Abstract
Uropathogenic Escherichia coli (UPEC) is a major bacterial pathogen that causes urinary tract infections (UTIs). The mouse is an available UTI model for studying the pathogenicity; however, Caenorhabditis elegans represents as an alternative surrogate host with the capacity for high-throughput analysis. Then, we established a simple assay for a UPEC infection model with C. elegans for large-scale screening. A total of 133 clinically isolated E. coli strains, which included UTI-associated and fecal isolates, were applied to demonstrate the simple pathogenicity assay. From the screening, several virulence factors (VFs) involved with iron acquisition (chuA, fyuA, and irp2) were significantly associated with high pathogenicity. We then evaluated whether the VFs in UPEC were involved in the pathogenicity. Mutants of E. coli UTI89 with defective iron acquisition systems were applied to a solid killing assay with C. elegans. As a result, the survival rate of C. elegans fed with the mutants significantly increased compared to when fed with the parent strain. The results demonstrated, the simple assay with C. elegans was useful as a UPEC infectious model. To our knowledge, this is the first report of the involvement of iron acquisition in the pathogenicity of UPEC in a C. elegans model.
1. Introduction
Urinary tract infections (UTIs) are very common [1], and the resulting direct medical cost is substantial [2]. Uropathogenic E. coli (UPEC) is one of the major etiologies of UTIs. A UPEC strain usually requires an array of virulence factors (VFs) with different functions to invade the urinary tract. The VFs of pathogenic bacteria are potential antimicrobial targets and markers of the pathogen. However, different UPEC strains may harbor varying combinations of VFs, and most of the factors only exist in a fraction of UPEC strains [3]. Thus, a combination of multiple VFs is required for developing effective and widely usable measures to prevent or treat UTIs caused by UPEC. Identifying how VFs contribute to UTIs and understanding their epidemiological distribution would facilitate the development of such novel strategies to manage the infection.
The mouse is generally used as an animal model for studying the pathogenicity of UPEC. From a technical, economical, and an ethical viewpoint, however, the model is not suitable for use in large-scale studies. On the other hand, the Caenorhabditis elegans model is cheap, easy to handle, and has been applied in various bacterial pathogenicity studies such as with Pseudomonas aeruginosa [4], Enterococcus [5], Serratia marcescens [6], and E. coli [7,8,9,10,11,12,13] including UPEC [11,12]. For the UPEC study, the results revealed that the pathogenicity in C. elegans was associated with the number of VFs, which were identified as virulence genes involved in the pathogenicity in mammals [11]. Namely, the VFs involved in the mammal infection are proposed to function in the C. elegans model, although the statistical association had not been proven in previous studies using mutant VF.
In most of the aforementioned C. elegans models, synchronized C. elegans animals were incubated with a lawn of interested bacteria on a plate, and the survival rates of the animals were determined during the examination (solid killing assay) [4,5,6,7,8,9,11,12]. The surviving C. elegans must be transferred to a fresh plate with the lawn every day to avoid affection of newborn C. elegans animals. Therefore, a liquid killing assay was developed [13], because the copious handling for transferring of C. elegans makes it challenging to use in large-scale screening. In the liquid killing assay, C. elegans incubated with the lawn of interested bacteria was harvested with liquid medium from the plate after incubation for 1 day, and the survivability of C. elegans in the liquid medium was observed in a time dependent manner. However, there are still rooms to simplify the assay further. We recently developed another liquid pathogenicity assay to screen a transposon mutant library of E. coli o157 comprised of 17,802 mutants [10]. Pathogenicity was evaluated by measuring the OD595nm value of a mixture of mutant E. coli and C. elegans in 96-well plates after 8 days of incubation.
In this study, we modified the liquid pathogenicity assay for high-throughput screening to apply it to 133 clinically isolated E. coli, including 83 UTI-associated isolates and 50 fecal isolates, and we analyzed the association between pathogenicity in C. elegans and the VFs in the isolates. In addition, we demonstrated that the iron acquisition systems associated with the pathogenicity of UPEC are involved in the virulence in C. elegans.
2. Materials and Methods
2.1. Strain and Culture of Bacteria and Nematodes
Eighty-one E. coli isolates from patients with UTI-related symptoms from National Cheng Kung University Hospital (NCKUH) in Taiwan from January to April 2006 were studied as UTI-associated isolates [14]. A total of 49 E. coli fecal isolates from healthy volunteers were also collected from NCKUH between June 2006 and April 2007 [14]. In addition, E. coli UTI89 and CFT073 were used as model UPEC, and E. coli K-12 MG1655 as a model non-pathogenic E. coli. These E. coli stains were cultured in lysogeny broth (LB) at 37 °C with vigorous shaking [15].
C. elegans N2 as wild type was used for the solid killing assay. The worm was maintained on NGM agar plates with E. coli OP50 as described previously [16]. C. elegans glp-4(bn2ts) was used for the liquid pathogenicity assay. The temperature-sensitive strain was maintained at 15 °C for animal preparation.
2.2. Phylogenetic and Virulence Factor Analyses
For the 133 E. coli used in this study, the phylogenetic group and 31 VFs identified previously in UPEC were determined by PCR as described previously [17,18,19,20,21]. The primers used in these analyses are listed in Table S1.
2.3. Mutant Construction of E. coli UTI89
Gene deletion in E. coli UTI89 was performed by applying the lambda red system with pKD46 as described previously [22]. To delete chuA, the CmR cassette on pKD3 was PCR-amplified with primers (chuA-P1 and chuA-P2), and the parent strain with pKD46 was mutated with the CmR cassette. The deletion of fyuA was performed similarly with chuA replaced with the KmR cassette amplified by PCR with pKD4 as template, and fyuA-P1 and fyuA-P2 as primers. A plasmid expressing Flp (pCP20) was used to remove the antibiotic markers flanked by FLPs. For entA deletion, the ∆entA::Km region from the KEIO collection (JW0588-KC) was amplified with 165-1 and 165-2, and applied for the lambda red recombination [23]. The genetic structures of all the recombination were confirmed by PCR. E. coli UTI89 and its derivatives that were used for solid killing assay are listed in Table S2.
2.4. Liquid Pathogenicity Assay
The pathogenicity assay method has been previously described in detail [10]. In brief, C. elegans glp-4(bn2ts), which is temperature-sensitive for pregnancy, was used to fix C. elegans generation during the assay. E. coli cells at an OD600nm of 0.2 that were in the stationary phase cultured in LB medium were transferred to a 96-well plate. Next, the plate was centrifuged (3700 rpm, 10 min, 25 °C), the resulting pellet of E. coli cells collected, and the supernatant discarded. Synchronized C. elegans in late L4 stage were prepared, and approximately 30 animals in 200 µL of S medium were added to the E. coli pellet. The bacteria and animals were co-incubated at 25 °C with shaking (100 rpm) for 8 days, at which time the OD592nm was measured and normalized with the value at 0 day. The experiments were performed independently three times.
2.5. Solid Killing Assay
The survivability of the C. elegans N2 strain feeding the E. coli UTI89 wild type or its mutants was measured as described previously [7], but with some modification. Briefly, E. coli strains were cultured in LB medium at 37 °C until at an OD600nm of 2.0, and 30 µL of the culture was spread on a 5.0 cm NGM agar plate and incubated at 37 °C overnight. On the next day, approximately 50 synchronized C. elegans N2 animals in late L4 stage were inoculated on each plate and incubated at 20 °C for 10 days. During the assay, the survivability of the C. elegans on the plate was measured daily, and living animals were transferred to a fresh plate. Nematoda that did not respond to gentle prodding were scored as dead. Animals that crawled off the plate were censored. The experiments were performed with approximately 100 worms total per E. coli strain.
2.6. Statistical Analysis
The statistical analysis of the phylogenetic and virulence factor data was performed with Fischer’s exact test. The Mann-Whitney U test was used for analysis of the liquid pathogenicity data. For the solid killing assay, the Mantel-Cox log-rank test was used to assess the statistical significance of the difference in survival of the C. elegans. These analyses were done by using GraphPad Prism (version 7.0, GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Epidemiological Characterization of the Clinically Isolated E. coli Strains
The phylogenetic group and VFs in 83 E. coli strains isolated from UTIs and 50 fecal isolates were determined to characterize them. Based on the syndromes of these UTI-associated strains, they were further divided into the lower UTI (cystitis associated strains), upper UTI (pyelonephritis associated strains), and urosepsis groups (Table S3). The phylogenetic groups of the clinical isolates are indicated in Table 1. Most of the UTI-associated isolates belonged to the B2 group, which mainly consists of extraintestinal pathogenic strains that display a high concentration of VFs [3]. In contrast, most of the fecal isolates are associated with phylogenetic group A, whose member strains are often devoid of extraintestinal VFs [24]. The distribution of 31 genes identified as uropathogenic VFs [18,19,20,21] were determined in the 133 E. coli isolates (Table 1). Eighteen genes showed significantly higher distribution in the UTI-associated isolates than was observed in the fecal isolates, while the level of ibeA was higher in the fecal isolates. The total number of VFs identified was significantly higher in the UTI-associated isolates than in the fecal isolates (Figure 1).

Table 1.
Characterization of the 133 E. coli clinical isolates.
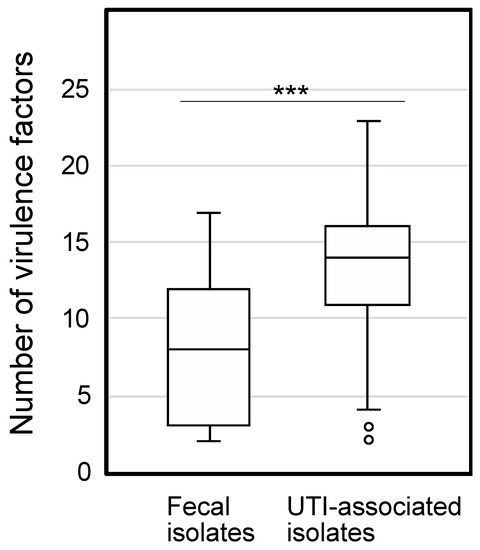
Figure 1.
The number of virulence factors in clinically isolated E. coli. The boxplot indicates the number of VFs in fecal and UTI-associated isolates. The numbers of VFs between fecal and UTI-associated isolates (average 8.1 and 13.2, respectively) show a significant difference by the Mann-Whitney U test (***, p < 0.001).
3.2. Liquid Pathogenicity Assay of E. coli with C. elegans
The pathogenicity of the E. coli isolates was determined by a liquid assay with C. elegans in which both species were incubated in S medium on 96-well plate for 8 days, followed by OD595nm measurement to determine E. coli survival. When a tested E. coli strain is non-pathogenic, the turbidity will be low after incubation because the E. coli cells are fed on by C. elegans. In contrast, when a C. elegans is not healthy with a pathogenic E. coli, the bacterial cells survive and show higher turbidity. As shown in Figure 2, the pathogenicity of UTI-associated isolates was significantly higher than that of the fecal isolates. The result is consistent with the higher number of VFs in UTI-associated isolates (Figure 1) and suggests that the C. elegans model reflects the pathogenicity in patients. In comparing symptoms, the isolates associated with lower and upper UTIs showed significantly higher pathogenicity than the fecal isolates, while the pathogenicity of urosepsis was comparable with that of commensal E. coli (Figure S1). Since the number of VFs in urosepsis was significantly higher than in the fecal isolates (Figure S2), the VFs for urosepsis might not be involved in the pathogenicity in C. elegans. Regarding the phylogenetic groups, the pathogenicity of groups B1, B2, and D was significantly higher than that of group A (Figure S1). However, the number of VFs in group B1 was comparable with group A (Figure S2). Perhaps, isolates in group B1 bear unidentified VFs for C. elegans. Seven VFs showed a significant association with the pathogenicity in C. elegans (Table 2). Three of the seven VFs belonged to the iron acquisition group, while the other four were in the other-group. These results suggest that the VFs for iron acquisition participate in pathogenicity in C. elegans.
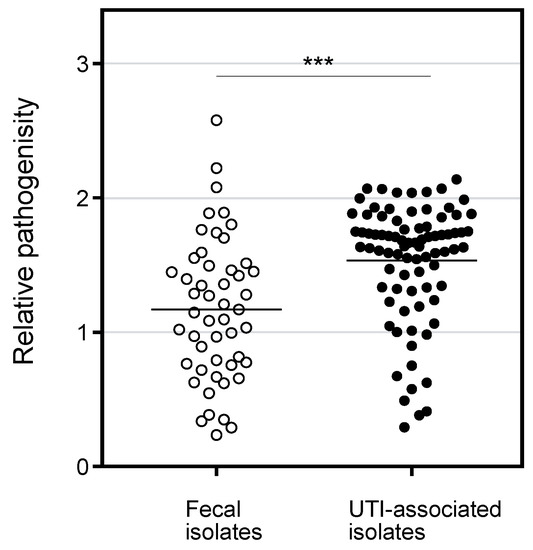
Figure 2.
Pathogenicity of E. coli clinical isolates in liquid killing assay with C. elegans. The pathogenicity (relative OD592nm value in the liquid assay) of the fecal and UTI-associated isolates were plotted. The value for MG1655 as a model strain for fecal isolate was 1.17, and that for UTI89 as a model strain for UTI associated isolate was 1.75. The horizontal bars represented the mean values. The results were analyzed statically by the Mann-Whitney U test. (***, p < 0.01). The pathogenicity of UTI-associated isolates was significantly higher than that of fecal isolates.

Table 2.
The association between pathogenicity and virulence factor.
3.3. Iron Acquisition of UPEC Involved in the Pathogenicity in C. elegans
To investigate whether VFs for iron acquisition (chuA, irp2, fyuA) are involved in the pathogenicity in C. elegans, UPEC mutants defective in the genes were applied to the animal model. E. coli UTI89 uses heme and produces the three siderophores yersiniabactin, enterobactin, and salmochelin [25]. One of the VFs, chuA, is a receptor for heme acquisition [26] and has been reported to be involved in kidney infection in the mouse model [27]. The other two genes are for yersiniabactin, and irp2 is for biosynthesis and fyuA is a receptor for the siderophore [28,29]. The genes for enterobactin were not analyzed in the liquid pathogenicity assay because it is found in most E. coli strains. However, because the involvement of enterobactin in mammal infection has been reported in UPEC [30], it was also investigated in this study. In addition, the mutant defective in enterobactin synthesis (∆entA) does not produce either enterobactin and salmochelin, because salmochelin is derived from enterobactin [31]. Multiple iron acquisition systems are generally identified in UPEC, and the redundancy is important for infection [32]. Therefore, the mutants combinatorially defective in heme acquisition (∆chuA), yersiniabactin acquisition (∆fyuA), and enterobactin and salmochelin production (∆entA) were constructed. As a result, all the mutant strains defective in the VFs for iron acquisition showed lower pathogenicity than wild type (Figure 3). The pathogenicity of a single mutation of chuA and fyuA, which were associated with pathogenicity in the liquid assay, were attenuated. A double mutant (∆chuA ∆fyuA) showed lower pathogenicity than each of the single mutants. The pathogenicity of the entA single mutation was also decreased compared to wild type. Although the difference in the pathogenicity of triple mutant ∆chuA ∆fyuA ∆entA was not statically significant compared with the double mutant, and the finding still supported the trend in decreasing pathogenicity. Taken together, these results demonstrated that iron acquisition is involved in the pathogenicity of UPEC in C. elegans. However, another mechanism might also be involved, because over 50% of animal survive for two weeks with harmless E. coli OP50.
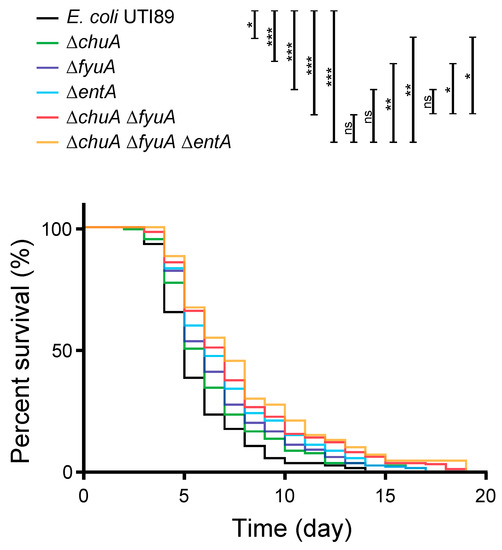
Figure 3.
UPEC mutants defective in iron acquisition decreased the pathogenicity in C. elegans. The survivability of C. elegans (n > 100) feeding on the wild type and mutants of E. coli UTI89 defective in iron acquisition, which shows association with pathogenicity in the liquid pathogenicity assay. The results were analyzed statically by the Mantel-Cox log-rank test (*, p > 0.05; **, p > 0.01; ***, p > 0.001; ns, not significant).
4. Discussion
4.1. Liquid Pathogenicity Assay
The pathogenicity of E. coli associated with UTIs was determined using C. elegans. Although several reports have been published on bacterial pathogenicity using C. elegans, the studies used C. elegans killing assay, which is based on counting the animals to determine survivability on a solid or in a liquid medium [4,5,6,7,8,9,11,12]. Here, we adopted a liquid pathogenicity assay in which C. elegans and E. coli were co-incubated, and the turbidity was compared before and after the incubation. Although the method has been applied for a mutant library derived from one parent strain [10], this is the first report to apply it to numerous clinical isolates. Since the clinically isolated E. coli associated with UTIs showed significantly higher pathogenicity than the commensal strains, the results of the simple method in this study reflected the pathogenicity in the patient (Figure 2). Besides, we found that the numbers of VFs belonging to the toxin, adhesin, and iron acquisition were detected with higher frequency in UTI-associated isolates than in feces isolates (Table 1). However, only 7 VFs belonging to the iron acquisition and other-group showed significant association with high pathogenicity in C. elegans (Table 2). Perhaps the VFs toxin and adhesin are not involved in the pathogenicity in C. elegans. Alternatively, the sensitivity of the method used in this study may not be enough to detect the difference.
4.2. Iron Acquisition
To demonstrate whether the iron acquisition systems participate in the pathogenicity of UPEC in C. elegans, E. coli UTI89 mutants defective in the VFs were applied in the C. elegans solid killing assay. The statistical associations between the number of VFs and pathogenicity in C. elegans have been reported in UPEC [9,11,12]. However, it has not been reported that a mutant defective in iron acquisition decreases pathogenicity in the C. elegans-E. coli model, while it is important in infection in a mammal. To the best of our knowledge, this is the first study to demonstrate that iron acquisition in E. coli is involved in the pathogenicity in C. elegans.
Iron is an essential element for all organisms. Iron homeostasis in mammals serves as an innate immune response to prevent bacterial infection. Therefore, iron acquisition in bacteria is important for virulence [33]. In C. elegans, many ortholog genes for iron homeostasis in mammal were identified, and the mammalian iron-metabolism is generally conserved [34]. In this context, iron deprivation prolonged the survivability of C. elegans infected with Salmonella enterica serovar Typhimurium [35]. The expression of smf-3 and smf-1 in C. elegans which are orthologs of DMT-1 in mammal were induced by exposure to Staphylococcus aureus, and mutants of the transporters showed hypersensitivity to the pathogen [36]. In addition, a ferritin homolog ftn-2 involved in cellular iron storage has been shown to be necessary for the full protective response to E. coli and S. aureus [37]. Consequently, iron homeostasis in C. elegans also serves as innate immunity.
In this study, the involvement of iron acquisition to UPEC pathogenicity was demonstrated in C. elegans. The association between seven iron acquisition genes and pathogenicity in C. elegans were analyzed (Table 2), and chuA for heme acquisition, irp2 for yersiniabactin synthesis, and fyuA for its receptor were found to be involved in the pathogenicity (Figure 3). Heme is the most abundant iron source in a mammal, and it is an important iron source for bacterial infection [32]. Since the worm is heme auxotroph [38], it is consistent with that heme acquisition by the pathogen affects the infection. In addition, involvements of siderophore in C. elegans infection was also revealed in this study. While four siderophores have been identified in E. coli, UTI89 produces enterobactin, salmochelin, and yersiniabactin, but not aerobactin [25]. Namely, UTI89 ∆entA ∆fyuA, which does not produce enterobactin and salmochelin nor uptake yersiniabactin, is defective in any siderophore. Enterobactin is a major iron acquisition system that is produced by most E. coli; however, it is sequestrated by lipocalin 2 during mammal infection [39] On the other hand, salmochelin is a glucosylated enterobactin that is not bound by lipocalin 2, and can therefore escape the sequestration [40]. However, the worm does not produce lipocalin 2 to sequestrate enterobactin. In addition to the iron acquisition by siderophores, copper acquisition by yersiniabactin was also reported to be involved in UTIs [41]. While further study is necessary to investigate the molecular mechanism of iron acquisition in UPEC infection in C. elegans, availability of C. elegans for the model is demonstrated here.
5. Conclusions
The liquid pathogenicity assay applied in this study is a simple method to determine bacterial pathogenicity in C. elegans. The assay was applied to numerous clinically isolated E. coli, and the strains associated with UTIs showed significantly high pathogenicity. Through the high-throughput screening, genes involved in the iron acquisition were identified as VFs in the C. elegans model. The combination of mutations for multiple iron acquisition systems in E. coli UTI89 decreased pathogenicity in C. elegans. In urinary tract infection, available iron is limited, and iron acquisition by bacteria is essential. Furthermore, multiple iron acquisition systems showed different roles in infection, so the redundancy is important for successful infection [32]. Therefore, the results in this study with the C. elegans model is consistent with UTIs in mammal. The C. elegans model will accelerate to investigate the molecular mechanism of UPEC pathogenicity.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/9/2/310/s1, Figure S1: The pathogenicity of E. coli clinical isolates in different symptoms and phylogenetic groups, Figure S2: The number of virulence factors in clinically isolated E. coli, Table S1: Primers used in this study, Table S2: E. coli strains used for the solid pathogenic assay, Table S3: Symptoms of clinically isolated 133 E. coli strains.
Author Contributions
Conceived and designed the experiments, M.H., C.-S.C. and C.-H.T.; performed the experiments, M.H., Y.-F.M., and S.-T.W.; analyzed the data, M.H. and Y.-F.M.; wrote the paper, M.H., C.-S.C. and C.-H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology, Taiwan MOST [MOST 106-2320-B-006-033] (MH), [MOST 106-2320-B-006-032] (CHT), and [MOST 108-2319-B-002-004] (CSC).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are within the manuscript and its Supplementary Information.
Acknowledgments
We are grateful to W.P. Zhang for his wonderful technical assistance. This work received funding in part from the Center of Allergy and Clinical Immunology Research (ACIR) and the Headquarters of University Advancement at the National Cheng Kung University, which is sponsored by the Ministry of Education in Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, 5–13. [Google Scholar] [CrossRef]
- Francois, M.; Hanslik, T.; Dervaux, B.; Le Strat, Y.; Souty, C.; Vaux, S.; Maugat, S.; Rondet, C.; Sarazin, M.; Heym, B.; et al. The economic burden of urinary tract infections in women visiting general practices in France: A cross-sectional survey. BMC Health Serv. Res. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iv, H.L.S.; Conover, M.S.; Chou, W.-C.; Hibbing, M.E.; Manson, A.L.; Dodson, K.W.; Hannan, T.J.; Roberts, P.L.; Stapleton, A.E.; Hooton, T.M.; et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci. Transl. Med. 2017, 9, eaaf1283. [Google Scholar] [CrossRef]
- Tan, M.-W.; Mahajan-Miklos, S.; Ausubel, F.M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Garsin, D.A.; Sifri, C.D.; Mylonakis, E.; Qin, X.; Singh, K.V.; Murray, B.E.; Calderwood, S.B.; Ausubel, F.M. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 2001, 98, 10892–10897. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Chauvet, S.; Andrès, E.; Aurouze, M.; Vallet, I.; Michel, G.P.; Uh, M.; Celli, J.; Filloux, A.; De Bentzmann, S.; et al. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 2003, 22, 1451–1460. [Google Scholar] [CrossRef]
- Chou, T.-C.; Chiu, H.-C.; Kuo, C.-J.; Wu, C.-M.; Syu, W.-J.; Chiu, W.-T.; Chen, C.-S. Enterohaemorrhagic Escherichia coli O157:H7 Shiga-like toxin 1 is required for full pathogenicity and activation of the p38 mitogen-activated protein kinase pathway in Caenorhabditis elegans. Cell. Microbiol. 2013, 15, 82–97. [Google Scholar] [CrossRef]
- Couillault, C.; Ewbank, J.J. Diverse Bacteria Are Pathogens of Caenorhabditis elegans. Infect. Immun. 2002, 70, 4705–4707. [Google Scholar] [CrossRef]
- Diard, M.; Baeriswyl, S.; Clermont, O.; Gouriou, S.; Picard, B.; Taddei, F.; Denamur, E.; Matic, I. Caenorhabditis elegans as a simple model to study phenotypic and genetic virulence determinants of extraintestinal pathogenic Escherichia coli. Microbes Infect. 2007, 9, 214–223. [Google Scholar] [CrossRef]
- Kuo, C.-J.; Wang, S.-T.; Lin, C.-M.; Chiu, H.-C.; Huang, C.-R.; Lee, D.-Y.; Chang, G.-D.; Chou, T.-C.; Chen, J.-W.; Chen, C.-S. A multi-omic analysis reveals the role of fumarate in regulating the virulence of enterohemorrhagic Escherichia coli. Cell Death Dis. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Lavigne, J.-P.; Blanc-Potard, A.-B.; Bourg, G.; Moreau, J.; Chanal, C.; Bouziges, N.; O’Callaghan, D.; Sotto, A. Virulence genotype and nematode-killing properties of extra-intestinal Escherichia coli producing CTX-M β-lactamases. Clin. Microbiol. Infect. 2006, 12, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, J.-P.; Vergunst, A.C.; Goret, L.; Sotto, A.; Combescure, C.; Blanco, J.; O’Callaghan, D.; Nicolas-Chanoine, M.-H. Virulence Potential and Genomic Mapping of the Worldwide Clone Escherichia coli ST131. PLoS ONE 2012, 7, e34294. [Google Scholar] [CrossRef] [PubMed]
- Merkx-Jacques, A.; Coors, A.; Brousseau, R.; Masson, L.; Mazza, A.; Tien, Y.-C.; Topp, E. Evaluating the Pathogenic Potential of Environmental Escherichia coli by Using the Caenorhabditis elegans Infection Model. Appl. Environ. Microbiol. 2013, 79, 2435–2445. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.-H.; Chang, Y.-F.; Scaria, J.; Chang, C.-C.; Chou, L.-W.; Tien, N.; Wu, J.-J.; Tseng, C.-C.; Wang, M.-C.; Hsu, Y.-M.; et al. Identification of Escherichia coli Genes Associated with Urinary Tract Infections. J. Clin. Microbiol. 2012, 50, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Brenner, S. The Genetics of Caenorhabditis Elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef]
- Johnson, J.R.; Stell, A.L. Extended Virulence Genotypes of Escherichia coli Strains from Patients with Urosepsis in Relation to Phylogeny and Host Compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef]
- Johnson, J.R.; O’Bryan, T.T.; Delavari, P.; Kuskowski, M.; Stapleton, A.; Carlino, U.; Russo, T.A. Clonal relationships and extended virulence genotypes among Escherichia coli isolates from women with a first or recurrent episode of cystitis. J. Infect. Dis. 2001, 183, 1508–1517. [Google Scholar] [CrossRef]
- Johnson, J.R.; Porter, S.; Johnston, B.; Kuskowski, M.A.; Spurbeck, R.R.; Mobley, H.L.; Williamson, D.A. Host characteristics and bacterial traits predict experimental virulence for Escherichia coli bloodstream isolates from patients with urosepsis. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Luo, Y.; Ma, Y.; Zhao, Q.; Wang, L.; Guo, L.; Ye, L.; Zhang, Y.; Yang, J. Similarity and Divergence of Phylogenies, Antimicrobial Susceptibilities, and Virulence Factor Profiles of Escherichia coli Isolates Causing Recurrent Urinary Tract Infections That Persist or Result from Reinfection. J. Clin. Microbiol. 2012, 50, 4002–4007. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Bingen, E.; Picard, B.; Brahimi, N.; Mathy, S.; Desjardins, P.; Elion, J.; Denamur, E. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 1998, 177, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.P.; Crowley, J.R.; Pinkner, J.S.; Walker, J.N.; Tsukayama, P.; Stamm, W.E.; Hooton, T.M.; Hultgren, S.J. Quantitative Metabolomics Reveals an Epigenetic Blueprint for Iron Acquisition in Uropathogenic Escherichia coli. PLoS Pathog. 2009, 5, e1000305. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Payne, S.M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 1997, 23, 825–833. [Google Scholar] [CrossRef]
- Hagan, E.C.; Mobley, H.L.T. Haem acquisition is facilitated by a novel receptor Hma and required by uropathogenic Escherichia colifor kidney infection. Mol. Microbiol. 2009, 71, 79–91. [Google Scholar] [CrossRef]
- Brumbaugh, A.R.; Smith, S.N.; Subashchandrabose, S.; Himpsl, S.D.; Hazen, T.H.; Rasko, D.A.; Mobley, H.L.T. Blocking Yersiniabactin Import Attenuates Extraintestinal Pathogenic Escherichia coli in Cystitis and Pyelonephritis and Represents a Novel Target to Prevent Urinary Tract Infection. Infect. Immun. 2015, 83, 1443–1450. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.G.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Genet. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Watts, R.E.; Totsika, M.; Challinor, V.L.; Mabbett, A.N.; Ulett, G.C.; De Voss, J.J.; Schembri, M.A. Contribution of Siderophore Systems to Growth and Urinary Tract Colonization of Asymptomatic Bacteriuria Escherichia coli. Infect. Immun. 2012, 80, 333–344. [Google Scholar] [CrossRef]
- Hantke, K.; Nicholson, G.; Rabsch, W.; Winkelmann, G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. USA 2003, 100, 3677–3682. [Google Scholar] [CrossRef]
- Garcia, E.C.; Brumbaugh, A.R.; Mobley, H.L.T. Redundancy and Specificity of Escherichia coli Iron Acquisition Systems during Urinary Tract Infection. Infect. Immun. 2011, 79, 1225–1235. [Google Scholar] [CrossRef]
- Cassat, J.E.; Skaar, E.P. Iron in Infection and Immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.P.; Leibold, E.A. Mechanisms of iron metabolism in Caenorhabditis elegans. Front. Pharmacol. 2014, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Kortman, G.A.M.; Mulder, M.L.M.; Richters, T.J.W.; Shanmugam, N.K.N.; Trebicka, E.; Boekhorst, J.; Timmerman, H.M.; Roelofs, R.; Wiegerinck, E.T.; Laarakkers, C.M.; et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur. J. Immunol. 2015, 45, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, J.; Song, H.-O.; Park, B.-J.; Singaravelu, G.; Sun, J.L.; Ahnn, J.; Cho, J.H. Functional assessment of Nramp-like metal transporters and manganese in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2009, 390, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, K.T.; Møller-Jensen, J.; Kristensen, A.R.; Andersen, J.S.; Riddle, N.L.; Kallipolitis, B.H. Quantitative proteomics identifies ferritin in the innate immune response of C. elegans. Virulence 2011, 2, 120–130. [Google Scholar] [CrossRef]
- Rao, A.U.; Carta, L.K.; Lesuisse, E.; Hamza, I. Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. USA 2005, 102, 4270–4275. [Google Scholar] [CrossRef]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nat. Cell Biol. 2004, 432, 917–921. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Lin, H.; Zhou, L.; Yu, Y.; Abergel, R.J.; Liu, D.R.; Raymond, K.N.; Wanner, B.L.; Strong, R.K.; Walsh, C.T.; et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc. Natl. Acad. Sci. USA 2006, 103, 16502–16507. [Google Scholar] [CrossRef]
- Robinson, A.E.; Heffernan, J.R.; Henderson, J.P. The iron hand of uropathogenic Escherichia coli: The role of transition metal control in virulence. Future Microbiol. 2018, 13, 745–756. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).