Invasive Group B Streptococcal Disease in Neonates and Infants, Italy, Years 2015–2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Bacterial Collection Typing
2.3. Statistical Analysis
3. Results
3.1. Characteristics of iGBS
3.2. Prevention Strategies and Missed Opportunities in GBS-EOD
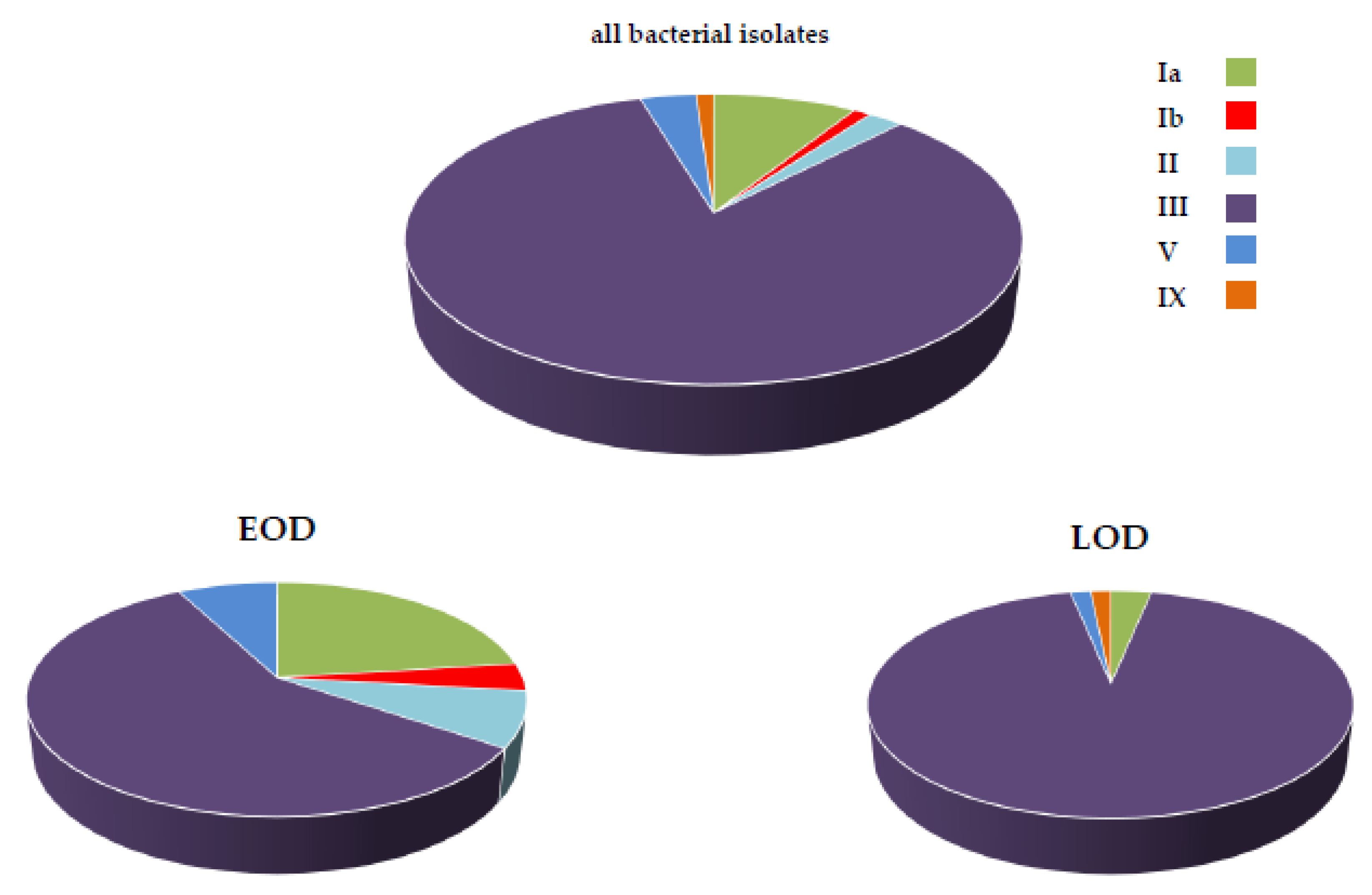
3.3. Bacterial Typing and Antimicrobial Resistance
4. Discussion
4.1. Prevention
4.2. Clinical Epidemiology and Risk Factors
4.3. Microbiological Epidemiology, Antibiotic Resistance and the Emergence of MDR CC-17 Sub-Lineage
4.4. GBS Vaccine
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seale, A.C.; Bianchi-Jassir, F.; Russell, N.J.; Kohli-Lynch, M.; Tann, C.J.; Hall, J.; Madrid, L.; Blencowe, H.; Cousens, S.; Baker, C.J.; et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin. Infect. Dis. 2017, 6, S200–S219. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.J. The spectrum of perinatal group B streptococcal disease. Vaccine 2013, 31 (Suppl. 4), D3–D6. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; Schrag, S.J. Group B streptococcal disease in infants: Progress in prevention and continued challenges. Clin. Perintol. 2010, 37, 375–392. [Google Scholar] [CrossRef]
- Puopolo, K.M.; Lynfield, R.; Cummings, J.J.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Infants at Risk for Group B Streptococcal Disease. Pediatrics 2019, 144, e20191881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhudasia, M.B.; Flannery, D.D.; Pfeifer, M.R.; Puopolo, K.M. Updated Guidance: Prevention and Management of Perinatal Group B Streptococcus Infection. Neoreviews 2021, 22, e177–e188. [Google Scholar] [CrossRef] [PubMed]
- Russell, N.J.; Seale, A.C.; O’Sullivan, C.; Le Doare, K.; Heath, P.T.; Lawn, J.E.; Bartlett, L.; Cutland, C.; Gravett, M.; Ip, M.; et al. Risk of Early-Onset Neonatal Group B Streptococcal Disease With Maternal Colonization Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. 2), S152–S159. [Google Scholar] [CrossRef] [Green Version]
- Le Doare, K.; O’Driscoll, M.; Turner, K.; Seedat, F.; Russell, N.J.; Seale, A.C.; Heath, P.T.; Lawn, J.E.; Baker, C.J.; Bartlett, L.; et al. Intrapartum Antibiotic Chemoprophylaxis Policies for the Prevention of Group B Streptococcal Disease Worldwide: Systematic Review. Clin. Infect. Dis. 2017, 65 (Suppl. 2), S143–S151. [Google Scholar] [CrossRef] [Green Version]
- Verani, J.R.; McGee, L.; Schrag, S.J.; Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease—Revised guidelines from CDC, 2010. MMWR Recomm. Rep. 2010, 59, 1–36. [Google Scholar]
- Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797. Obs. Gynecol. 2020, 135, e51–e72. [CrossRef]
- Schrag, S.J.; Verani, J.R. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: Experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine 2013, 31 (Suppl. 4), D20–D26. [Google Scholar] [CrossRef] [Green Version]
- Berardi, A.; Trevisani, V.; Di Caprio, A.; Bua, J.; China, M.; Perrone, B.; Pagano, R.; Lucaccioni, L.; Fanaro, S.; Iughetti, L.; et al. Understanding Factors in Group B Streptococcus Late-Onset Disease. Infect. Drug Resist. 2021, 14, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Rossi, C.; Lugli, L.; Creti, R.; Reggiani, M.L.B.; Lanari, M.; Memo, L.; Pedna, M.F.; Venturelli, C.; Perrone, E.; et al. Group B streptococcus late-onset disease: 2003–2010. Pediatrics 2013, 131, e361–e368. [Google Scholar] [CrossRef] [Green Version]
- Berardi, A.; Spada, C.; Creti, R.; Auriti, C.; Gambini, L.; Rizzo, V.; Capretti, M.G.; Laforgia, N.; Papa, I.; Tarocco, A.; et al. Maternal Carriage in Late-Onset Group B Streptococcus Disease, Italy. Emerg. Infect. Dis. 2021, 27, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Madrid, L.; Seale, A.C.; Kohli-Lynch, M.; Edmond, K.M.; Lawn, J.E.; Heath, P.T.; Madhi, S.A.; Baker, C.J.; Bartlett, L.; Cutland, C.; et al. Infant GBS Disease Investigator Group. Infant Group B Streptococcal Disease Incidence and Serotypes Worldwide: Systematic Review and Meta-analyses. Clin. Infect. Dis. 2017, 65 (Suppl. 2), S160–S172. [Google Scholar] [CrossRef] [Green Version]
- Bianchi-Jassir, F.; Paul, P.; To, K.N.; Carreras-Abad, C.; Seale, A.C.; Jauneikaite, E.; Madhi, S.A.; Russell, N.J.; Hall, J.; Madrid, L.; et al. Systematic review of Group B Streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine 2020, 38, 6682–6694. [Google Scholar] [CrossRef] [PubMed]
- Creti, R.; Berardi, A.; Baldassarri, L.; Imperi, M.; Pataracchia, M.; Alfarone, G.; Recchia, S.; GBS Prevention Working Group. Emilia-Romagna and the Neonatal GBS Italian network. Characteristics of neonatal GBS disease during a multicentre study (2007–2010) and in the year 2012. Ann. Ist. Super. Sanita 2013, 49, 370–375. [Google Scholar] [CrossRef]
- Creti, R.; Imperi, M.; Berardi, A.; Pataracchia, M.; Recchia, S.; Alfarone, G.; Baldassarri, L.; Italian Neonatal GBS Infections Working Group. Neonatal Group B Streptococcus Infections: Prevention Strategies, Clinical and Microbiologic Characteristics in 7 Years of Surveillance. Pediatr. Infect. Dis. J. 2017, 36, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Guideline 20: Management of uncomplicated pregnancy. In National System of Guidelines; Italian Ministry of Health: Rome, Italy, 2011; pp. 154–157.
- Berardi, A.; Lugli, L.; Baronciani, D.; Rossi, C.; Ciccia, M.; Creti, R.; Gambini, L.; Mariani, S.; Papa, I.; Tridapalli, E.; et al. Group B Streptococcus early-onset disease in Emilia-Romagna: Review after introduction of a screening-based approach. Pediatr. Infect. Dis. J. 2010, 29, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Lugli, L.; Rossi, C.; Guidotti, I.; Lanari, M.; Creti, R.; Perrone, E.; Biasini, A.; Sandri, F.; Volta, A.; et al. Impact of perinatal practices for early-onset group B Streptococcal disease prevention. Pediatr. Infect. Dis. J. 2013, 32, e265–e271. [Google Scholar] [CrossRef]
- Berardi, A.; Baroni, L.; Reggiani, M.L.B.; Ambretti, S.; Biasucci, G.; Bolognesi, S.; Capretti, M.G.; Carretto, E.; Ciccia, M.; Fiorini, V.; et al. The burden of early-onset sepsis in Emilia-Romagna (Italy): A 4-year, population-based study. J. Matern. Fetal Neonatal Med. 2016, 29, 3126–3131. [Google Scholar] [CrossRef]
- Berardi, A.; Rossi, C.; Reggiani, M.L.B.; Bastelli, A.; Capretti, M.G.; Chiossi, C.; Fiorini, V.; Gambini, L.; Gavioli, S.; Lanari, M.; et al. An area-based study on intrapartum antibiotic prophylaxis for preventing group B streptococcus early-onset disease: Advances and limitations. J. Matern. Fetal Neonatal Med. 2017, 30, 1739–1744. [Google Scholar] [CrossRef]
- Berardi, A.; Spada, C.; Creti, R.; Ambretti, S.; Chiarabini, R.; Barozzi, A.; Pagano, R.; Sarti, M.; Pedna, M.F.; Fornaciari, S.; et al. Risk factors for group B streptococcus early-onset disease: An Italian, area-based, case-control study. J. Matern. Fetal Neonatal Med. 2020, 33, 2480–2486. [Google Scholar] [CrossRef]
- Berardi, A.; Lugli, L.; Baronciani, D.; Creti, R.; Rossi, K.; Ciccia, M.; Gambini, L.; Mariani, S.; Papa, I.; Serra, L.; et al. Group B streptococcal infections in a northern region of Italy. Pediatrics 2007, 120, e487–e493. [Google Scholar] [CrossRef] [PubMed]
- Afshar, B.; Broughton, K.; Creti, R.; Decheva, A.; Hufnagel, M.; Kriz, P.; Lambertsen, L.; Lovgren, M.; Melin, P.; Orefici, G.; et al. International external quality assurance for laboratory identification and typing of Streptococcus agalactiae (Group B streptococci). J. Clin. Microbiol. 2011, 49, 1475–1482. [Google Scholar] [CrossRef] [Green Version]
- Slotved, H.C.; Hoffmann, S. Evaluation of procedures for typing of group B Streptococcus: A retrospective study. PeerJ 2017, 5, e3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imperi, M.; Pataracchia, M.; Alfarone, G.; Baldassarri, L.; Orefici, G.; Creti, R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J. Microbiol. Methods 2010, 80, 212–214. [Google Scholar] [CrossRef]
- Lamy, M.C.; Dramsi, S.; Billoët, A.; Réglier-Poupet, H.; Tazi, A.; Raymond, J.; Guérin, F.; Couvé, E.; Kunst, F.; Glaser, P.; et al. Rapid detection of the “highly virulent” group B Streptococcus ST-17 clone. Microbes Infect. 2006, 8, 1714–1722. [Google Scholar] [CrossRef]
- Springman, A.C.; Lacher, D.W.; Waymire, E.A.; Wengert, S.L.; Singh, P.; Zadoks, R.N.; Davies, H.D.; Manning, S.D. Pilus distribution among lineages of group b streptococcus: An evolutionary and clinical perspective. BMC Microbiol. 2014, 14, 159. [Google Scholar] [CrossRef] [Green Version]
- Berardi, A.; Guidotti, I.; Creti, R.; Alfarone, G.; Grottola, A.; Venturelli, C.; Fregni Serpini, G.; Della Casa, E.; Vecchi, E.; Boncompagni, A.; et al. Two Overlapping Clusters of Group B Streptococcus Late-onset Disease in a Neonatal Intensive Care Unit. Pediatr. Infect. Dis. J. 2018, 37, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Campisi, E.; Rosini, R.; Ji, W.; Guidotti, S.; Rojas-López, M.; Geng, G.; Deng, Q.; Zhong, H.; Wang, W.; Liu, H.; et al. Genomic Analysis Reveals Multi-Drug Resistance Clusters in Group B Streptococcus CC17 Hypervirulent Isolates Causing Neonatal Invasive Disease in Southern Mainland China. Front. Microbiol. 2016, 7, 1265. [Google Scholar] [CrossRef] [PubMed]
- Teatero, S.; Ramoutar, E.; McGeer, A.; Li, A.; Melano, R.G.; Wasserscheid, J.; Dewar, K.; Fittipaldi, N. Clonal Complex 17 Group B Streptococcus strains causing invasive disease in neonates and adults originate from the same genetic pool. Sci. Rep. 2016, 6, 20047. [Google Scholar] [CrossRef] [Green Version]
- Martins, E.R.; Pedroso-Roussado, C.; Melo-Cristino, J.; Ramirez, M.; Portuguese Group for the Study of Streptococcal Infections. Streptococcus agalactiae Causing Neonatal Infections in Portugal (2005–2015): Diversification and Emergence of a CC17/PI-2b Multidrug Resistant Sublineage. Front. Microbiol. 2017, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Stoll, B.J.; Hansen, N.I.; Sánchez, P.J.; Faix, R.G.; Poindexter, B.B.; Van Meurs, K.P.; Bizzarro, M.J.; Goldberg, R.N.; Frantz, I.D., 3rd; Hale, E.C.; et al. Early onset neonatal sepsis: The burden of group B Streptococcal and E. coli disease continues. Pediatrics 2011, 127, 817–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polcwiartek, L.B.; Smith, P.B.; Benjamin, D.K.; Zimmerman, K.; Love, A.; Tiu, L.; Murray, S.; Kang, P.; Ebbesen, F.; Hagstrøm, S.; et al. Early-onset sepsis in term infants admitted to neonatal intensive care units (2011–2016). J. Perinatol. 2021, 41, 157–163. [Google Scholar] [CrossRef]
- Colomer, B.F.; Badia, M.C.; Cotallo, D.C.; Sastre, J.L.; Grupo Castrillo Network. The Spanish National Network “Grupo Castrillo”: 22 Years of Nationwide Neonatal Infection Surveillance. Am. J. Perinatol. 2020, 37 (Suppl. 02), S71–S75. [Google Scholar] [CrossRef]
- Jamrozy, D.; Bijlsma, M.W.; de Goffau, M.C.; van de Beek, D.; Kuijpers, T.W.; Parkhill, J.; van der Ende, A.; Bentley, S.D. Increasing incidence of group B streptococcus neonatal infections in the Netherlands is associated with clonal expansion of CC17 and CC23. Sci. Rep. 2020, 10, 9539. [Google Scholar] [CrossRef]
- O’Sullivan, C.P.; Lamagni, T.; Patel, D.; Efstratiou, A.; Cunney, R.; Meehan, M.; Ladhani, S.; Reynolds, A.J.; Campbell, R.; Doherty, L.; et al. Group B streptococcal disease in UK and Irish infants younger than 90 days, 2014–2015: A prospective surveillance study. Lancet. Infect. Dis. 2019, 19, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Nanduri, S.A.; Petit, S.; Smelser, C.; Apostol, M.; Alden, N.B.; Harrison, L.H.; Lynfield, R.; Vagnone, P.S.; Burzlaff, K.; Spina, N.L.; et al. Epidemiology of Invasive Early-Onset and Late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-Based Surveillance. JAMA Pediatr. 2019, 173, 224–233. [Google Scholar] [CrossRef]
- Filkins, L.; Hauser, J.R.; Robinson-Dunn, B.; Tibbetts, R.; Boyanton, B.L.; Revell, P. American Society for Microbiology Provides 2020 Guidelines for Detection and Identification of Group B Streptococcus. J. Clin. Microbiol. 2020, 59, e01230-20. [Google Scholar] [CrossRef] [PubMed]
- Detection of Carriage of Group B Streptococci (Streptococcus agalactiae). In UK Standards for Microbiology Investigations. B 58 Issue SMI B 58: Detection of Carriage of Group B Streptococci (Streptococcus agalactiae); 3.1; Public Health England: Colindale, UK, 2018; pp. 1–24.
- Tzialla, C.; Berardi, A.; Farina, C.; Clerici, P.; Borghesi, A.; Viora, E.; Scollo, P.; Stronati, M. Task Force for group B streptococcal infections for the Italian Society of Neonatology; Italian Society of Obstetricians and Gynecologists; Italian Association of Clinical Microbiologists. Strategies for preventing group B streptococcal infections in newborns: A nation-wide survey of Italian policies. Ital. J. Pediatr. 2017, 43, 98. [Google Scholar] [CrossRef] [Green Version]
- Baeringsdottir, B.; Erlendsdottir, H.; Bjornsdottir, E.S.; Martins, E.R.; Ramirez, M.; Haraldsson, A.; Thorkelsson, T. Group B streptococcal infections in infants in Iceland: Clinical and microbiological factors. J. Med. Microbiol. 2021, 70, 001426. [Google Scholar] [CrossRef]
- Kadambari, S.; Trotter, C.L.; Heath, P.T.; Goldacre, M.J.; Pollard, A.J.; Goldacre, R. Group B Streptococcal Disease in England (1998–2017): A Population-based Observational Study. Clin. Infect. Dis. 2021, 72, e791–e798. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsdottir, M.J.; Hentz, E.; Adlerberth, I.; Tessin, I.; Trollfors, B.; Elfvin, A. Late-onset Neonatal Infections 1997 to 2017 Within a Cohort in Western Sweden—The Last 21 Years of a 43-Year Surveillance. Pediatr. Infect. Dis. J. 2021, 40, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Collin, S.M.; Lamb, P.; Jauneikaite, E.; Le Doare, K.; Creti, R.; Berardi, A.; Heath, P.T.; Sriskandan, S.; Lamagni, T. Hospital clusters of invasive Group B Streptococcal disease: A systematic review. J. Infect. 2019, 79, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Collin, S.M.; Groves, N.; O’Sullivan, C.; Jauneikaite, E.; Patel, D.; Cunney, R.; Meehan, M.; Reynolds, A.; Smith, A.; Lindsay, D.; et al. Uncovering Infant Group B Streptococcal (GBS) Disease Clusters in the United Kingdom and Ireland Through Genomic Analysis: A Population-based Epidemiological Study. Clin. Infect. Dis. 2021, 72, e296–e302. [Google Scholar] [CrossRef] [PubMed]
- Horváth-Puhó, E.; van Kassel, M.N.; Gonçalves, B.P.; de Gier, B.; Procter, S.R.; Paul, P.; van der Ende, A.; Søgaard, K.K.; Hahné, S.J.M.; Chandna, J.; et al. Mortality, neurodevelopmental impairments, and economic outcomes after invasive group B streptococcal disease in early infancy in Denmark and the Netherlands: A national matched cohort study. Lancet Child Adolesc. Health 2021, 5, 398–407. [Google Scholar] [CrossRef]
- McGee, L.; Chochua, S.; Li, Z.; Mathis, S.; Rivers, J.; Metcalf, B.; Ryan, A.; Alden, N.; Farley, M.M.; Harrison, L.H.; et al. Multistate, Population-Based Distributions of Candidate Vaccine Targets, Clonal Complexes, and Resistance Features of Invasive Group B Streptococci within the United States, 2015–2017. Clin. Infect. Dis. 2021, 72, 1004–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.; Liu, H.; Madhi, S.A.; Cunnington, M.; Zhang, Z.; Dangor, Z.; Zhou, H.; Mu, X.; Jin, Z.; Wang, A.; et al. Clinical and Molecular Epidemiology of Invasive Group B Streptococcus Disease among Infants, China. Emerg. Infect. Dis. 2019, 25, 2021–2030. [Google Scholar] [CrossRef] [Green Version]
- de Cambronne, R.D.; Fouet, A.; Picart, A.; Bourrel, A.S.; Anjou, C.; Bouvier, G.; Candeias, C.; Bouaboud, A.; Costa, L.; Boulay, A.C.; et al. CC17 group B Streptococcus exploits integrins for neonatal meningitis development. J. Clin. Investig. 2021, 131, e136737. [Google Scholar] [CrossRef]
- Almeida, A.; Rosinski-Chupin, I.; Plainvert, C.; Douarre, P.E.; Borrego, M.J.; Poyart, C.; Glaser, P. Parallel Evolution of Group B Streptococcus Hypervirulent Clonal Complex 17 Unveils New Pathoadaptive Mutations. MSystems 2017, 2, e00074-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelan, F.; Lafita, A.; Griffiths, S.C.; Cooper, R.E.M.; Whittingham, J.L.; Turkenburg, J.P.; Manfield, I.W.; St John, A.N.; Paci, E.; Bateman, A.; et al. Defining the remarkable structural malleability of a bacterial surface protein Rib domain implicated in infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26540–26548. [Google Scholar] [CrossRef] [Green Version]
- Plainvert, C.; Hays, C.; Touak, G.; Joubrel-Guyot, C.; Dmytruk, N.; Frigo, A.; Poyart, C.; Tazi, A. Multidrug-Resistant Hypervirulent Group B Streptococcus in Neonatal Invasive Infections, France, 2007–2019. Emerg. Infect. Dis. 2020, 26, 2721–2724. [Google Scholar] [CrossRef] [PubMed]
- Meehan, M.; Eogan, M.; McCallion, N.; Cunney, R.; Bray, J.E.; Jolley, K.A.; Unitt, A.; Maiden, M.C.J.; Harrison, O.B.; Drew, R.J. Genomic epidemiology of group B streptococci spanning 10 years in an Irish maternity hospital, 2008–2017. J. Infect. 2021, 83, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Berardi, A.; Cassetti, T.; Creti, R.; Vocale, C.; Ambretti, S.; Sarti, M.; Facchinetti, F.; Cose, S.; The Prepare Network; Heath, P.; et al. The Italian arm of the PREPARE study: An international project to evaluate and license a maternal vaccine against group B streptococcus. Ital. J. Pediatr. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Carreras-Abad, C.; Ramkhelawon, L.; Heath, P.T.; Le Doare, K. A Vaccine against Group B Streptococcus: Recent Advances. Infect. Drug Resist. 2020, 13, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.E.; Heath, P.T.; Le Doare, K. GBS and CMV Vaccines in Pipeline Development. In Pediatric Vaccines and Vaccinations; Vesikari, T., Van Damme, P., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
| EOD n = 67 (35%) | LOD n = 124 (65%) | All Cases (n = 191) | |
|---|---|---|---|
| Males, n (%) | 32 (47.8) | 63 (50.8) | 95 (49.7) |
| Gestational age at delivery, median, week (IQ) | 39 (37.7–40) | 38 (33–39) | 38 (34.8–40) |
| Age at onset of disease, median, (IQ) | 4 h (0–18.5) | 30 days (17.5–44) | not done |
| Ethnicity n (%) | |||
| African | 5 (7.5) | 16 (12.9) | 21 (11.0) |
| Arab | 1 (1.5) | 4 (3.2) | 5 (2.6) |
| Asian | 3 (4.5) | 3 (2.4) | 6 (3.1) |
| White | 58 (86.6) | 101 (81.4) | 159 (83.3) |
| Clinical diagnosis, n (%) | |||
| Sepsis | 35 (52.2) | 78 (62.9) | 113 (59.2) |
| Asymptomatic bacteremia | 21 (31.3) | 2 (1.6) | 23 (12.0) |
| Meningitis | 9 (13.4) | 32 (25.8) | 41 (21.5) |
| Septic shock | 2 (3.0) | 10 (8.0) | 12 (6.3) |
| Arthritis, osteomyelitis | 0 | 2 (1.6) | 2 (1.0) |
| Bacterial isolation, n (%) | |||
| Blood | 60 (89.5) | 89 (71.8) | 149 (78.0) |
| Cerebrospinal fluid | 2 (3.0) | 2 (1.6) | 4 (2.1) |
| Blood and cerebrospinal fluid | 5 (7.5) | 33 (26.6) | 38 (19.9) |
| Outcome, n (%) | |||
| Full recovery | 57 (85.1) | 108 (87.1) | 165 (86.4) |
| Brain lesions | 4 (6.0) | 12 (9.7) | 16 (8.4) |
| Deceased | 6 (8.9) | 4 (3.2) | 10 (5.2) |
| Risk factors n (%) | |||
| None | 37 (55.2) | 64 (51.6) | 101 (52.9) |
| 1 | 21 (31.3) | 49 (39.5) | 70 (36.6) |
| >1 | 7 (10.4) | 5 (4.0) | 12 (6.3) |
| Not reported | 2 (3.0) | 6 (4.8) | 8 (4.2) |
| Risk factors, specified, n (%) | |||
| Prematurity (<37 weeks) | 12 (17.9) | 49 (39.5) | 61 (31.9) |
| Intrapartum fever ≥ 38 °C | 12 (17.9) | 1 (0.8) | 13 (6.8) |
| Bacteriuria | 4 (6.0) | 2 (1.6) | 6 (3.1) |
| Amniotic membrane rupture > 18h | 8 (11.9) | 7 (5.6) | 15 (7.8) |
| GBS antenatal screening, n (%) * | |||
| Not done | 4/53 (7.5) | 9/74 (12.1) | 13/127 (10.2) |
| Not reported | 2/53 (3.8) | 5/74 (6.7) | 7/127 (5.5) |
| Negative | 28/47 (59.6) | 30/60 (50.0) | 58/107 (54.2) |
| Positive | 19/47 (40.4) | 30/60 (50.0) | 49/107 (45.8) |
| Mode of delivery, n (%) | |||
| Vaginal | 44 (65.7) | 67 (54.0) | 111 (58.1) |
| Planned caesarean section | 1 (1.5) | 26 (21.0) | 27 (14.1) |
| Emergency caesarean section | 20 (29.8) | 24 (19.3) | 44 (23.0) |
| Not reported | 2 (3.0) | 7 (5.6) | 9 (4.7) |
| Pre-Term Infants (n = 61, 31.9%) | Term Infants (n = 119, 62.3%) | p Value | |
|---|---|---|---|
| Hospital stay, median, days (IQ) | 36.5 (14.7–64.5) | 12 (10–16) | |
| Mode of delivery, n (%) | |||
| vaginal | 19 (31.1%) | 90 (75.6%) | <0.0001 |
| planned caesarean section | 14 (23.0%) | 12 (10.1%) | 0.04 |
| emergency caesarean section | 28 (45.9%) | 14 (11.8%) | <0.0001 |
| not available | -- | 3 | |
| Outcome | |||
| death | 5 (8.2%) | 3 (2.5%) | 0.12 |
| brain lesions at discharge from hospital | 10 (16.4%) | 6 (5.0%) | 0.02 |
| IAP Administrated | IAP Not Administrated | |
|---|---|---|
| any indication for IAP | 10 | 22 |
| (32 cases) | emergency CS (8 cases) | |
| unknown reason (14 cases) | ||
| no indication for IAP | 7 | 26 |
| (33 cases) | (maternal GBS negative status but fever) ** | unknown maternal GBS status and no risk factors (3 cases) |
| maternal GBS negative status and no risk factors (20 cases) | ||
| maternal GBS negative status but fever (3 cases) |
| Ery Res Strains (Number, %) | Serotypes | ST-17 | MDR* ST17 (Number, %) | Erythromycin Resistance Genes (Number, %) | Tet Res Strains (Number, %) | Tetracycline Resistant Genes (Number, %) | |
|---|---|---|---|---|---|---|---|
| EOD | 8 (30.8%) | Ib (1), II (1), III (6) | 6 | 4 (66.7%) | ermB (6, 75%); ermA (2, 25%) | 25 (96.1%) | tetM (21; 80.8%); tetO (6; 23.1%) |
| (26) | |||||||
| LOD | 17 (27.0%) | III (17) | 17 | 15 (88.2%) | ermB (17, 100%) | 57 (90.5%) | tetM (44, 69.8%); tetO (16, 25.4%); |
| (63) | tetM + tetO (1; 1.6%) | ||||||
| Total | 25 (28.1%) | 23 (92%) | 19 (76%) | ermB (23, 92%); ermA (2, 8%) | 82 (92.1%) | tetM (66, 74.1%); tetO (23, 25.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Creti, R.; Imperi, M.; Berardi, A.; Lindh, E.; Alfarone, G.; Pataracchia, M.; Recchia, S.; The Italian Network on Neonatal and Infant GBS Infections. Invasive Group B Streptococcal Disease in Neonates and Infants, Italy, Years 2015–2019. Microorganisms 2021, 9, 2579. https://doi.org/10.3390/microorganisms9122579
Creti R, Imperi M, Berardi A, Lindh E, Alfarone G, Pataracchia M, Recchia S, The Italian Network on Neonatal and Infant GBS Infections. Invasive Group B Streptococcal Disease in Neonates and Infants, Italy, Years 2015–2019. Microorganisms. 2021; 9(12):2579. https://doi.org/10.3390/microorganisms9122579
Chicago/Turabian StyleCreti, Roberta, Monica Imperi, Alberto Berardi, Erika Lindh, Giovanna Alfarone, Marco Pataracchia, Simona Recchia, and The Italian Network on Neonatal and Infant GBS Infections. 2021. "Invasive Group B Streptococcal Disease in Neonates and Infants, Italy, Years 2015–2019" Microorganisms 9, no. 12: 2579. https://doi.org/10.3390/microorganisms9122579
APA StyleCreti, R., Imperi, M., Berardi, A., Lindh, E., Alfarone, G., Pataracchia, M., Recchia, S., & The Italian Network on Neonatal and Infant GBS Infections. (2021). Invasive Group B Streptococcal Disease in Neonates and Infants, Italy, Years 2015–2019. Microorganisms, 9(12), 2579. https://doi.org/10.3390/microorganisms9122579






