in the presence of 25 mM Zn2+ and no survivors could be detected after 24 h of incubation. Although a drastically decreased cell survival in the first hours of incubation was also shown for CH34ZnR, a fraction of the cells did survive (2.85 ± 2.07 × 10−4 CFU/mL after 24 h). Pre-inducing the czc operon with 0.3 mM Zn2+ for 48 h [30,51,52] resulted in an increased cell survival of both the parental and zinc-resistant derivative (Figure 1). This derepression enables a fraction of the wild type cells to survive a very high concentration of Zn2+ because the Czc efflux pumps are already active before the cell encounters the toxic Zn2+ concentration [30,51,52]. However, cell survival for pre-induced CH34ZnR was still higher than for pre-induced CH34. These observations suggest that the mutation(s) in CH34ZnR do not target the czc operon and that its genetic background works synergistically with a zinc-induced czc operon, leading to more cells that survive.
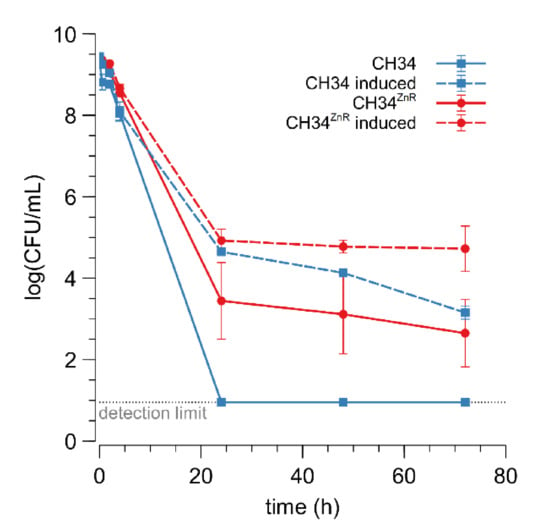
Figure 1.
Cell survival upon exposure to 25 mM Zn2+ of C. metallidurans CH34 (blue square) and CH34ZnR (red circles) without (full) or with (dashed) pre-induction with 300 µM Zn2+ for 48 h. The light grey dotted line represents the detection limit. The average values of three independent experiments with standard deviations are shown. CFU: colony forming unit.
3.2. Whole-Genome Expression Profile and Genome Sequence Analysis of CH34ZnR
As the increased resistance stemmed from natural selection of resistance-conferring mutations [53], the global shift in transcriptome resulting from the altered genotype of the evolved strain (CH34ZnR) as compared to the parental strain was examined in non-selective conditions [54,55,56]. This revealed 61 coding sequences (CDSs) that were significantly differentially expressed in comparison with the parental CH34 (>1 log2 fold with an adjusted p-value < 0.05), of which 21 were upregulated and 40 downregulated (Table S2). The ABC-type transporter (Rmet_2229_2234) (Figure 2) and the flagellar filament structural protein (fliC2) were the most upregulated genes. The cupRAC operon was the most downregulated, with copK and copM also being downregulated, but no alteration in copper resistance was observed (data not shown). Interestingly, no overlap was observed between the transcriptional profile of CH34ZnR in non-selective conditions and the previously established transcriptional response of the parental CH34 strain to zinc stress [48].
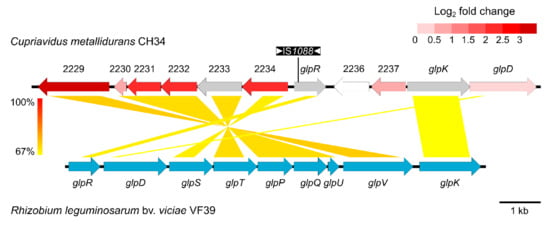
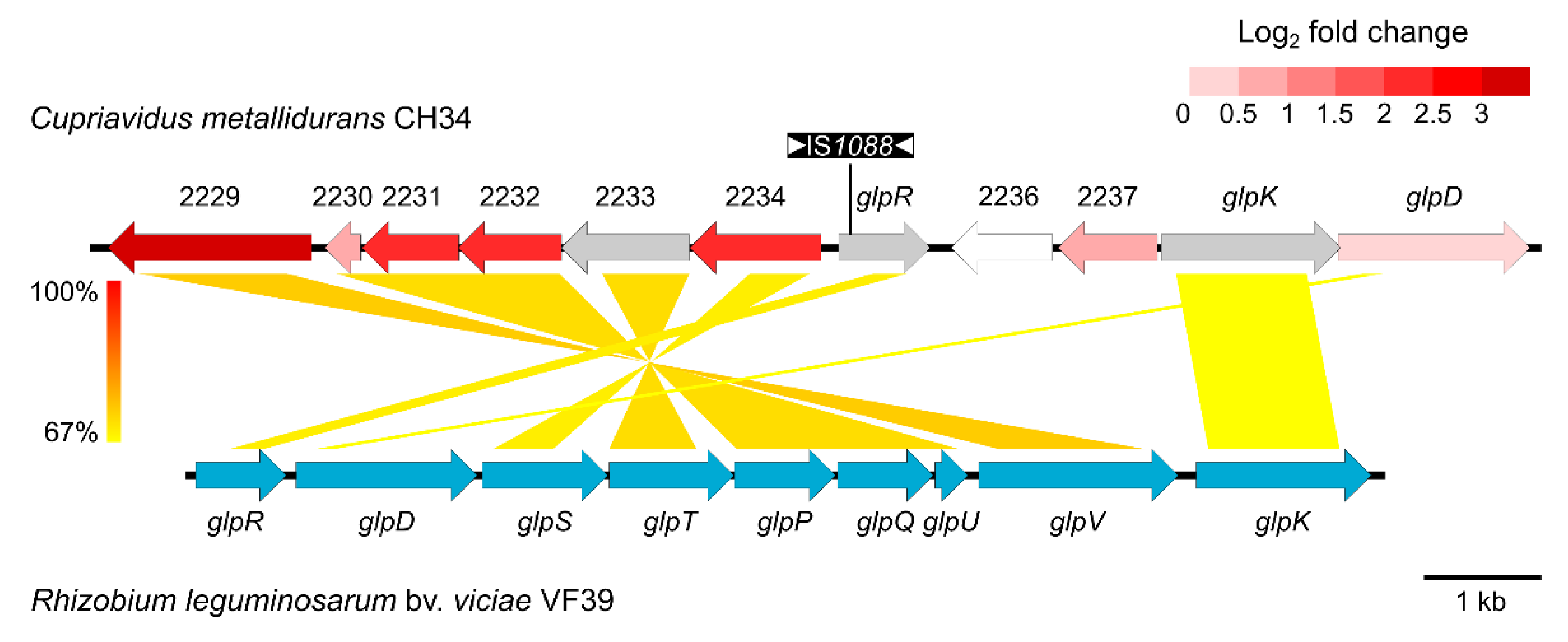
Figure 2.
BLASTn comparison of the ABC-type transporter of C. metallidurans CH34/CH34ZnR and the glycerol utilization cluster of the plasmid pRleVF39c from R. leguminosarum bv. viciae VF39 (accession no. JN390944). The colored shading indicates nucleotide identity between the sequences. Coding sequences (CDSs) of C. metallidurans CH34 are color-coded based on their log2 fold change (CH34ZnR vs. CH34 expression), with grey being not detected. Insertion of IS1088 in glpR of CH34ZnR is shown.
Subsequent whole genome sequencing of strain CH34ZnR revealed seven mutations, all of which were caused by transposition of insertion sequences (IS), more specifically ISRme5, ISRme15 and IS1088 (Table 4). Taking into account the expression profile, the inactivation of Rmet_2235 (glpR) by insertion of IS1088 stands out. Therefore, we hypothesized that inactivation of Rmet_2235 resulted in derepressed transcription of the adjacently encoded ABC-type transporter (Rmet_2229-2234) and subsequent increased zinc and cadmium tolerance (Figure 2). This derepression was confirmed by qRT-PCR as transcription of Rmet_2229 was increased 3-fold in CH34 ΔglpR compared to the parental CH34 strain (Figure S2). Existing tagRNA-seq data used to identify the 5′ ends of RNAs in C. metallidurans CH34 corroborated that Rmet_2229-2234 was an operon transcribed from the same promoter into one polycistronic mRNA [57]. Furthermore, this dataset indicated that the main transcription start site of the glpR gene (for strain CH34 in non-selective growth conditions) was found 9 bp downstream of the currently annotated start codon, indicating translation of a 3-aa shorter GlpR from a leaderless transcript (Figure S3).

Table 4.
Mutations identified in CH34ZnR.
A similar gene cluster, also containing a glycerol kinase (glpK) and glycerol 3-phosphate dehydrogenase (glpD), was detected in various α-proteobacteria enabling utilization of glycerol as a carbon source [58]. In fact, the cluster is similar to the plasmid-borne locus responsible for glycerol utilization from plasmid pRleVF39c in Rhizobium leguminosarum bv. viciae VF39 [58]. In this cluster, the glpR gene codes for the repressor of the glycerol-3-phosphate regulon and negatively regulates the adjacent genes involved in glycerol metabolism.
3.3. ABC-Type Transporter (Rmet_2229-2234) Is Responsible for Increased Zinc Resistance
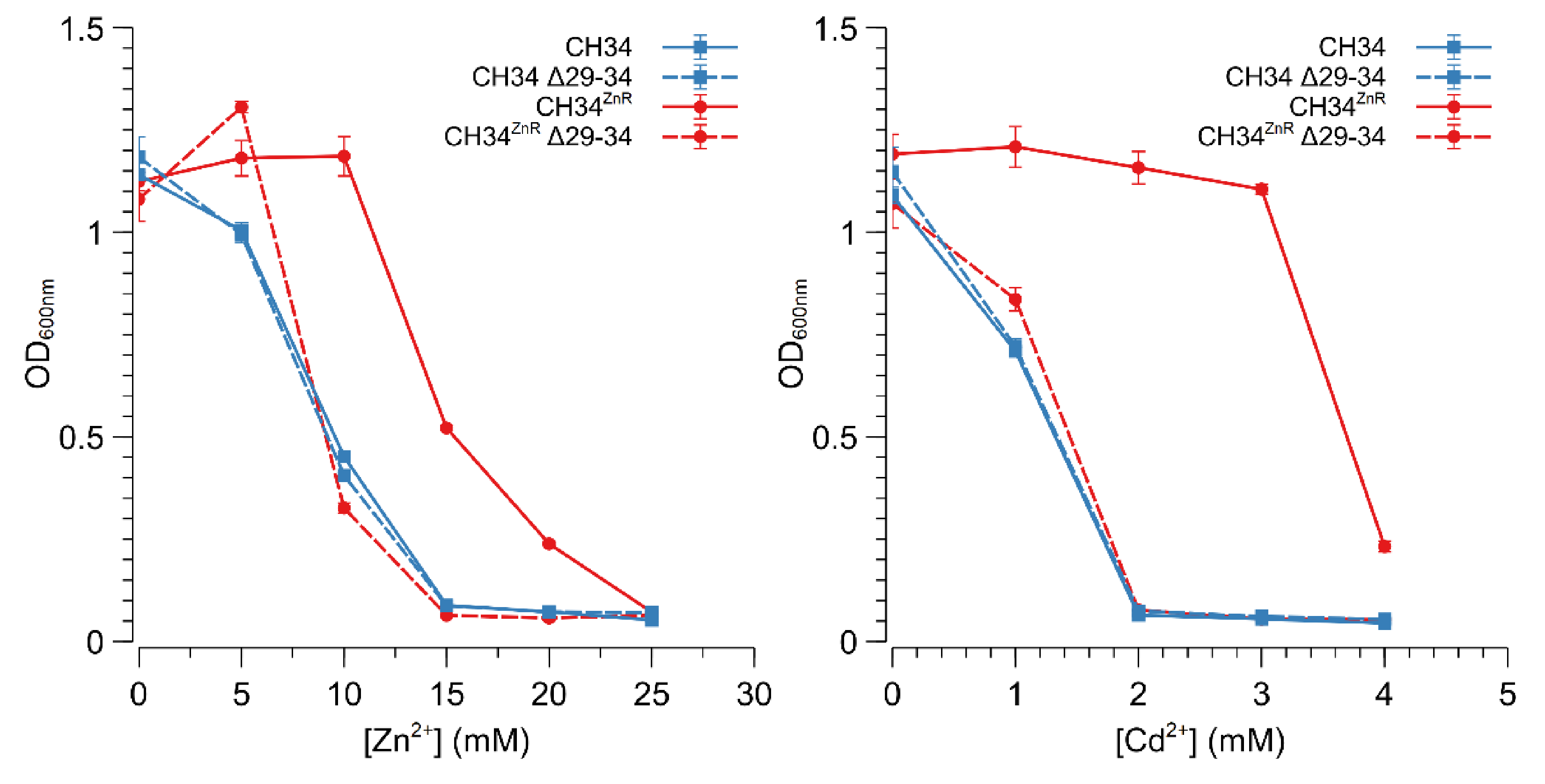
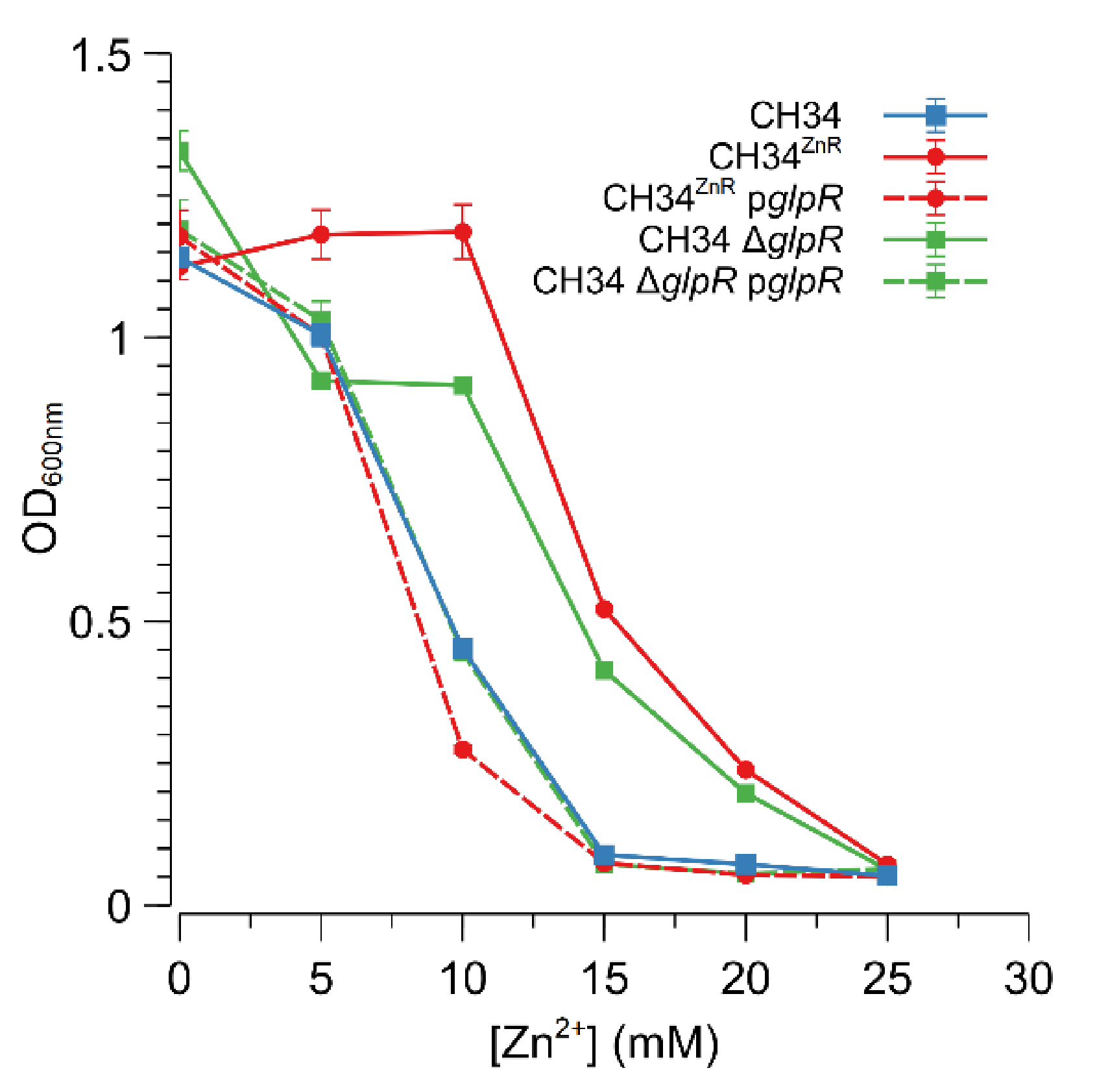
The Rmet_2229_2234 gene cluster was subsequently deleted by replacing it with a tetracycline resistance cassette (referred to as ∆29-34) in both CH34 and CH34ZnR to test the hypothesis that constitutive derepression of the ABC-type transporter is responsible for CH34ZnR’s increased zinc resistance. First of all, no differences in zinc and cadmium resistance could be observed between CH34 ∆29-34 and its parental strain, indicating that the native ABC-type transporter exerts no role in the detoxification of zinc or cadmium in the parental CH34 strain (Figure 3). However, when the Rmet_2229-2234 gene cluster was deleted in CH34ZnR, the zinc and cadmium resistance of the resulting CH34ZnR ∆29-34 mutant dropped to levels similar to those of the parental CH34, indicating that the ABC-type transporter is required for its increased zinc and cadmium resistance (Figure 3). In turn, deletion of the glpR gene in CH34 resulted in increased zinc resistance, while plasmid-based complementation of glpR reduced zinc resistance back to the parental level in both CH34 ΔglpR and CH34ZnR (Figure 4). The latter further confirms that loss of GlpR function results in increased zinc resistance.
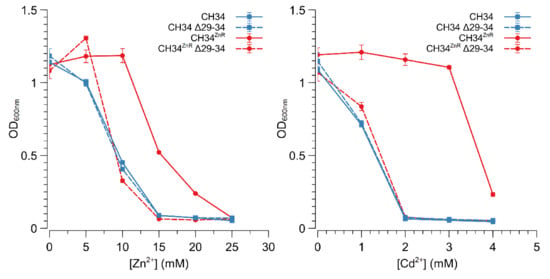
Figure 3.
Zinc and cadmium resistance of C. metallidurans CH34 and its indicated derivatives. Zinc (left) and cadmium (right) dose–response experiments with CH34 (blue full line, squares), CH34ZnR (red full line, circles) and their Δ29-34 derivatives (blue dashed line with squares and red dashed line with circles, respectively). The average values of three independent experiments with standard deviations are shown.
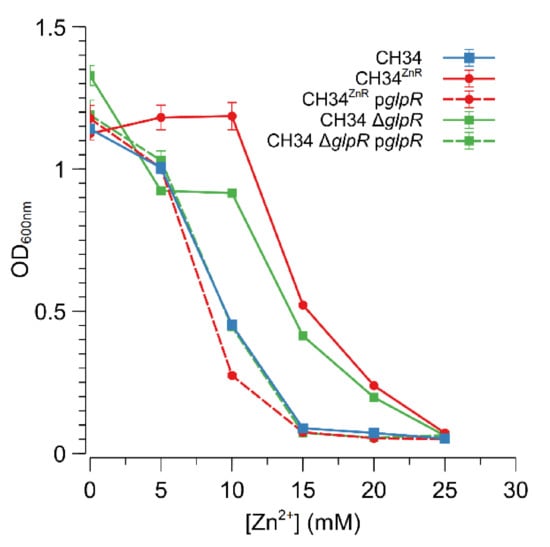
Figure 4.
Impact of GlpR function on the increased zinc resistance of C. metallidurans CH34. Dose–response experiments with CH34 ΔglpR (green, squares) and CH34ZnR (red, circles) without (full lines) or with (dashed lines) plasmid-based glpR complementation. The dose–response experiment with the parental CH34 strain is shown as a blue line (squares). The average values of three independent experiments with standard deviations are shown.
Finally, CH34 ∆29-34 was still able to utilize glycerol as a sole carbon source (data not shown), demonstrating that the ABC-type transporter is not essential for glycerol uptake in C. metallidurans (contrary to R. leguminosarum bv. viciae VF39).
3.4. Glycerol Induces Increased Zinc Resistance
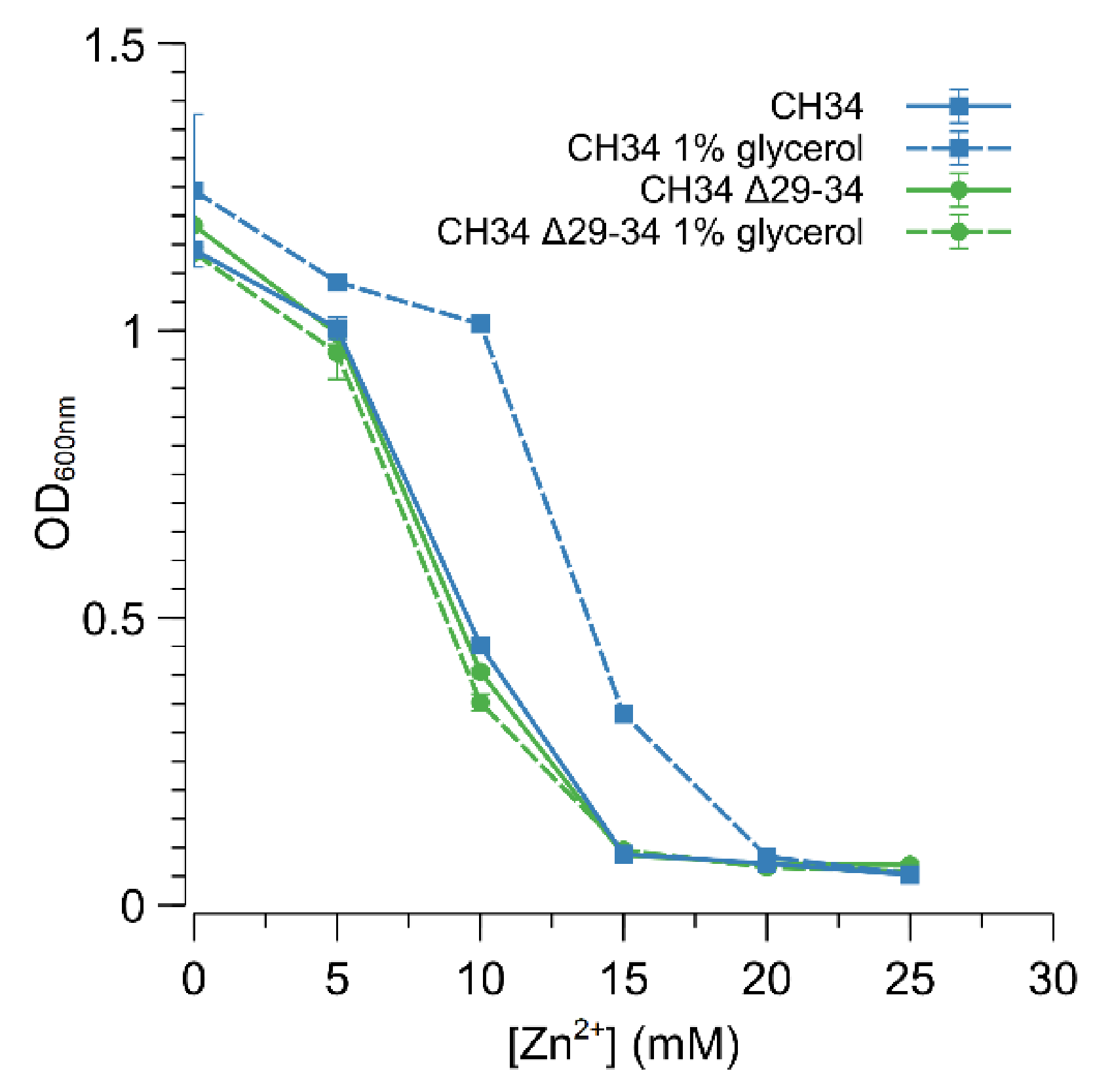
In E. coli, direct interaction with glycerol or glycerol-3-phosphate impedes the action of the GlpR repressor, and thus relieves the repression of the glycerol metabolism [59]. Therefore, C. metallidurans CH34 and CH34 ∆29-34 were grown in MM284 containing 1% (w/v) glycerol to examine if pre-induction with glycerol could promote derepression of the ABC-type transporter and consequently result in increased zinc resistance. Indeed, CH34 displayed a higher resistance to zinc in the continued presence of glycerol (Figure 5), putatively indicating that binding of GlpR to the operator of the ABC-type transporter is diminished in the presence of glycerol. Furthermore, glycerol had no direct effect on zinc resistance as no increase in zinc resistance was observed for CH34 ∆29-34 (Figure 5). However, a lower level of zinc resistance was reached for glycerol-induced CH34 compared to CH34ZnR or CH34 ∆glpR, respectively, suggesting that the presence of glycerol was not sufficient to bind with all available GlpR repressors or that glycerol–GlpR interaction allowed a reduced repression of the ABC-type transporter.
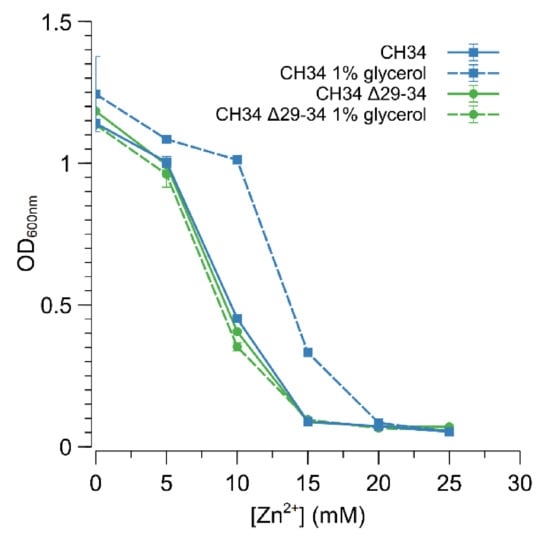
Figure 5.
Impact of glycerol on the increased zinc resistance of C. metallidurans CH34. Dose–response experiments with CH34 (blue) and CH34 Δ29-34 (green) without (full line) and with (dashed line) 1% glycerol. The average values of three independent experiments with standard deviations are shown.
4. Discussion
This study presents evidence that C. metallidurans CH34 can readily improve its extreme zinc and cadmium resistance through adaptive evolution. It is noteworthy that the high Zn2+ concentrations used resulted in precipitation (of zinc hydroxide) in an abiotic non-inoculated control. Therefore, in the presence of cells there will be a dynamic equilibrium between free Zn2+ ions, protein-bound Zn2+, and precipitation. As correct measurement of free Zn2+ is not straightforward [60], such analyses were out of the scope of this study. However, even without these analyses, the central observation of this study, which is the significant and consequent growth difference between the parental and adapted strain in the presence of high zinc concentrations, remains valid. Moreover, whole genome sequencing, expression profiling, and genetic analysis unexpectedly revealed that insertion sequence-mediated inactivation of the GlpR transcriptional repressor and subsequent upregulation of a neighboring ABC transporter (not previously involved in metal resistance) were causally responsible for the resistance phenotype.
GlpR belongs to the DeoR-family of transcriptional regulators that are widespread in bacteria and commonly function as specific regulators of carbon source uptake and catabolism, often playing a role in catabolite repression [59,61]. It shares 46% and 47% amino acid identity with the GlpR repressor of the glycerol 3-phosphate regulon in E. coli and the glycerol regulon in R. leguminosarum bv. viciae VF39, respectively. Glycerol uptake in R. leguminosarum bv. viciae VF39 is mediated by ABC-type active transport, in contrast to the GlpF-mediated facilitated diffusion in E. coli [62]. Uptake is followed by the enzymatic activity of glycerol kinase (glpK) and glycerol-3-phosphate dehydrogenase (glpD), for which expression is negatively regulated by GlpR binding to their operator sequences, enabling the use of glycerol as sole carbon source. In E. coli, glpD, but not glpK, is located near glpR. In contrast, in R. leguminosarum bv. viciae VF39, glpD and glpK flank the glpSTPQUV genes coding for an ABC transporter and form (together with the directly upstream located glpR) its plasmid-borne glp operon [58]. In both E. coli and R. leguminosarum, the glycerol utilization regulon is inducible by glycerol and glycerol-3-phosphate via the specific and direct interaction of these metabolites with the GlpR repressor, thereby causing derepression of the glp genes [58,62]. In C. metallidurans CH34, glpK (Rmet_2238) and glpD (Rmet_2239) are found in close proximity to glpR and to the genes coding for the ABC-type transporter (Rmet_2229-2234), although they do not form an operon with the transporter, reflecting a different genomic arrangement than in R. leguminosarum bv. viciae VF39 (Figure 2). Only a slight differential expression of glpD (1.3-fold) was detected in CH34ZnR (expression of glpK could not be determined). As glpK (Rmet_2238) and glpD (Rmet_2239) likely form an operon (transcription start sites not detected by the existing tagRNA-seq data [57]), it is conceivable that expression of glpK is also only slightly affected in CH34ZnR. In contrast to the derepression of the ABC-type transporter (Rmet_2229-2234), these data do not univocally show GlpR-mediated regulation of glpKD (Rmet_2238-39) in C. metallidurans. In addition, CH34 carries a second locus containing a glpK (Rmet_5445) and glpD (Rmet_5444) gene, as well as genes coding for a putative glycerol uptake operon antiterminator regulatory protein (Rmet_5442), a putative akylglycerone-phosphate synthase (Rmet_5443), and a Major Facilitator Superfamily (MFS) transporter (Rmet_5446). However, none of the genes in this second glp locus are differentially expressed in CH34ZnR. Furthermore, as glycerol can still be used as sole carbon source in the absence of the ABC-type transporter (CH34 Δ29-34), other glycerol transporters exist, e.g., the MFS transporter in the second glp locus (Rmet_5446).
The ABC-type transporter responsible for increased zinc and cadmium resistance in CH34ZnR comprises six proteins that are encoded by Rmet_2229 up to Rmet_2234. More specifically, one periplasmic protein, one small integral membrane protein, two permeases, and two ATPases form the ABC transporter. The six genes encoding the ABC transporter resemble the glycerol ABC transporter operon of R. leguminosarum bv. viciae VF39, with for instance 63% amino acid identity between the periplasmic protein encoded by Rmet_2229 of CH34 and glpV of VF39 [58]. These similarities clearly indicate a role in glycerol uptake for this uncharacterized ABC-type transporter. In fact, addition of glycerol to the growth medium improved zinc resistance in a Rmet_2229-2234-dependent fashion, indicating a role for glycerol-mediated relief of repression of the ABC-type transporter. This observation therefore reveals that bacterial metal resistance can also depend on the exact carbon sources in the environment.
ABC transporters are found in all taxa and form one of the largest transporter superfamilies, containing both uptake and efflux transport systems. These transporters couple hydrolysis of ATP to the translocation of various substrates, ranging from single ions to entire protein toxins, across cell membranes [63]. The best-studied metal ABC transporter is the high-affinity Zn2+ uptake system encoded by the znuABC genes and regulated by Zur, which was initially reported in E. coli [20]. ZnuB is the membrane permease and ZnuC is the ATPase component of the transporter, whereas ZnuA is a soluble periplasmic metallochaperone that efficiently captures zinc in this cellular compartment and then delivers the metal to the transmembrane component of the transporter [64]. In contrast to eukaryotes, no examples of ABC transporters involved in metal export are known in bacteria. Nevertheless, ABC transporters that export substrates (which are still to be elucidated) to the periplasm could mediate the repair of metal-induced protein damage [65].
Based on its similarity to the glycerol utilization operon of R. leguminosarum bv. viciae VF39, the Rmet_2229-2234 transporter is likely an uptake system, and the exact mechanism underlying the observed zinc and cadmium resistance requires further study. Nevertheless, a few hypotheses could be formulated that might explain preventing excess zinc and cadmium in the cytoplasm. The imported substrates or the metabolism of these substrates might yield binding sites for metals [66,67]. However, no glycerol or sugars were added to the selective medium. Alternatively, the increased pool of periplasmic binding proteins could bind Zn2+ (Cd2+) and prevent entry into the cytoplasm. Lastly, released inorganic phosphate (Pi) by ATP hydrolysis might bind excess Zn2+ (Cd2+), resulting in phosphate–zinc conjugates [68].
Finally, it is noteworthy that all of the mutations incurred by CH34ZnR were caused by the transposition of different insertion sequences (more specifically ISRme5, ISRme15, and IS1088), underscoring the dynamic nature of these elements. Moreover, we have recently shown zinc-induced promoter activity for the transposases of ISRme5 and IS1088 [69], indicating that IS dynamics can be boosted in times of stress to provide evolutionary escape routes for the host cell [69,70].
5. Conclusions
To conclude, we have demonstrated that derepression of the inconspicuous Rmet_2229-2234 ABC-type transporter (which is likely involved in glycerol uptake) can serve as an evolutionary road towards extreme zinc and cadmium resistance in C. metallidurans CH34. Moreover, Rmet_2229-2234-mediated protection against zinc can also become environmentally triggered in the presence of glycerol. Finally, our observations also underscore the importance of IS elements in the adaptive potential of C. metallidurans CH34.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/2/309/s1, Figure S1: Zinc dose–response experiments with CH34 (blue) and CH34ZnR (red). The abiotic (non-inoculated) control is shown in green. The average values of three independent experiments with standard deviations are shown. Figure S2: Relative fold change determined by quantitative real-time PCR (qRT-PCR) analysis of Rmet_2229 in CH34 DglpR and CH34ZnR. All data were normalized with 16S rRNA gene expression and given as relative to the parental CH34 strain. Figure S3: Transcription profile analysis of the Rmet_2234-glpR intergenic region from C. metallidurans CH34 (coordinates for the chromosomal region are shown at the bottom). Combined transcription start site (TSS) read counts of the three biological replicates are shown for positive (green) and negative (red) strands. The small black arrows indicate clearly identified primary and internal TSSs. The green arrow represents a re-annotated CDS. Data from [57], Table S1: Primers used in this study, Table S2: Differential gene expression analysis results.
Author Contributions
Conceptualization, R.V.H. and A.A.; methodology, R.V.H., J.V. and A.A.; validation, R.V.H., J.V. and P.M.; formal analysis, R.V.H., J.V. and P.M.; investigation, R.V.H., J.V. and P.M.; data curation, R.V.H.; writing—original draft preparation, R.V.H. and J.V.; writing—review and editing, R.V.H. and A.A.; visualization, R.V.H.; supervision, R.V.H. and A.A.; project administration, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The full description of the microarray data has been deposited at the Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE156826. Sequencing data are available within the Sequencing Read Archive (SRA) of NCBI using the accession number PRJNA658861.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moore, C.M.; Helmann, J.D. Metal ion homeostasis in Bacillus subtilis. Curr. Opin. Microbiol. 2005, 8, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Hobman, J.; Yamamoto, K.; Oshima, T. Transcriptomic Responses of Bacterial Cells to Sublethal Metal Ion Stress. In Molecular Microbiology of Heavy Metals; Nies, D., Silver, S., Eds.; Springer: Berlin/Heidelberg, Gemany, 2007; Volume 6, pp. 73–115. [Google Scholar]
- Nies, D.H. The biological chemistry of the transition metal “transportome” of Cupriavidus metallidurans. Metallomics 2016, 8, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Hantke, K. Bacterial zinc transporters and regulators. BioMetals 2001, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Staicu, L.C.; Wojtowicz, P.J.; Pósfai, M.; Pekker, P.; Gorecki, A.; Jordan, F.L.; Barton, L.L. PbS biomineralization using cysteine: Bacillus cereus and the sulfur rush. FEMS Microbiol. Ecol. 2020, 96, fiaa151. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M.; Baker-Austin, C.; Koppineedi, P.R.; Bond, P.L. Growth in sulfidic mineral environments: Metal resistance mechanisms in acidophilic micro-organisms. Microbiology 2003, 149, 1959–1970. [Google Scholar] [CrossRef]
- Monsieurs, P.; Hobman, J.; Vandenbussche, G.; Mergeay, M.; Van Houdt, R. Response of Cupriavidus metallidurans to Metals. In Metal Response in Cupriavidus Metallidurans, Volume I: From Habitats to Genes and Proteins; Mergeay, M., Van Houdt, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 45–89. [Google Scholar]
- Grosse, C.; Friedrich, S.; Nies, D.H. Contribution of extracytoplasmic function sigma factors to transition metal homeostasis in Cupriavidus metallidurans strain CH34. J. Mol. Microbiol. Biotechnol. 2007, 12, 227–240. [Google Scholar] [CrossRef]
- Malle, K.G. Zink in der Umwelt. Acta Hydrochim. Hydrobiol. 1992, 20, 196–204. [Google Scholar] [CrossRef]
- Tan, S.Y.; Praveena, S.M.; Abidin, E.Z.; Cheema, M.S. A review of heavy metals in indoor dust and its human health-risk implications. Rev. Environ. Health 2016, 31, 447–456. [Google Scholar] [CrossRef]
- Gillan, D.C.; Van Camp, C.; Mergeay, M.; Provoost, A.; Thomas, N.; Vermard, L.; Billon, G.; Wattiez, R. Paleomicrobiology to investigate copper resistance in bacteria: Isolation and description of Cupriavidus necator B9 in the soil of a medieval foundry. Environ. Microbiol. 2017, 19, 770–787. [Google Scholar] [CrossRef]
- John, M.; Heuss-Aßbichler, S.; Ullrich, A. Recovery of Zn from wastewater of zinc plating industry by precipitation of doped ZnO nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 2127–2134. [Google Scholar] [CrossRef]
- Gillan, D.C.; Van Houdt, R. The Impact of Metal Contamination on Soil Microbial Community Dynamics. In Modern Soil Microbiology, 3rd ed.; Van Elsas, J.D., Trevors, J.T., Rosado, A.S., Nannipieri, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 403–419. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 548. [Google Scholar]
- Ellis, R.J.; Morgan, P.; Weightman, A.J.; Fry, J.C. Cultivation-Dependent and -Independent Approaches for Determining Bacterial Diversity in Heavy-Metal-Contaminated Soil. Appl. Environ. Microbiol. 2003, 69, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Rogiers, T.; Claesen, J.; Van Gompel, A.; Vanhoudt, N.; Mysara, M.; Williamson, A.; Leys, N.; Van Houdt, R.; Boon, N.; Mijnendonckx, K. Soil microbial community structure and functionality changes in response to long-term metal and radionuclide pollution. Environ. Microbiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Bronzetti, G.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, D.K.; Morby, A.P. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 291–311. [Google Scholar] [CrossRef]
- Mergeay, M.; Nies, D.; Schlegel, H.G.; Gerits, J.; Charles, P.; Vangijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy-metals. J. Bacteriol. 1985, 162, 328–334. [Google Scholar] [CrossRef]
- Patzer, S.I.; Hantke, K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 1998, 28, 1199–1210. [Google Scholar] [CrossRef]
- Saier, M.H., Jr.; Tran, C.V.; Barabote, R.D. TCDB: The Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006, 34, D181–D186. [Google Scholar] [CrossRef]
- Herzberg, M.; Bauer, L.; Nies, D.H. Deletion of the zupT gene for a zinc importer influences zinc pools in Cupriavidus metallidurans CH34. Metallomics 2014, 6, 421–436. [Google Scholar] [CrossRef]
- Kirsten, A.; Herzberg, M.; Voigt, A.; Seravalli, J.; Grass, G.; Scherer, J.; Nies, D.H. Contributions of five secondary metal uptake systems to metal homeostasis of Cupriavidus metallidurans CH34. J. Bacteriol. 2011, 193, 4652–4663. [Google Scholar] [CrossRef]
- Schmidt, C.; Schwarzenberger, C.; Grosse, C.; Nies, D.H. FurC regulates expression of zupT for the central zinc importer ZupT of Cupriavidus metallidurans. J. Bacteriol. 2014, 196, 3461–3471. [Google Scholar] [CrossRef]
- Janssen, P.J.; Van Houdt, R.; Moors, H.; Monsieurs, P.; Morin, N.; Michaux, A.; Benotmane, M.A.; Leys, N.; Vallaeys, T.; Lapidus, A.; et al. The complete genome sequence of Cupriavidus metallidurans strain CH34, a master survivalist in harsh and anthropogenic environments. PLoS ONE 2010, 5, e10433. [Google Scholar] [CrossRef] [PubMed]
- Mergeay, M.; Monchy, S.; Vallaeys, T.; Auquier, V.; Benotmane, A.; Bertin, P.; Taghavi, S.; Dunn, J.; van der Lelie, D.; Wattiez, R. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: Towards a catalogue of metal-responsive genes. FEMS Microbiol. Rev. 2003, 27, 385–410. [Google Scholar] [CrossRef]
- Nies, D.H. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev. 2003, 27, 313–339. [Google Scholar] [CrossRef]
- Diels, L.; Van Roy, S.; Taghavi, S.; Van Houdt, R. From industrial sites to environmental applications with Cupriavidus metallidurans. Antonie van Leeuwenhoek 2009, 96, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hynninen, A.; Touze, T.; Pitkanen, L.; Mengin-Lecreulx, D.; Virta, M. An efflux transporter PbrA and a phosphatase PbrB cooperate in a lead-resistance mechanism in bacteria. Mol. Microbiol. 2009, 74, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Grass, G.; Anton, A.; Franke, S.; Santos, A.N.; Lawley, B.; Brown, N.L.; Nies, D.H. Transcriptional organization of the czc heavy-metal homeostasis determinant from Alcaligenes eutrophus. J. Bacteriol. 1999, 181, 2385–2393. [Google Scholar] [CrossRef]
- Nies, D.; Mergeay, M.; Friedrich, B.; Schlegel, H.G. Cloning of plasmid genes encoding resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus CH34. J. Bacteriol. 1987, 169, 4865–4868. [Google Scholar] [CrossRef]
- Tseng, T.T.; Gratwick, K.S.; Kollman, J.; Park, D.; Nies, D.H.; Goffeau, A.; Saier, M.H., Jr. The RND permease superfamily: An ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1999, 1, 107–125. [Google Scholar]
- Vandenbussche, G.; Mergeay, M.; Van Houdt, R. Metal Response in Cupriavidus Metallidurans, Volume II: Insights into the Structure-Function Relationship of Proteins; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Rensing, C.; Pribyl, T.; Nies, D.H. New functions for the three subunits of the CzcCBA cation-proton antiporter. J. Bacteriol. 1997, 179, 6871–6879. [Google Scholar] [CrossRef]
- Seeger, M.A.; Schiefner, A.; Eicher, T.; Verrey, F.; Diederichs, K.; Pos, K.M. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 2006, 313, 1295–1298. [Google Scholar] [CrossRef]
- Murakami, S.; Nakashima, R.; Yamashita, E.; Matsumoto, T.; Yamaguchi, A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 2006, 443, 173–179. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Totrov, M. Vacuuming the periplasm. J Bacteriol 2005, 187, 1879–1883. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scherer, J.; Nies, D.H. CzcP is a novel efflux system contributing to transition metal resistance in Cupriavidus metallidurans CH34. Mol. Microbiol. 2009, 73, 601–621. [Google Scholar] [CrossRef]
- van der Lelie, D.; Schwuchow, T.; Schwidetzky, U.; Wuertz, S.; Baeyens, W.; Mergeay, M.; Nies, D.H. Two-component regulatory system involved in transcriptional control of heavy-metal homoeostasis in Alcaligenes eutrophus. Mol. Microbiol. 1997, 23, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Legatzki, A.; Grass, G.; Anton, A.; Rensing, C.; Nies, D.H. Interplay of the Czc system and two P-type ATPases in conferring metal resistance to Ralstonia metallidurans. J. Bacteriol. 2003, 185, 4354–4361. [Google Scholar] [CrossRef] [PubMed]
- Munkelt, D.; Grass, G.; Nies, D.H. The chromosomally encoded cation diffusion facilitator proteins DmeF and FieF from Wautersia metallidurans CH34 are transporters of broad metal specificity. J. Bacteriol. 2004, 186, 8036–8043. [Google Scholar] [CrossRef]
- De Angelis, F.; Lee, J.K.; O’Connell, J.D., 3rd; Miercke, L.J.; Verschueren, K.H.; Srinivasan, V.; Bauvois, C.; Govaerts, C.; Robbins, R.A.; Ruysschaert, J.M.; et al. Metal-induced conformational changes in ZneB suggest an active role of membrane fusion proteins in efflux resistance systems. Proc. Natl. Acad. Sci. USA 2010, 107, 11038–11043. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.E.; Ekende, E.N.; Kifle, E.G.; O’Connell, J.D., 3rd; De Angelis, F.; Tessema, M.B.; Derfoufi, K.M.; Robles-Colmenares, Y.; Robbins, R.A.; Goormaghtigh, E.; et al. Structures of intermediate transport states of ZneA, a Zn(II)/proton antiporter. Proc. Natl. Acad. Sci. USA 2013, 110, 18484–18489. [Google Scholar] [CrossRef]
- Mergeay, M.; Van Houdt, R. Metal Response in Cupriavidus Metallidurans: Volume I: From Habitats to Genes and Proteins; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop, R.M., 2nd; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Chang, A.C.; Cohen, S.N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 1978, 134, 1141–1156. [Google Scholar] [CrossRef]
- Katzen, F.; Becker, A.; Ielmini, M.V.; Oddo, C.G.; Ielpi, L. New mobilizable vectors suitable for gene replacement in gram-negative bacteria and their use in mapping of the 3′ end of the Xanthomonas campestris pv. campestris gum operon. Appl. Environ. Microb. 1999, 65, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Monsieurs, P.; Moors, H.; Van Houdt, R.; Janssen, P.J.; Janssen, A.; Coninx, I.; Mergeay, M.; Leys, N. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. BioMetals 2011, 24, 1133–1151. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol. 1992, 174, 8102–8110. [Google Scholar] [CrossRef] [PubMed]
- Monchy, S.; Benotmane, M.A.; Janssen, P.; Vallaeys, T.; Taghavi, S.; van der Lelie, D.; Mergeay, M. Plasmids pMOL28 and pMOL30 of Cupriavidus metallidurans are specialized in the maximal viable response to heavy metals. J. Bacteriol. 2007, 189, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Lenski, R.E. What is adaptation by natural selection? Perspectives of an experimental microbiologist. PLoS Genet. 2017, 13, e1006668. [Google Scholar] [CrossRef]
- LaCroix, R.A.; Sandberg, T.E.; O’Brien, E.J.; Utrilla, J.; Ebrahim, A.; Guzman, G.I.; Szubin, R.; Palsson, B.O.; Feist, A.M. Use of adaptive laboratory evolution to discover key mutations enabling rapid growth of Escherichia coli K-12 MG1655 on glucose minimal medium. Appl. Environ. Microbiol. 2015, 81, 17–30. [Google Scholar] [CrossRef]
- McCloskey, D.; Xu, S.; Sandberg, T.E.; Brunk, E.; Hefner, Y.; Szubin, R.; Feist, A.M.; Palsson, B.O. Growth Adaptation of gnd and sdhCB Escherichia coli Deletion Strains Diverges From a Similar Initial Perturbation of the Transcriptome. Front. Microbiol. 2018, 9, 1793. [Google Scholar] [CrossRef]
- Sandberg, T.E.; Pedersen, M.; LaCroix, R.A.; Ebrahim, A.; Bonde, M.; Herrgard, M.J.; Palsson, B.O.; Sommer, M.; Feist, A.M. Evolution of Escherichia coli to 42 degrees C and subsequent genetic engineering reveals adaptive mechanisms and novel mutations. Mol. Biol. Evol. 2014, 31, 2647–2662. [Google Scholar] [CrossRef]
- Maertens, L.; Leys, N.; Matroule, J.Y.; Van Houdt, R. The Transcriptomic Landscape of Cupriavidus metallidurans CH34 Acutely Exposed to Copper. Genes 2020, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yip, C.B.; Geddes, B.A.; Oresnik, I.J.; Hynes, M.F. Glycerol utilization by Rhizobium leguminosarum requires an ABC transporter and affects competition for nodulation. Microbiology 2012, 158, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Ye, S.; Larson, T.J. Repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K-12: Primary structure and identification of the DNA-binding domain. J. Bacteriol. 1996, 178, 7080–7089. [Google Scholar] [CrossRef] [PubMed]
- Ollig, J.; Kloubert, V.; Weßels, I.; Haase, H.; Rink, L. Parameters Influencing Zinc in Experimental Systems in Vivo and in Vitro. Metals 2016, 6, 71. [Google Scholar] [CrossRef]
- Martin, J.H.; Sherwood Rawls, K.; Chan, J.C.; Hwang, S.; Martinez-Pastor, M.; McMillan, L.J.; Prunetti, L.; Schmid, A.K.; Maupin-Furlow, J.A. GlpR Is a Direct Transcriptional Repressor of Fructose Metabolic Genes in Haloferax volcanii. J. Bacteriol. 2018, 200, e00218–e00244. [Google Scholar] [CrossRef]
- Lin, E.C. Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 1976, 30, 535–578. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 354–411. [Google Scholar] [CrossRef]
- Ammendola, S.; Pasquali, P.; Pistoia, C.; Petrucci, P.; Petrarca, P.; Rotilio, G.; Battistoni, A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect. Immun. 2007, 75, 5867–5876. [Google Scholar] [CrossRef]
- Mikolay, A.; Nies, D.H. The ABC-transporter AtmA is involved in nickel and cobalt resistance of Cupriavidus metallidurans strain CH34. Antonie Van Leeuwenhoek 2009, 96, 183–191. [Google Scholar] [CrossRef]
- Edgar, R.J.; van Hensbergen, V.P.; Ruda, A.; Turner, A.G.; Deng, P.; Le Breton, Y.; El-Sayed, N.M.; Belew, A.T.; McIver, K.S.; McEwan, A.G.; et al. Discovery of glycerol phosphate modification on streptococcal rhamnose polysaccharides. Nat. Chem. Biol. 2019, 15, 463–471. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Trivedi, D.; Bewsher, A.; Lloyd, J.R. Biostimulation by Glycerol Phosphate to Precipitate Recalcitrant Uranium(IV) Phosphate. Environ. Sci. Technol. 2015, 49, 11070–11078. [Google Scholar] [CrossRef] [PubMed]
- Sarret, G.; Saumitou-Laprade, P.; Bert, V.; Proux, O.; Hazemann, J.L.; Traverse, A.; Marcus, M.A.; Manceau, A. Forms of zinc accumulated in the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2002, 130, 1815–1826. [Google Scholar] [CrossRef]
- Vandecraen, J.; Monsieurs, P.; Mergeay, M.; Leys, N.; Aertsen, A.; Van Houdt, R. Zinc-induced transposition of insertion sequence elements contributes to increased adaptability of Cupriavidus metallidurans. Front. Microbiol. 2016, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Vandecraen, J.; Chandler, M.; Aertsen, A.; Van Houdt, R. The impact of insertion sequences on bacterial genome plasticity and adaptability. Crit. Rev. Microbiol. 2017, 43, 709–730. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).