Streptococcus suis Induces Expression of Cyclooxygenase-2 in Porcine Lung Tissue
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Bacterial Strains and Recombinant Suilysin (SLY) Protein
2.3. Generation of Precision-Cut Lung Slice (PCLS) and Infection with Streptococcus suis (S. suis)
2.4. Isolatation of Primary Brochial Fibroblasts and Infection with S. suis
2.5. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
2.6. Prostaglandin E2 (PGE2) Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Immunofluorescence Staining of Histological Sections and Primary Fibroblasts
2.8. Microscopy and Image Analysis
2.9. Statistical Analysis
3. Results
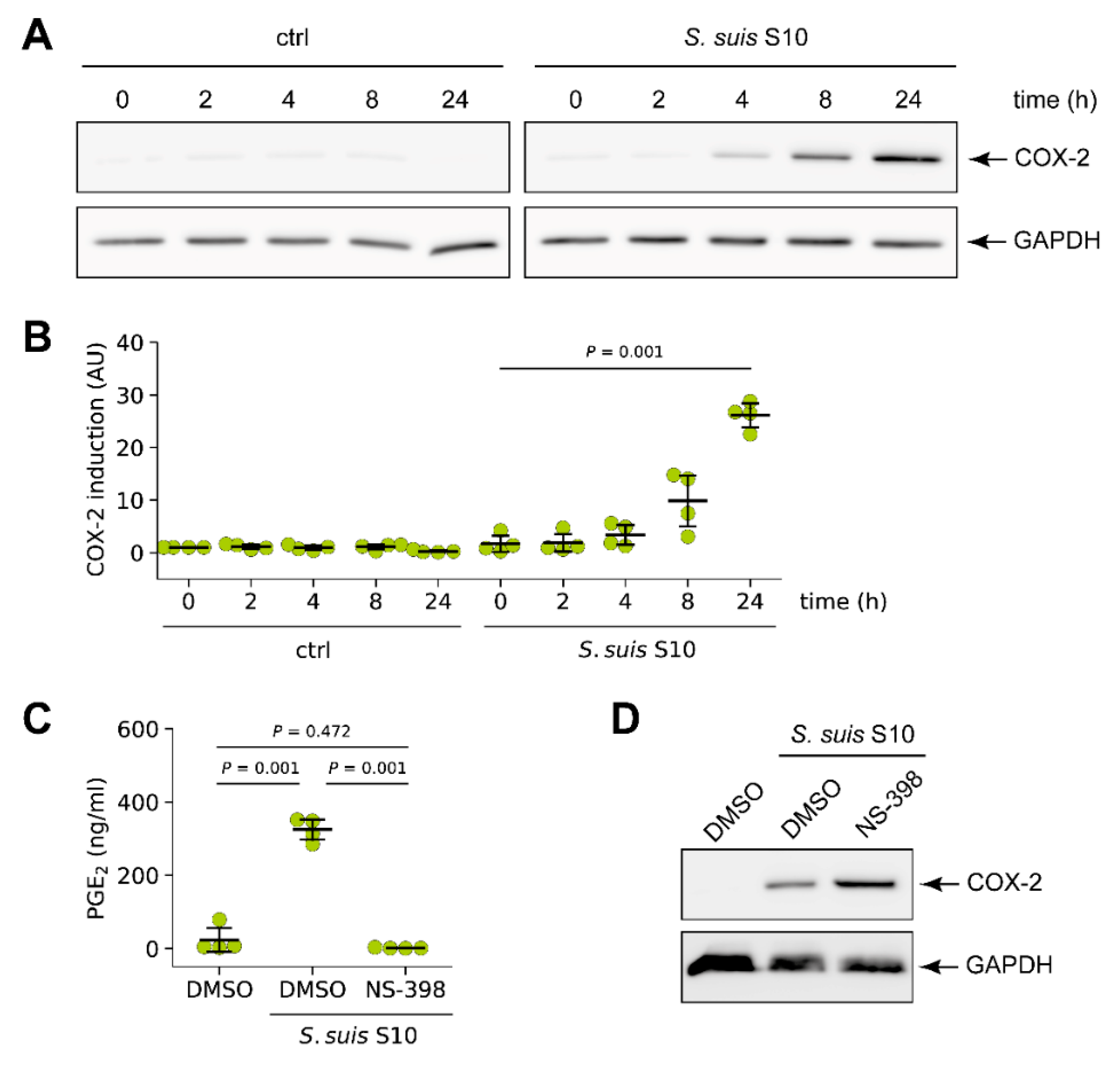
3.1. S. suis Induced Expression of Cyclooxygenase-2 (COX-2) in Porcine Lung Tissue
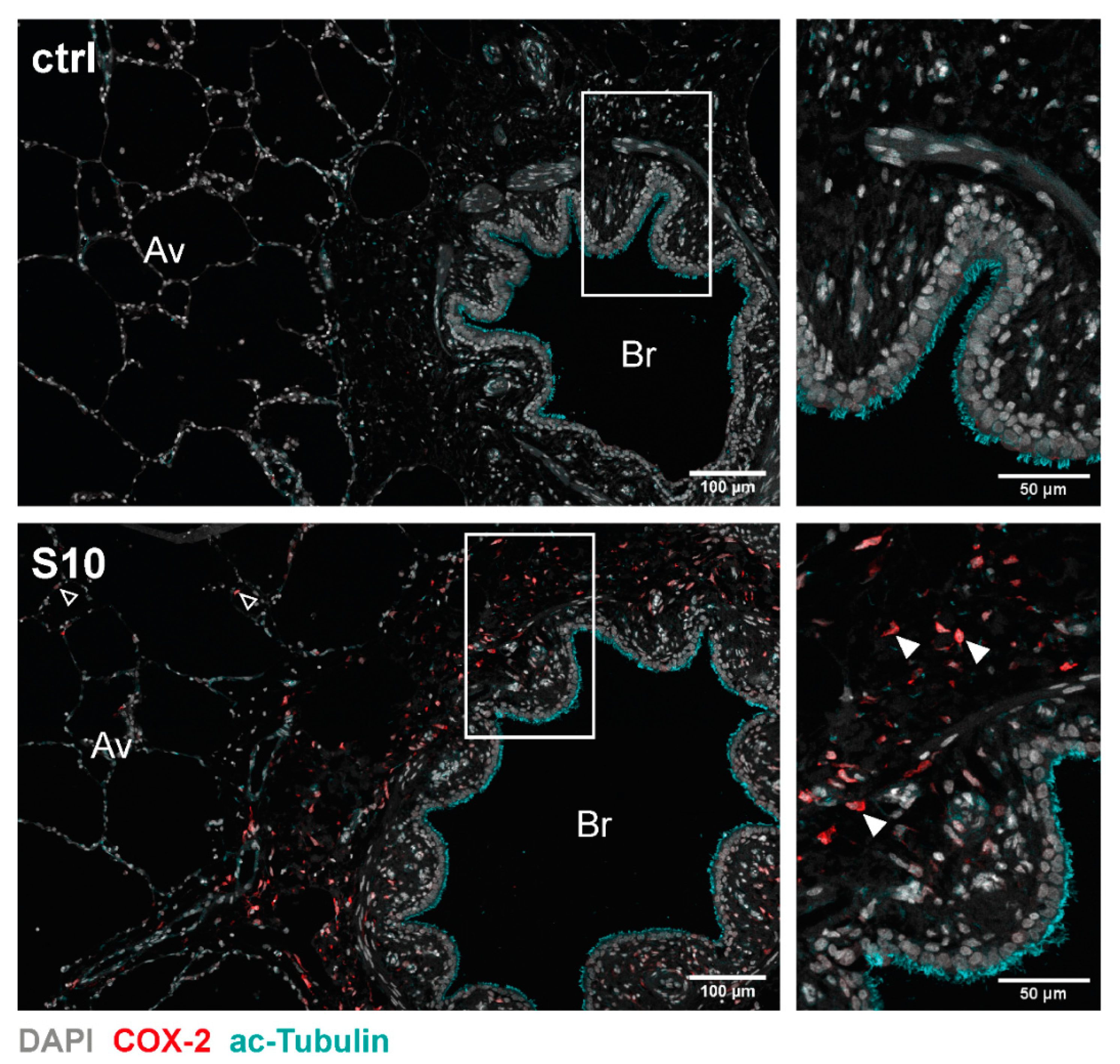
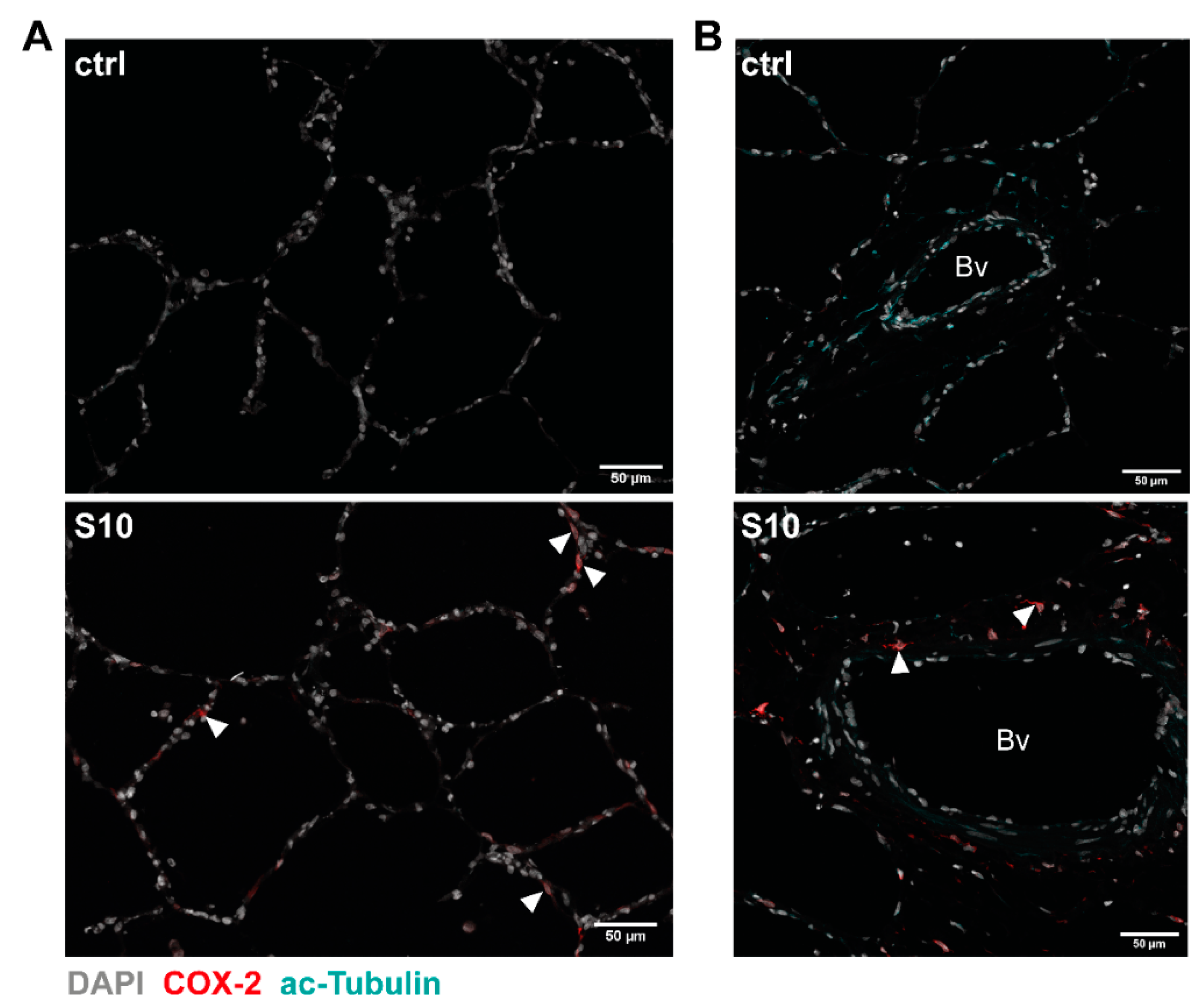
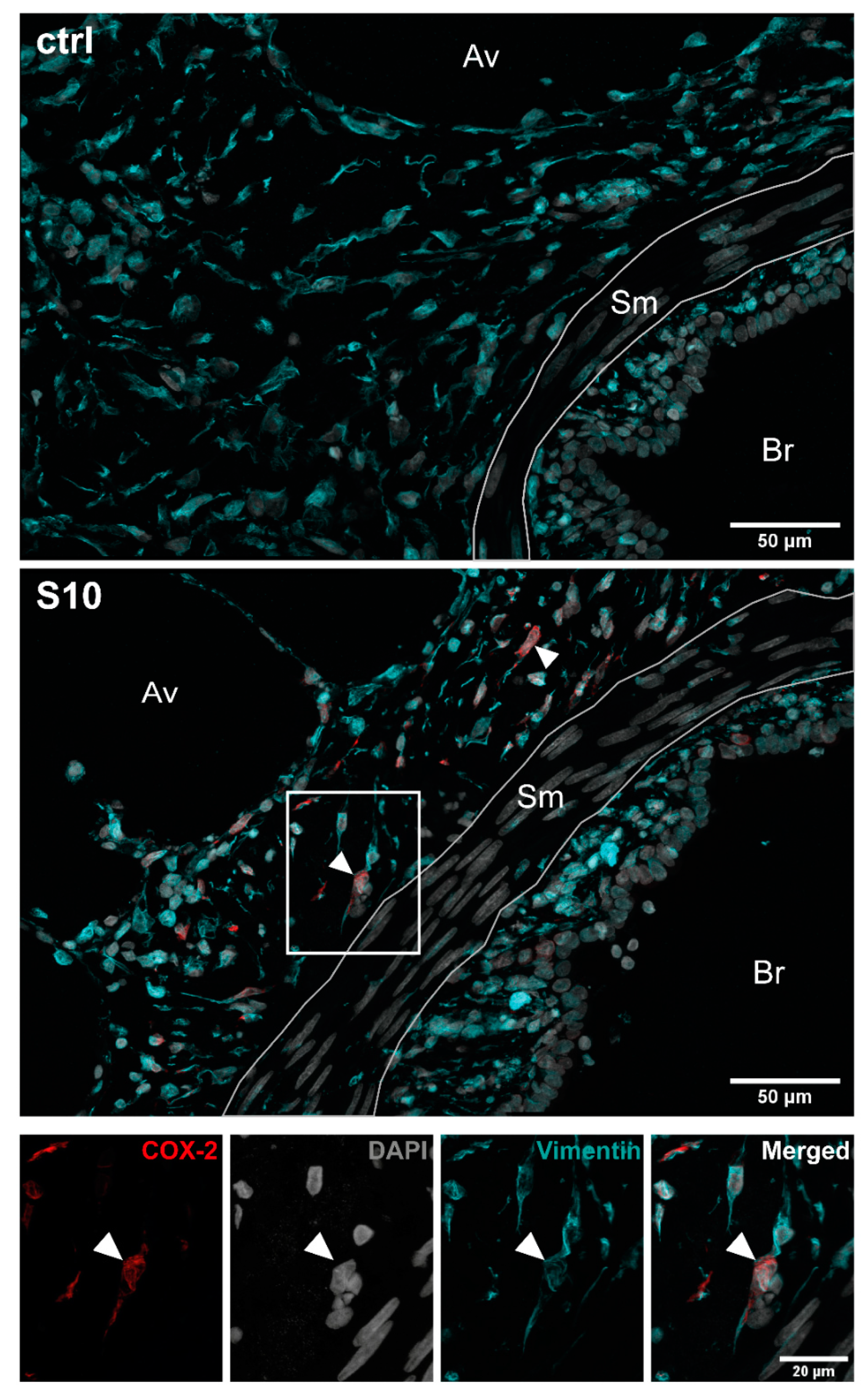
3.2. Localisation of COX-2 Induction in S. suis-Infected PCLS
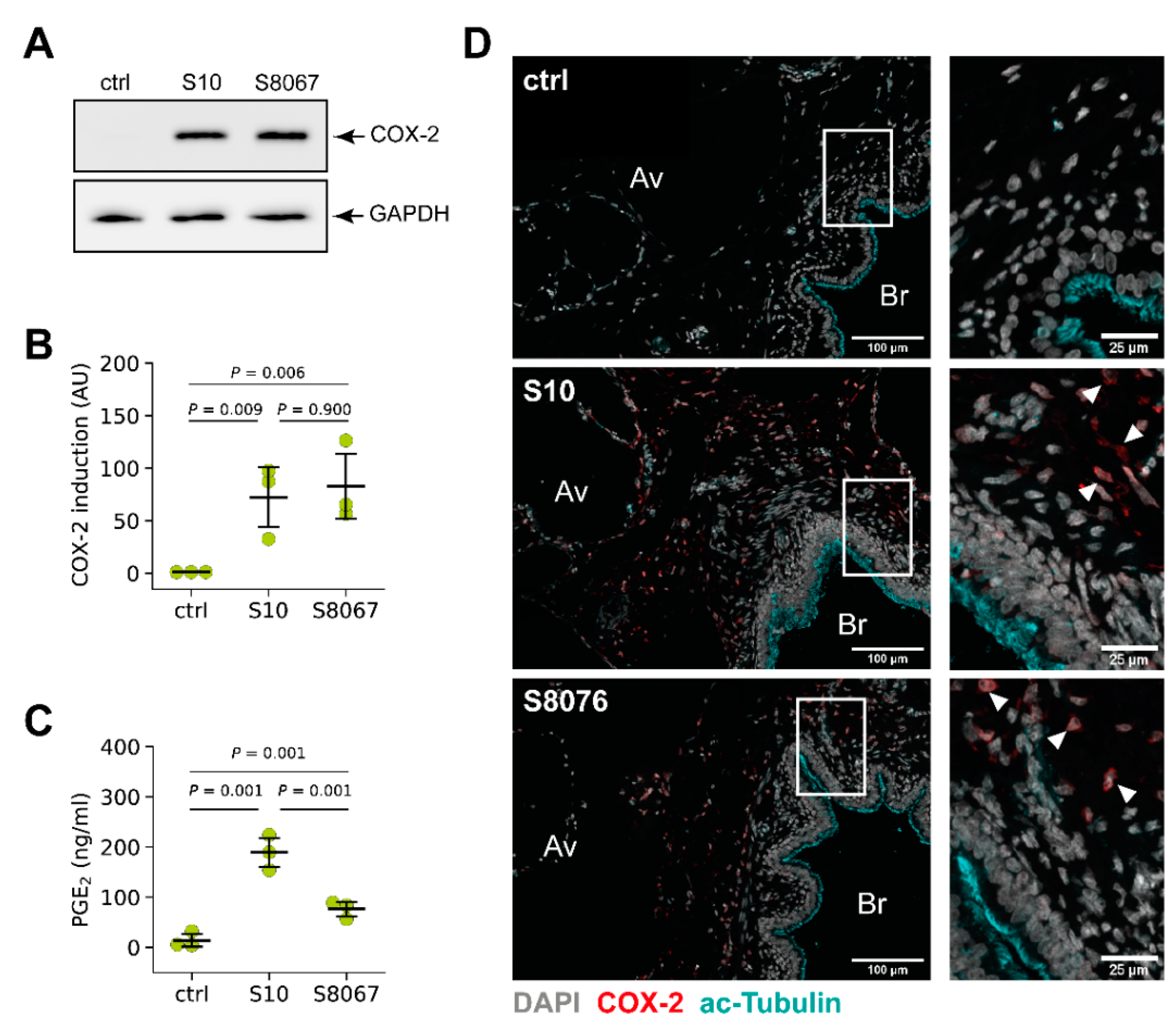
3.3. COX-2 is Induced by Two Prevalent S. suis Serotypes in Porcine PCLS
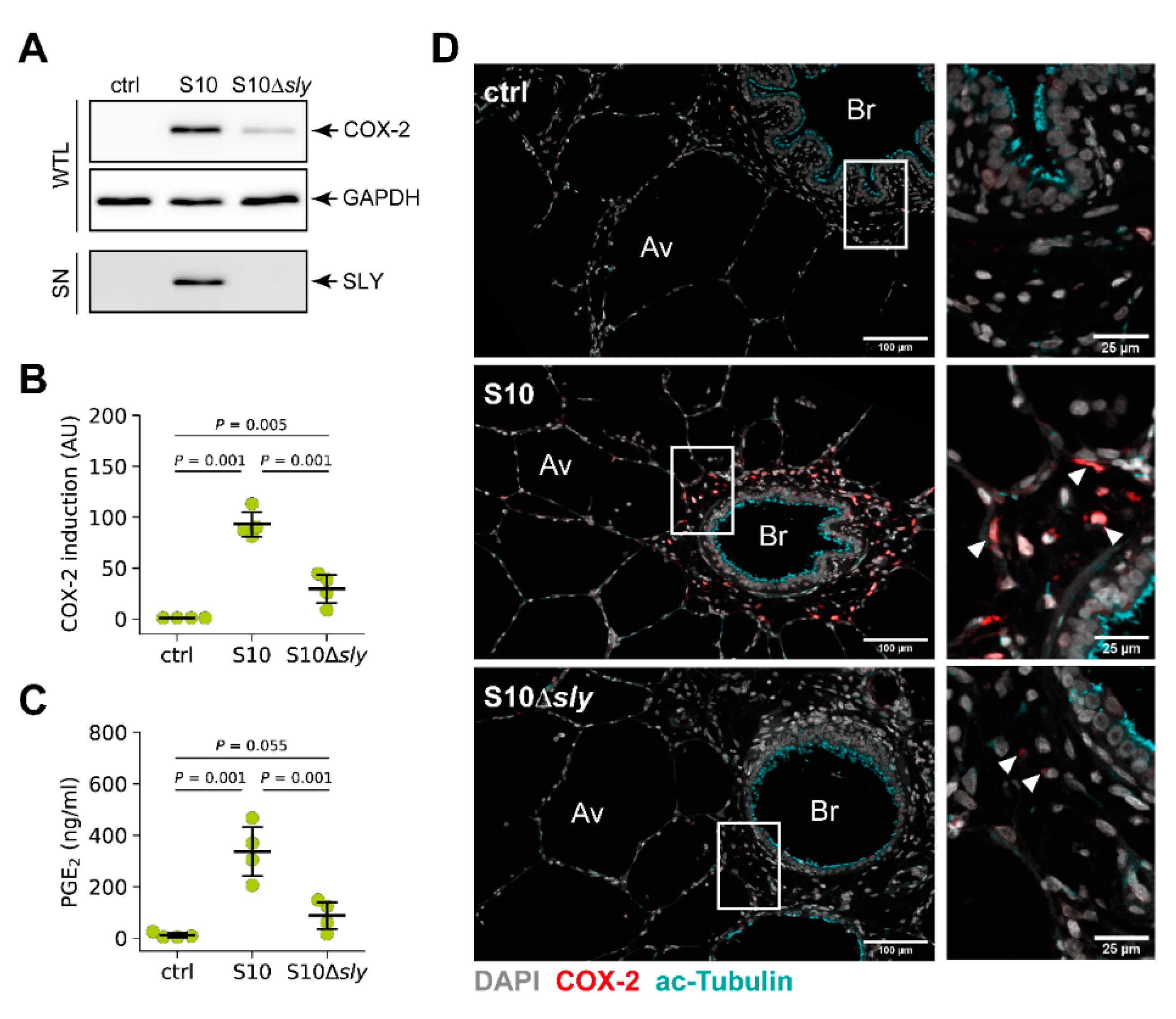
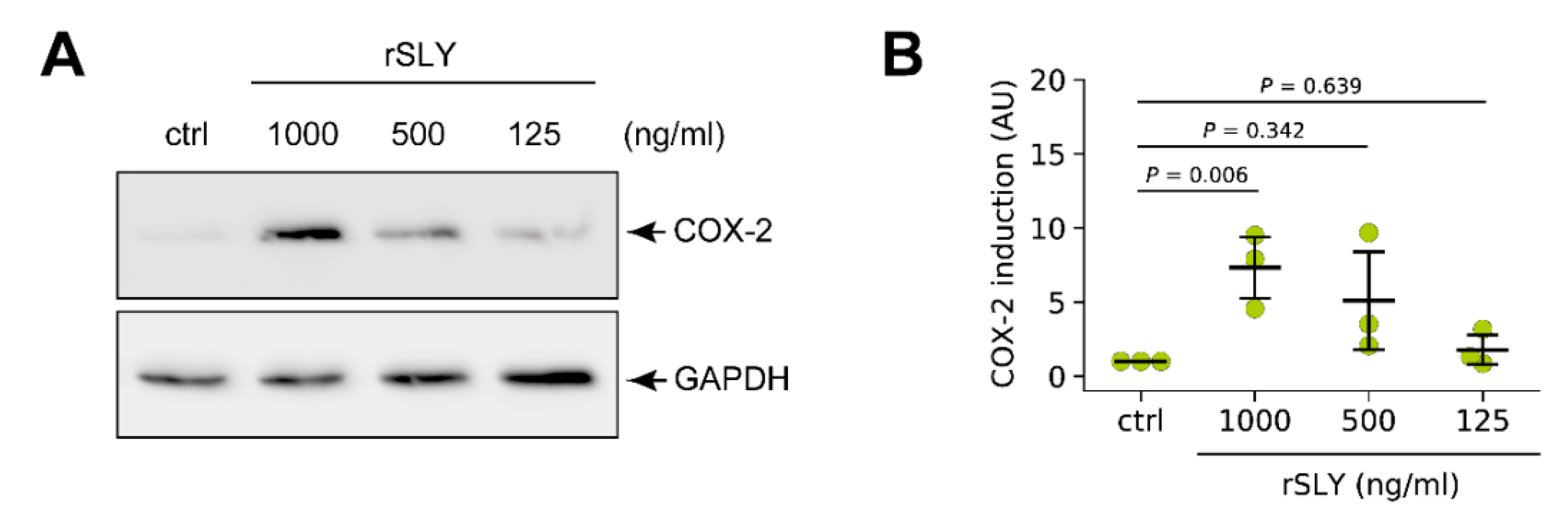
3.4. S. suis-Induced Expression of COX-2 in Porcine Lung Tissue is Modulated by SLY
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Vötsch, D.; Willenborg, M.; Weldearegay, Y.B.; Valentin-Weigand, P. Streptococcus suis—The "Two Faces" of a Pathobiont in the Porcine Respiratory Tract. Front. Microbiol. 2018, 9, 480. [Google Scholar] [CrossRef]
- Tang, J.; Wang, C.; Feng, Y.; Yang, W.; Song, H.; Chen, Z.; Yu, H.; Pan, X.; Zhou, X.; Wang, H.; et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med. 2006, 3, e151. [Google Scholar] [CrossRef]
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef]
- Okura, M.; Osaki, M.; Nomoto, R.; Arai, S.; Osawa, R.; Sekizaki, T.; Takamatsu, D. Current Taxonomical Situation of Streptococcus suis. Pathogens 2016, 5, 45. [Google Scholar] [CrossRef]
- Schultsz, C.; Jansen, E.; Keijzers, W.; Rothkamp, A.; Duim, B.; Wagenaar, J.A.; van der Ende, A. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS ONE 2012, 7, e33854. [Google Scholar] [CrossRef]
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Heuck, A.P.; Moe, P.C.; Johnson, B.B. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Sub-Cell. Biochem. 2010, 51, 551–577. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Park, G.Y.; Christman, J.W. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L797–L805. [Google Scholar] [CrossRef]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh, S.; et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 2000, 275, 32783–32792. [Google Scholar] [CrossRef]
- Martínez-Colón, G.J.; Moore, B.B. Prostaglandin E(2) as a Regulator of Immunity to Pathogens. Pharmacol. Ther. 2018, 185, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, O.; Hertzen, E.; Hecht, A.; Schmidt, H.; Lehne, S.; Norrby-Teglund, A.; Medina, E. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J. Immunol. 2010, 185, 2372–2381. [Google Scholar] [CrossRef]
- Szymanski, K.V.; Toennies, M.; Becher, A.; Fatykhova, D.; N’Guessan, P.D.; Gutbier, B.; Klauschen, F.; Neuschaefer-Rube, F.; Schneider, P.; Rueckert, J.; et al. Streptococcus pneumoniae-induced regulation of cyclooxygenase-2 in human lung tissue. Eur. Respir. J. 2012, 40, 1458–1467. [Google Scholar] [CrossRef]
- Andrada, M.; Quesada-Canales, O.; Suárez-Bonnet, A.; Paz-Sánchez, Y.; Espinosa de Los Monteros, A.; Rodríguez, F. Cyclooxygenase-2 expression in pigs infected experimentally with Mycoplasma hyopneumoniae. J. Comp. Pathol. 2014, 151, 271–276. [Google Scholar] [CrossRef]
- Cho, W.S.; Chae, C. Expression of nitric oxide synthase 2 and cyclooxygenase-2 in swine experimentally infected with Actinobacillus pleuropneumoniae. Vet. Pathol. 2004, 41, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Benga, L.; Fulde, M.; Neis, C.; Goethe, R.; Valentin-Weigand, P. Polysaccharide capsule and suilysin contribute to extracellular survival of Streptococcus suis co-cultivated with primary porcine phagocytes. Vet. Microbiol. 2008, 132, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.E.; Damman, M.; van der Velde, J.; Wagenaar, F.; Wisselink, H.J.; Stockhofe-Zurwieden, N.; Smits, M.A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: The capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 1999, 67, 1750–1756. [Google Scholar] [CrossRef]
- Meng, F.; Wu, N.H.; Seitz, M.; Herrler, G.; Valentin-Weigand, P. Efficient suilysin-mediated invasion and apoptosis in porcine respiratory epithelial cells after streptococcal infection under air-liquid interface conditions. Sci. Rep. 2016, 6, 26748. [Google Scholar] [CrossRef]
- Wisselink, H.J.; Smith, H.E.; Stockhofe-Zurwieden, N.; Peperkamp, K.; Vecht, U. Distribution of capsular types and production of muramidase-released protein (MRP) and extracellular factor (EF) of Streptococcus suis strains isolated from diseased pigs in seven European countries. Vet. Microbiol. 2000, 74, 237–248. [Google Scholar] [CrossRef]
- Meng, F.; Wu, N.H.; Nerlich, A.; Herrler, G.; Valentin-Weigand, P.; Seitz, M. Dynamic Virus-Bacterium Interactions in a Porcine Precision-Cut Lung Slice Coinfection Model: Swine Influenza Virus Paves the Way for Streptococcus suis Infection in a Two-Step Process. Infect. Immun. 2015, 83, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Paddenberg, R.; Mermer, P.; Goldenberg, A.; Kummer, W. Videomorphometric analysis of hypoxic pulmonary vasoconstriction of intra-pulmonary arteries using murine precision cut lung slices. J. Vis. Exp. 2014, e50970. [Google Scholar] [CrossRef]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Nerlich, A.; von Wunsch Teruel, I.; Mieth, M.; Hönzke, K.; Rückert, J.C.; Mitchell, T.J.; Suttorp, N.; Hippenstiel, S.; Hocke, A.C. Reversion of pneumolysin induced executioner caspase activation redirects cells to survival. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Vallat, R. Pingouin: Statistics in Python. J. Open Source Softw. 2018, 3, 1026. [Google Scholar] [CrossRef]
- Blaschke, U.; Beineke, A.; Klemens, J.; Medina, E.; Goldmann, O. Induction of Cyclooxygenase 2 by Streptococcus pyogenes Is Mediated by Cytolysins. J. Innate Immun. 2017, 9, 587–597. [Google Scholar] [CrossRef]
- Lyons-Cohen, M.R.; Thomas, S.Y.; Cook, D.N.; Nakano, H. Precision-cut Mouse Lung Slices to Visualize Live Pulmonary Dendritic Cells. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Suyarnsesthakorn, C.; Pittayakhajonwut, D.; Sriklung, K.; Kirtikara, K. Positive feedback regulation of COX-2 expression by prostaglandin metabolites. Inflamm. Res. 2005, 54, 163–172. [Google Scholar] [CrossRef]
- Ferguson, S.; Hébert, R.L.; Laneuville, O. NS-398 upregulates constitutive cyclooxygenase-2 expression in the M-1 cortical collecting duct cell line. J. Am. Soc. Nephrol. 1999, 10, 2261–2271. [Google Scholar]
- Pang, L.; Nie, M.; Corbett, L.; Knox, A.J. Cyclooxygenase-2 expression by nonsteroidal anti-inflammatory drugs in human airway smooth muscle cells: Role of peroxisome proliferator-activated receptors. J. Immunol. 2003, 170, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, T.; Legesse-Miller, A.; Hameed, M.R.; Aisner, S.C.; Randolph-Habecker, J.; Coller, H.A. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J. Histochem. Cytochem. 2008, 56, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Lacy, S.H.; Woeller, C.F.; Thatcher, T.H.; Maddipati, K.R.; Honn, K.V.; Sime, P.J.; Phipps, R.P. Human lung fibroblasts produce proresolving peroxisome proliferator-activated receptor-γ ligands in a cyclooxygenase-2-dependent manner. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L855–Ll867. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.F.; Allen, E.K.; Randolph, A.G.; Guo, X.J.; Weng, Y.; Sanders, C.J.; Bajracharya, R.; Lee, N.K.; Guy, C.S.; Vogel, P.; et al. Exuberant fibroblast activity compromises lung function via ADAMTS4. Nature 2020. [Google Scholar] [CrossRef]
- Zhang, Q.; Young, T.F.; Ross, R.F. Glycolipid receptors for attachment of Mycoplasma hyopneumoniae to porcine respiratory ciliated cells. Infect. Immun. 1994, 62, 4367–4373. [Google Scholar] [CrossRef]
- Jakobsson, P.J.; Thorén, S.; Morgenstern, R.; Samuelsson, B. Identification of human prostaglandin E synthase: A microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA 1999, 96, 7220–7225. [Google Scholar] [CrossRef]
- Kojima, F.; Naraba, H.; Miyamoto, S.; Beppu, M.; Aoki, H.; Kawai, S. Membrane-associated prostaglandin E synthase-1 is upregulated by proinflammatory cytokines in chondrocytes from patients with osteoarthritis. Arthritis Res. Ther. 2004, 6, R355–R365. [Google Scholar] [CrossRef]
- Wright, K.L.; Weaver, S.A.; Patel, K.; Coopman, K.; Feeney, M.; Kolios, G.; Robertson, D.A.; Ward, S.G. Differential regulation of prostaglandin E biosynthesis by interferon-gamma in colonic epithelial cells. Br. J. Pharmacol. 2004, 141, 1091–1097. [Google Scholar] [CrossRef]
- Lee, I.Y.; Cho, W.; Kim, J.; Park, C.S.; Choe, J. Human follicular dendritic cells interact with T cells via expression and regulation of cyclooxygenases and prostaglandin E and I synthases. J. Immunol. 2008, 180, 1390–1397. [Google Scholar] [CrossRef]
- Auger, J.P.; Payen, S.; Roy, D.; Dumesnil, A.; Segura, M.; Gottschalk, M. Interactions of Streptococcus suis serotype 9 with host cells and role of the capsular polysaccharide: Comparison with serotypes 2 and 14. PLoS ONE 2019, 14, e0223864. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Soyoola, E.; Chanmugam, P.; Hart, S.; Sun, W.; Zhong, H.; Liou, S.; Simmons, D.; Hwang, D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 1992, 267, 25934–25938. [Google Scholar] [CrossRef]
- Kim, H.; Rhee, S.H.; Kokkotou, E.; Na, X.; Savidge, T.; Moyer, M.P.; Pothoulakis, C.; LaMont, J.T. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J. Biol. Chem. 2005, 280, 21237–21245. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.; Segura, M.; Lacouture, S.; Gottschalk, M. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology 2000, 146 Pt 8, 1913–1921. [Google Scholar] [CrossRef]
- Charland, N.; Nizet, V.; Rubens, C.E.; Kim, K.S.; Lacouture, S.; Gottschalk, M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect. Immun. 2000, 68, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Lavagna, A.; Auger, J.P.; Dumesnil, A.; Roy, D.; Girardin, S.E.; Gisch, N.; Segura, M.; Gottschalk, M. Interleukin-1 signaling induced by Streptococcus suis serotype 2 is strain-dependent and contributes to bacterial clearance and inflammation during systemic disease in a mouse model of infection. Vet. Res. 2019, 50, 52. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresen, M.; Schenk, J.; Berhanu Weldearegay, Y.; Vötsch, D.; Baumgärtner, W.; Valentin-Weigand, P.; Nerlich, A. Streptococcus suis Induces Expression of Cyclooxygenase-2 in Porcine Lung Tissue. Microorganisms 2021, 9, 366. https://doi.org/10.3390/microorganisms9020366
Dresen M, Schenk J, Berhanu Weldearegay Y, Vötsch D, Baumgärtner W, Valentin-Weigand P, Nerlich A. Streptococcus suis Induces Expression of Cyclooxygenase-2 in Porcine Lung Tissue. Microorganisms. 2021; 9(2):366. https://doi.org/10.3390/microorganisms9020366
Chicago/Turabian StyleDresen, Muriel, Josephine Schenk, Yenehiwot Berhanu Weldearegay, Désirée Vötsch, Wolfgang Baumgärtner, Peter Valentin-Weigand, and Andreas Nerlich. 2021. "Streptococcus suis Induces Expression of Cyclooxygenase-2 in Porcine Lung Tissue" Microorganisms 9, no. 2: 366. https://doi.org/10.3390/microorganisms9020366
APA StyleDresen, M., Schenk, J., Berhanu Weldearegay, Y., Vötsch, D., Baumgärtner, W., Valentin-Weigand, P., & Nerlich, A. (2021). Streptococcus suis Induces Expression of Cyclooxygenase-2 in Porcine Lung Tissue. Microorganisms, 9(2), 366. https://doi.org/10.3390/microorganisms9020366






