Exploring the Chemical Space of Macro- and Micro-Algae Using Comparative Metabolomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Selection
2.2. Culture Conditions and Metabolite Extraction
2.3. DNA Extraction and 18S rRNA Gene Amplification
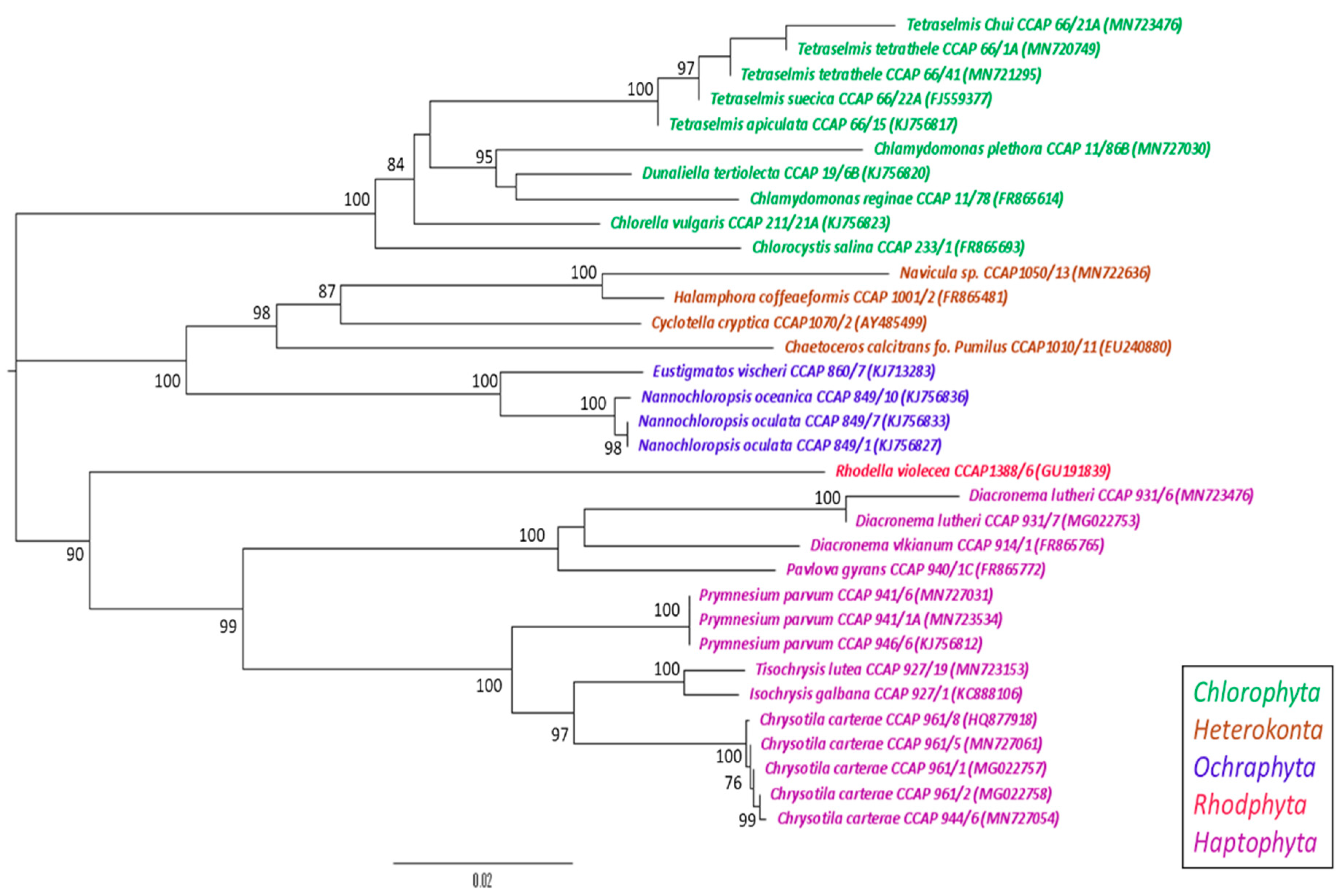
2.4. Phylogenetic Analysis
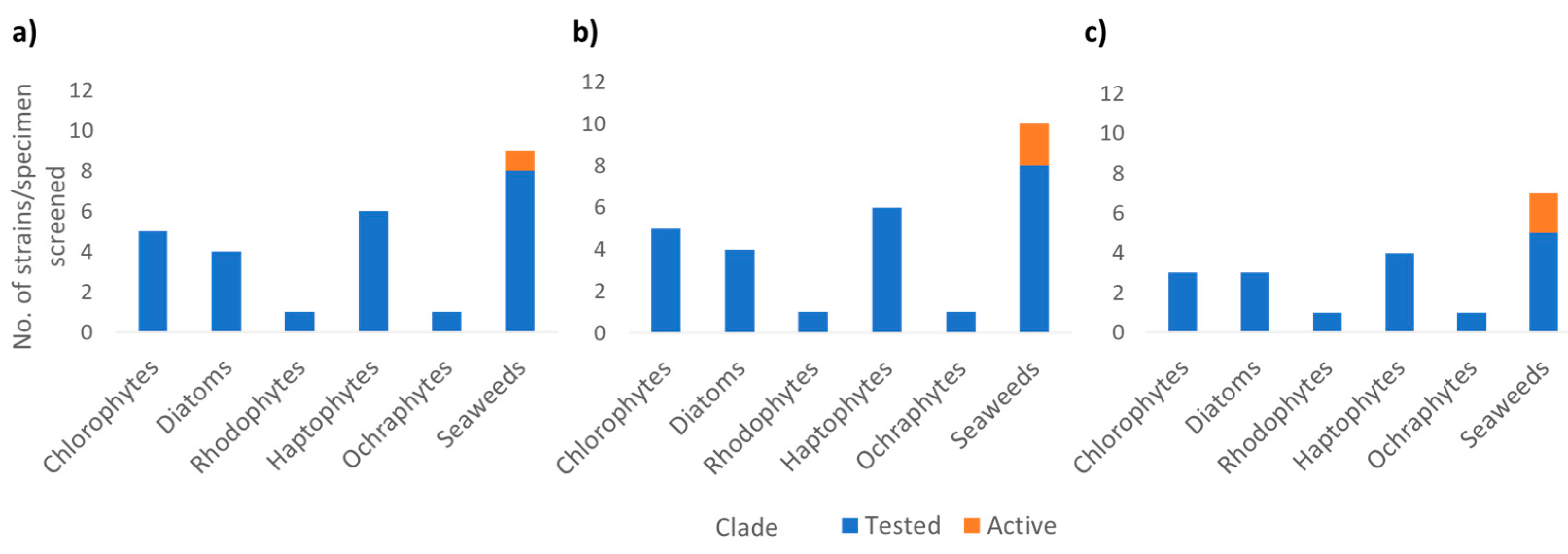
2.5. Bioassay Screening of Metabolite Extracts
2.6. Tandem High-Resolution Mass Spectrometry Data Acquisition
2.7. Processing of Raw Liquid Chromatography-Mass Spectrometry (LC-MS) Data Using MZmine
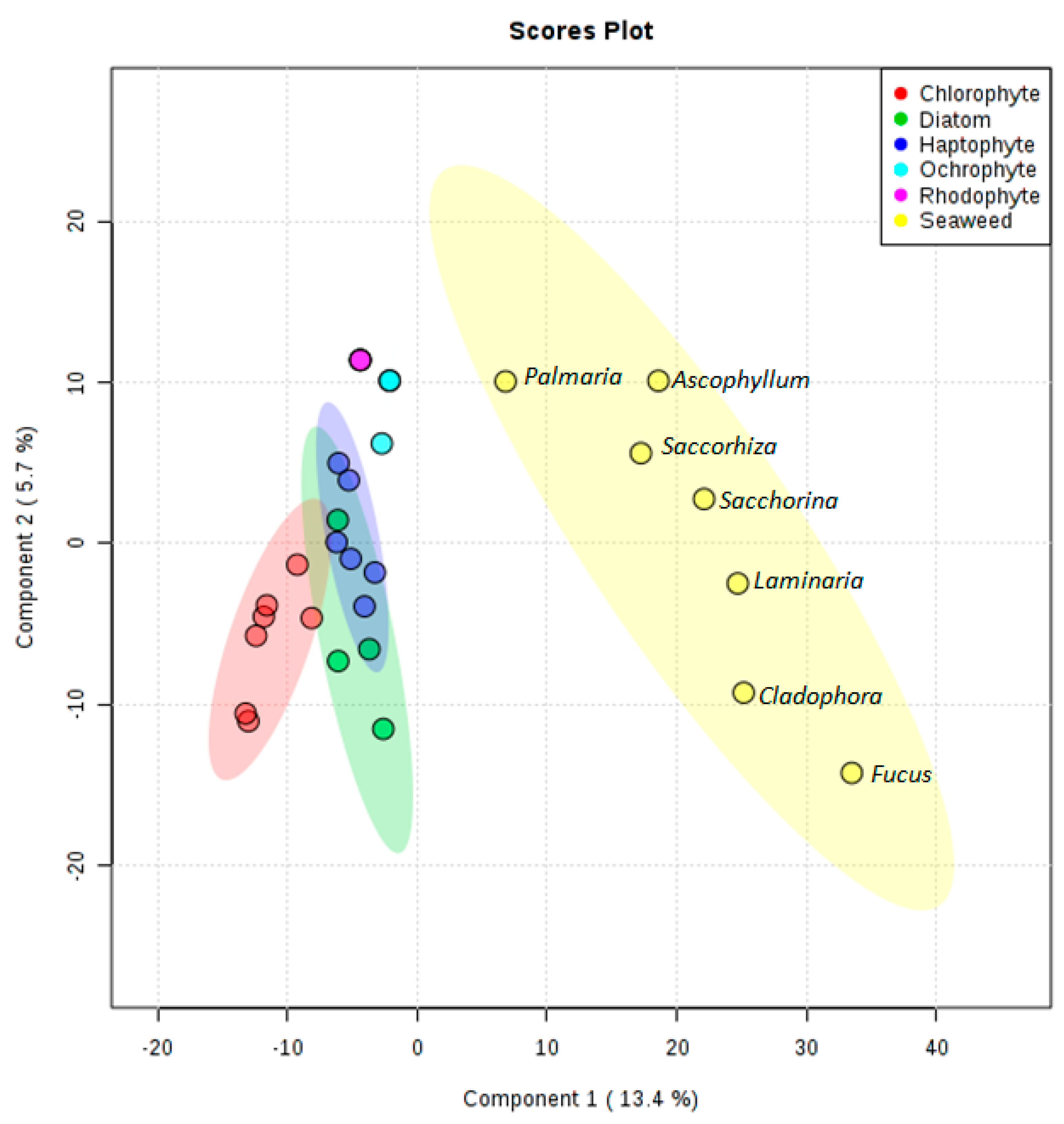
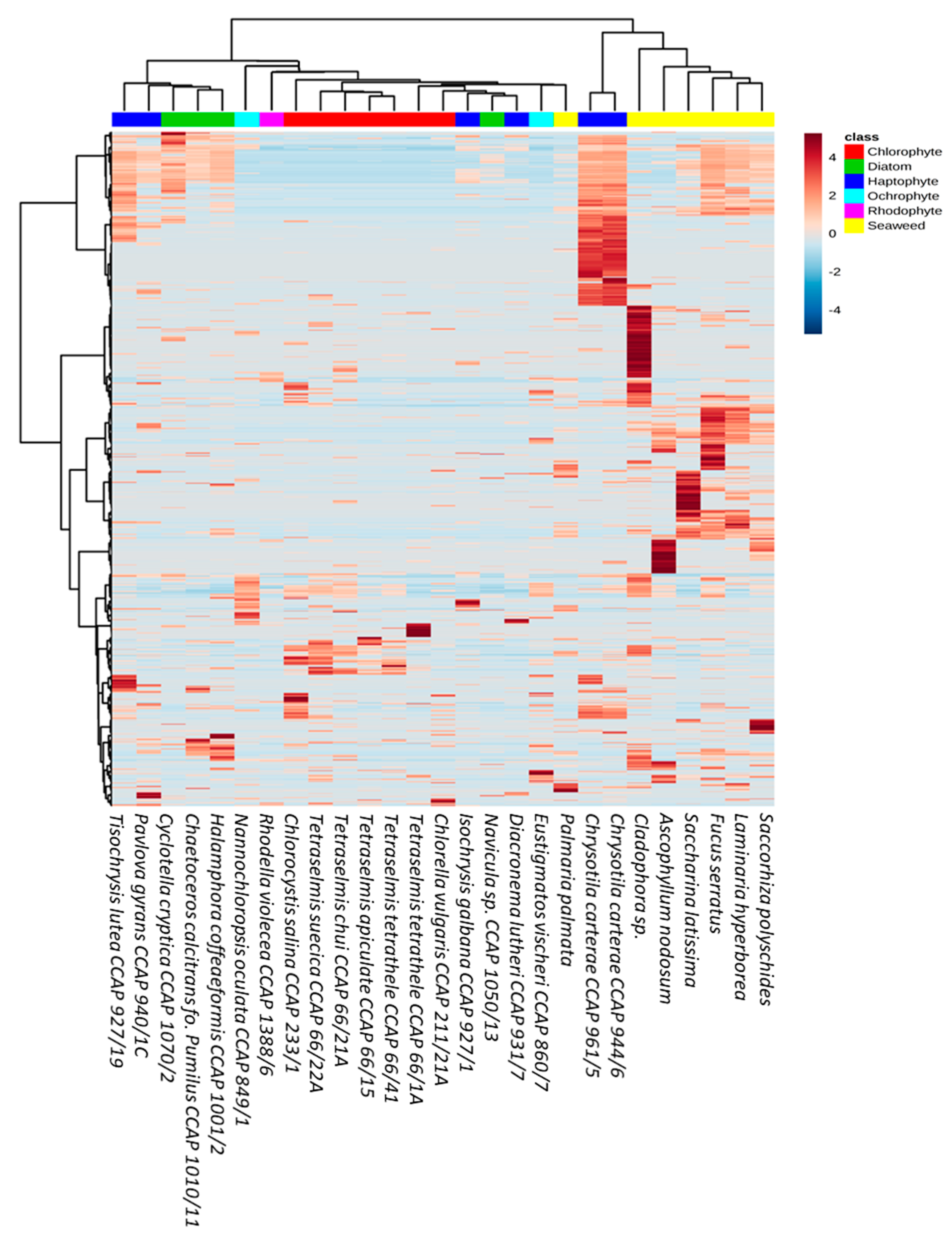
2.8. Multivariate Statistical Analysis
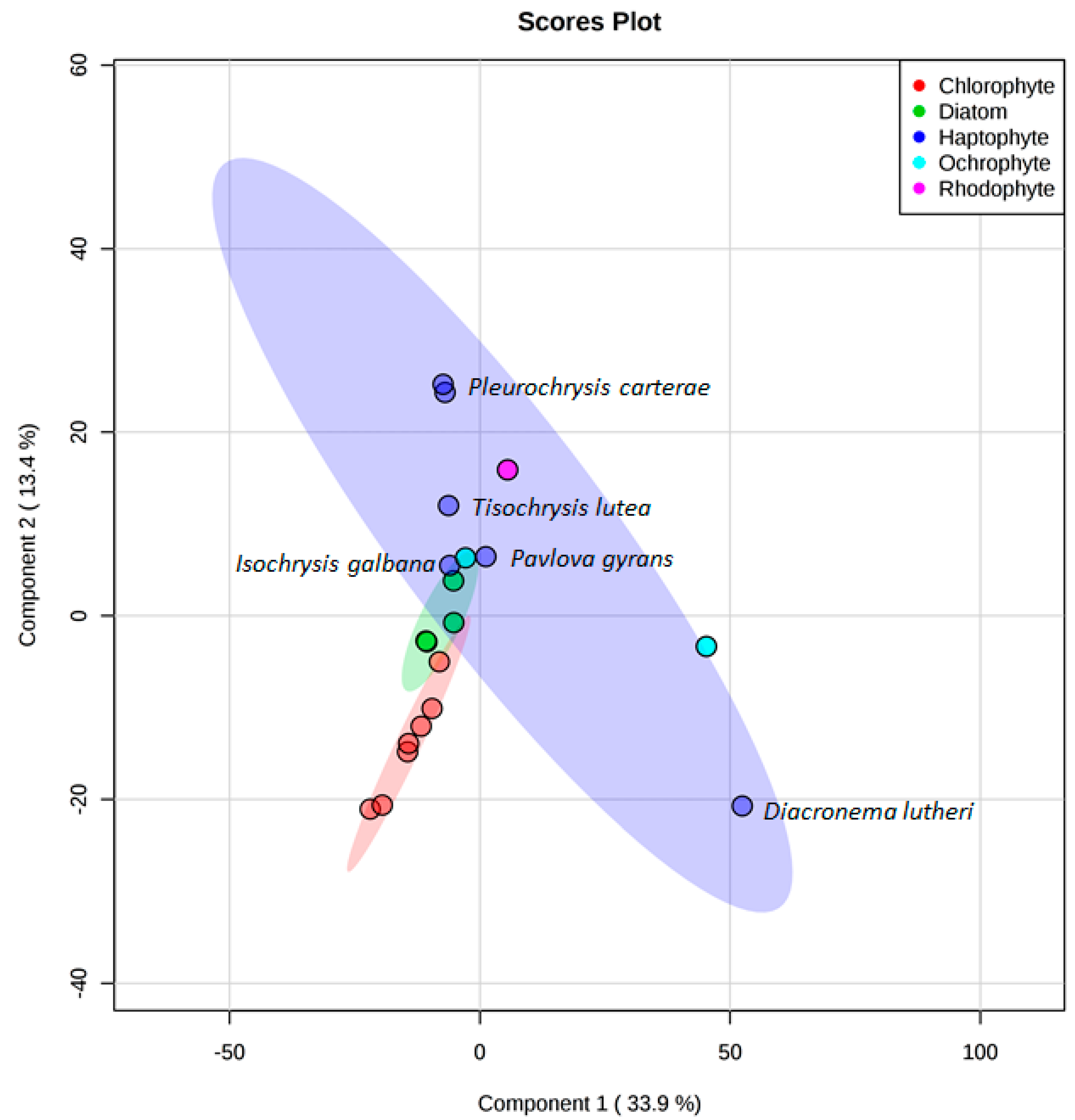
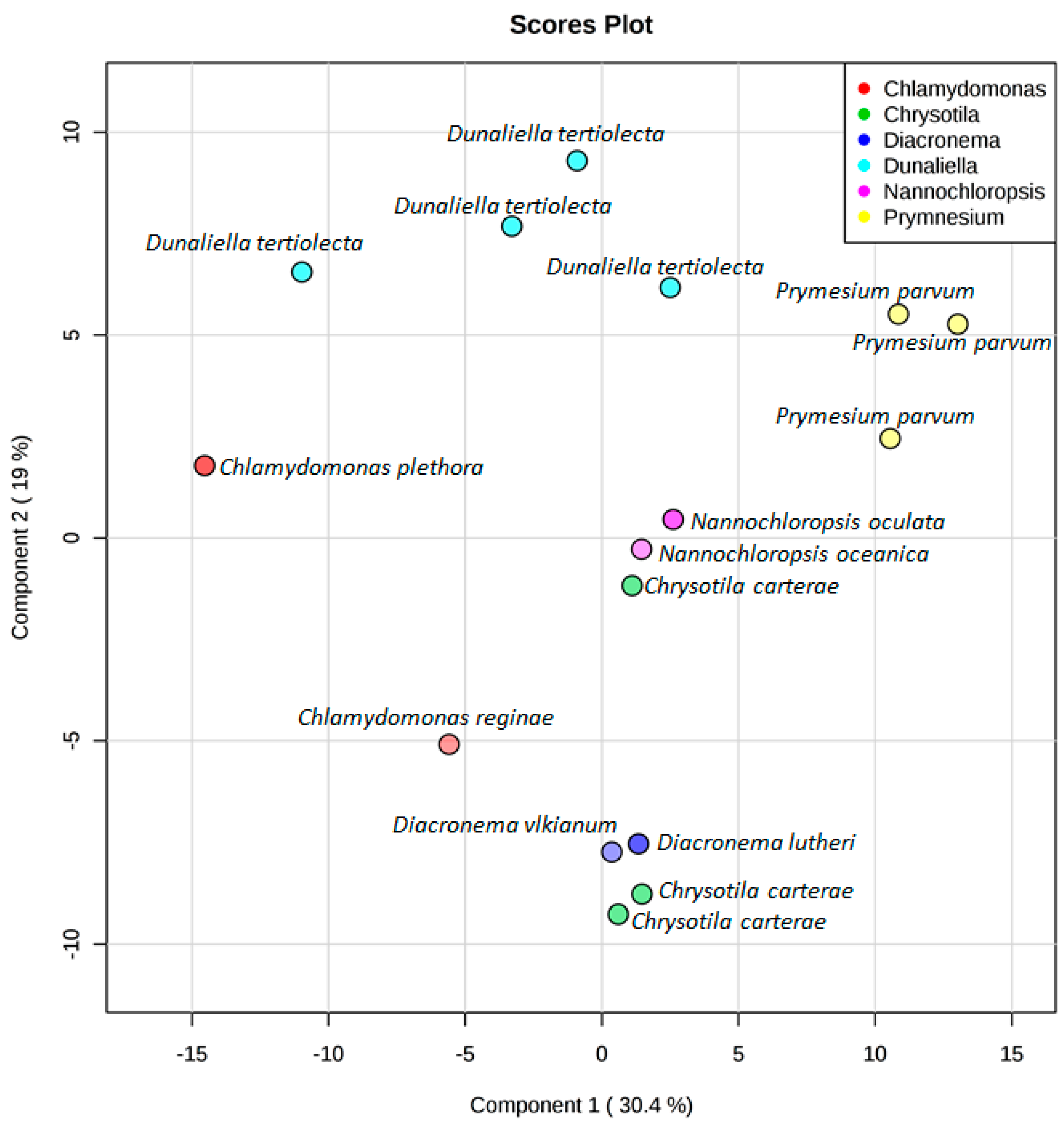
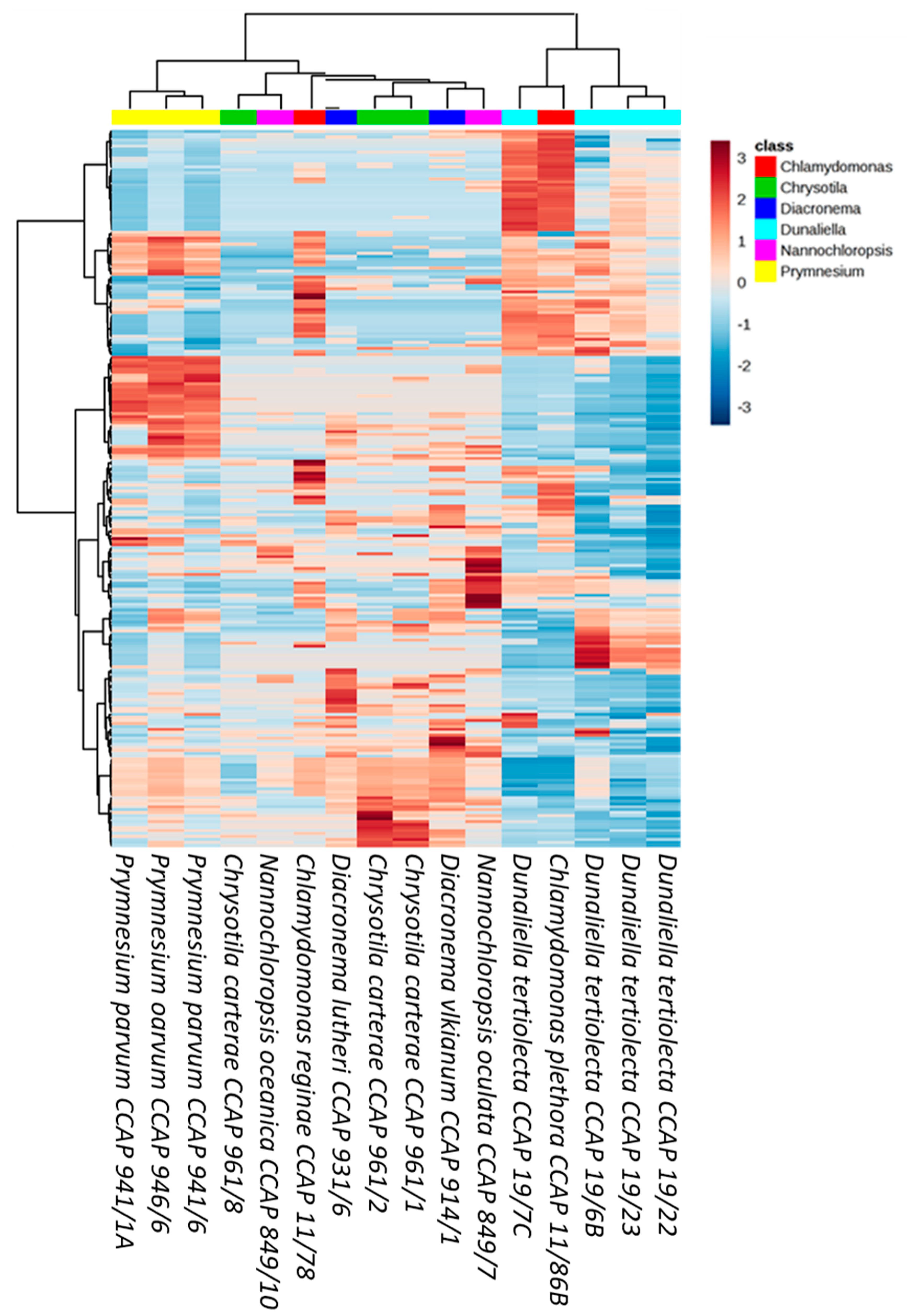
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guiry, M.D.; Guiry, G.M. Algaebase: Listing the World’s Algae. Available online: http://www.algaebase.org/ (accessed on 24 June 2018).
- Keeling, P.J. Diversity and Evolutionary History of Plastids and Their Hosts. Am. J. Bot. 2004, 91, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Blight, A.J.; Thompson, R.C. Epibiont Species Richness Varies between Holdfasts of a Northern and a Southerly Distributed Kelp Species. J. Mar. Biol. Assoc. UK 2008, 88, 469–475. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.; Zhang, H. Natural Products and Drug Discovery: Can Thousands of Years of Ancient Medical Knowledge Lead Us to New and Powerful Drug Combinations in the Fight against Cancer and Dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz, B.D.S.; de Morais, E.G.; Costa, J.A.V. Biologically Active Metabolites Synthesized by Microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Rocha, D.; Seca, A.; Pinto, D. Seaweed Secondary Metabolites In Vitro and In Vivo Anticancer Activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Ingebrigtsen, R.A.; Hansen, E.; Andersen, J.H.; Eilertsen, H.C. Light and Temperature Effects on Bioactivity in Diatoms. J. Appl. Phycol. 2016, 28, 939–950. [Google Scholar] [CrossRef]
- The Global Status of Seaweed Production, Trade and Utilization—Volume 124|GLOBEFISH|Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/in-action/globefish/publications/details-publication/en/c/1154074/ (accessed on 10 December 2019).
- Mobin, S.; Alam, F. Some Promising Microalgal Species for Commercial Applications: A Review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old Sustainable Food and Fashion Nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Adjallé, K.; Barnabé, S.; Raghavan, G.S.V. Microalgae Biomass Production for a Biorefinery System: Recent Advances and the Way towards Sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Willamme, R.; Alsafra, Z.; Arumugam, R.; Eppe, G.; Remacle, F.; Levine, R.D.; Remacle, C. Metabolomic Analysis of the Green Microalga Chlamydomonas Reinhardtii Cultivated under Day/Night Conditions. J. Biotechnol. 2015, 215, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Jacinto, V.; García-Barrera, T.; Garbayo-Nores, I.; Vilchez-Lobato, C.; Gómez-Ariza, J.-L. Metal-Metabolomics of Microalga Chlorella Sorokiniana Growing in Selenium- and Iodine-Enriched Media. Chem. Pap. 2012, 66. [Google Scholar] [CrossRef]
- Liland, K.H. Multivariate Methods in Metabolomics—From Pre-Processing to Dimension Reduction and Statistical Analysis. TrAC Trends Anal. Chem. 2011, 30, 827–841. [Google Scholar] [CrossRef]
- Keller, L.; Canuto, K.M.; Liu, C.; Suzuki, B.M.; Almaliti, J.; Sikandar, A.; Naman, C.B.; Glukhov, E.; Luo, D.; Duggan, B.M.; et al. Tutuilamides A–C: Vinyl-Chloride-Containing Cyclodepsipeptides from Marine Cyanobacteria with Potent Elastase Inhibitory Properties. ACS Chem. Biol. 2020, 15, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Luzzatto-Knaan, T.; Garg, N.; Wang, M.; Glukhov, E.; Peng, Y.; Ackermann, G.; Amir, A.; Duggan, B.M.; Ryazanov, S.; Gerwick, L.; et al. Digitizing Mass Spectrometry Data to Explore the Chemical Diversity and Distribution of Marine Cyanobacteria and Algae. eLife 2017, 6, e24214. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.-B.; Zhang, Y.; Leao, T.; Glukhov, E.; Pierce, M.L.; Zhang, C.; Kim, H.; Mao, H.H.; Fang, F.; et al. Pagoamide A, a Cyclic Depsipeptide Isolated from a Cultured Marine Chlorophyte, Derbesia sp., Using MS/MS-Based Molecular Networking. J. Nat. Prod. 2020, 83, 617–625. [Google Scholar] [CrossRef]
- Keller, L.; Siqueira-Neto, J.L.; Souza, J.M.; Eribez, K.; LaMonte, G.M.; Smith, J.E.; Gerwick, W.H. Palstimolide A: A Complex Polyhydroxy Macrolide with Antiparasitic Activity. Molecules 2020, 25, 1604. [Google Scholar] [CrossRef]
- Marin, B.; Palm, A.; Klingberg, M.; Melkonian, M. Phylogeny and Taxonomic Revision of Plastid-Containing Euglenophytes based on SSU rDNA Sequence Comparisons and Synapomorphic Signatures in the SSU rRNA Secondary Structure. Protist 2003, 154, 99–145. [Google Scholar] [CrossRef]
- Coleman, A.W.; Suarez, A.; Goff, L.J. Molecular Delineation of Species and Syngens in Volvocacean Green Algae (Chlorophyta). J. Phycol. 1994, 30, 80–90. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open Source Software for Rapid Proteomics Tools Development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinformatics 2010, 11, 395. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68. [Google Scholar] [CrossRef]
- Huang, J.; Qian, H.-Y.; Li, Z.-Z.; Zhang, J.-M.; Wang, S.; Tao, Y.; Gao, Y.-L.; Yin, C.-Q.; Que, B.; Sun, T.; et al. Role of Endothelial lipase in atherosclerosis. Transl. Res. 2010, 156, 1–6. [Google Scholar] [CrossRef]
- Hong, C.; Deng, R.; Wang, P.; Lu, X.; Wang, X.; Cai, R.; Lin, J. LIPG: An inflammation and cancer modulator. Cancer Gene Ther. 2020. [Google Scholar] [CrossRef]
- Bai, T.; Zhang, D.; Lin, S.; Long, Q.; Wang, Y.; Ou, H.; Kang, Q.; Deng, Z.; Liu, W.; Tao, M. Operon for biosynthesis of lipstatin, the Beta-lactone inhibitor of human pancreatic lipase. Appl. Environ. Microbiol. 2014, 80, 7473–7483. [Google Scholar] [CrossRef]
- Nomura, D.K.; Casida, J.E. Lipases and their inhibitors in health and disease. Chem. Biol. Interact. 2016, 259 Pt B, 211–222. [Google Scholar] [CrossRef]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 2017, 13, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hanh, J.; Váradi, L.; Cairns, R.; Sjöström, H.; Liao, V.W.; Wood, P.; Balaban, S.; Ong, J.A.; Lin, H.Y.; et al. Identification of dual PPARα/γ agonists and their effects on lipid metabolism. Bioorg. Med. Chem. 2015, 23, 7676–7684. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Jung, Y.; Kim, M.C.; Kwon, H.C.; Kang, K.S.; Kim, Y.K.; Kim, S.N. Sargahydroquinoic acid inhibits TNFα-induced AP-1 and NF-κB signaling in HaCaT cells through PPARα activation. Biochem. Biophys. Res. Commun. 2014, 450, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.; Leal, E.C.; Carvalho, E. Protein Tyrosine Phosphatase 1B Inhibition as a Potential Therapeutic Target for Chronic Wounds in Diabetes. Pharmacol. Res. 2020, 159, 104977. [Google Scholar] [CrossRef]
- Hess, S.K.; Lepetit, B.; Kroth, P.G.; Mecking, S. Production of Chemicals from Microalgae Lipids—Status and Perspectives: Production of Chemicals from Microalgae—Status and Perspectives. Eur. J. Lipid Sci. Technol. 2018, 120, 1700152. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial Potential of Carotenoid Pigments from Microalgae: Current Trends and Future Prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef]
- Mc Gee, D.; Archer, L.; Paskuliakova, A.; Mc Coy, G.R.; Fleming, G.T.A.; Gillespie, E.; Touzet, N. Rapid Chemotaxonomic Profiling for the Identification of High-Value Carotenoids in Microalgae. J. Appl. Phycol. 2018, 30, 385–399. [Google Scholar] [CrossRef]
- Reggasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.; Kang, Q.; Lu, J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Disctyopteris divaricate. Int. J. Biol. Macromol. 2018, 117, 256–263. [Google Scholar] [CrossRef]
- Calvo, M.M.; Dado, D.; Santa-María, G. Influence of Extraction with Ethanol or Ethyl Acetate on the Yield of Lycopene, β-Carotene, Phytoene and Phytofluene from Tomato Peel Powder. Eur. Food Res. Technol. 2007, 224, 567–571. [Google Scholar] [CrossRef]
- Granéli, E.; Edvardsen, B.; Roelke, D.L.; Hagström, J.A. The Ecophysiology and Bloom Dynamics of Prymnesium spp. Harmful Algae 2012, 14, 260–270. [Google Scholar] [CrossRef]
- Edrada-Ebel, R.; Evarsson, A.; Polymenakou, P.; Hentschel, U.; Carettoni, D.; Day, J.; Green, D.; Hreggvidsson, G.O.; Harvey, L.; McNeill, B. SeaBioTech: From Seabed to Test-Bed: Harvesting the Potential of Marine Biodiversity for Industrial Biotechnology. In Grand Challenges in Marine Biotechnology; Rampelotto, P., Trincone, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 451–504. [Google Scholar] [CrossRef]
- Romano, S.; Jackson, S.; Patry, S.; Dobson, A. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef]
- Wandy, J.; Zhu, Y.; van der Hooft, J.J.J.; Daly, R.; Barrett, M.P.; Rogers, S. Ms2lda.Org: Web-Based Topic Modelling for Substructure Discovery in Mass Spectrometry. Bioinformatics 2018, 34, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leysson, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [PubMed]
| Name | Sequence |
|---|---|
| PCR | - |
| EAF3 | TCGACAATCTGGTTGATCCTGCCAG [22] |
| ITS055R | CTCCTTGGTCCGTGTTTCAAGACGGG [22] |
| Sequencing | - |
| E528F | TGCCAGCAGCYGCGGTAATTCCAGC [22] |
| 920F | GAAACTTAAAKGAATTG [22] |
| 920R | ATTCCTTTRAGTTTC [22] |
| BR | TTGATCCTTCTGCAGGTTCACCTAC [22] |
| 536R | GWATTACCGCGGCKGCTG [22] |
| GF | GGGATCCGTTTCCGTAGGTGAACCTGC [23] |
| GR | GGGATCCATATGCTTAAGTTCAGCGGGT [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, A.H.; Magot, F.; Tawfike, A.F.; Rad-Menéndez, C.; Thomas, N.; Young, L.C.; Stucchi, L.; Carettoni, D.; Stanley, M.S.; Edrada-Ebel, R.; et al. Exploring the Chemical Space of Macro- and Micro-Algae Using Comparative Metabolomics. Microorganisms 2021, 9, 311. https://doi.org/10.3390/microorganisms9020311
Hughes AH, Magot F, Tawfike AF, Rad-Menéndez C, Thomas N, Young LC, Stucchi L, Carettoni D, Stanley MS, Edrada-Ebel R, et al. Exploring the Chemical Space of Macro- and Micro-Algae Using Comparative Metabolomics. Microorganisms. 2021; 9(2):311. https://doi.org/10.3390/microorganisms9020311
Chicago/Turabian StyleHughes, Alison H., Florent Magot, Ahmed F. Tawfike, Cecilia Rad-Menéndez, Naomi Thomas, Louise C. Young, Laura Stucchi, Daniele Carettoni, Michele S. Stanley, RuAngelie Edrada-Ebel, and et al. 2021. "Exploring the Chemical Space of Macro- and Micro-Algae Using Comparative Metabolomics" Microorganisms 9, no. 2: 311. https://doi.org/10.3390/microorganisms9020311
APA StyleHughes, A. H., Magot, F., Tawfike, A. F., Rad-Menéndez, C., Thomas, N., Young, L. C., Stucchi, L., Carettoni, D., Stanley, M. S., Edrada-Ebel, R., & Duncan, K. R. (2021). Exploring the Chemical Space of Macro- and Micro-Algae Using Comparative Metabolomics. Microorganisms, 9(2), 311. https://doi.org/10.3390/microorganisms9020311








