Abstract
Streptococcal peptide of virulence (SpoV) is a Streptococcus pyogenes (group A streptococcus (GAS))-specific peptide that is important for GAS survival in murine blood, and the expression of the virulence factors streptolysin O (slo) and streptolysin S (sagA). We used a spoV mutant in isolate MGAS315 to assess the contribution of the SpoV peptide to virulence by using a murine model of invasive disease and an ex vivo human model (Lancefield assay). We then used antibodies to SpoV in both models to evaluate their ability to decrease morbidity and mortality. Results showed that SpoV is essential for GAS virulence, and targeting the peptide has therapeutic potential.
1. Introduction
Streptococcus pyogenes (group A streptococcus (GAS)) is an exclusively human pathogen that causes both mild and invasive multiple diseases. Mild infections of the throat and skin include pharyngitis and impetigo. Life-threatening invasive GAS (iGAS) diseases include bacteremia, streptococcal toxic shock syndrome, pneumonia, and necrotizing fasciitis (“flesh-eating disease”). iGAS diseases are significantly concerning because they have high mortality rates despite the availability of antibiotics that are effective ex vivo [1,2]. The diversity and severity of GAS diseases is partly attributed to the pathogens’ ability to regulate the expression of a variety of virulence factors, including adherence and invasion proteins, toxins, superantigens, proteases, and immune-modulating proteins [3]. Consequently, to cause disease, GAS must be able to adapt to and grow in many different environments within the human host.
GAS uses extracellular peptides as signaling molecules to regulate the expression of virulence genes [4,5]. Propeptides are synthesized and then post-translationally processed during secretion to biologically active extracellular signaling peptides. Extracellular peptides can be detected either at the cell surface or intracellularly [5]. Peptides are typically detected at the cell surface by a membrane-bound sensor kinase. The sensor kinase responds by transferring a phosphoryl group to a response regulator protein to change its DNA-binding specificity, which results in the activation or repression of target genes. Alternatively, peptides can be actively transported into the cell, where the peptide can directly interact with a transcriptional regulator to alter target gene expression [6,7,8]. Several characterized GAS signaling peptides influence pathogenesis by utilizing both mechanisms [9,10,11,12,13,14,15].
We previously identified the streptococcal peptide of virulence (SpoV) in culture supernatants of MGAS315 when screening for GAS signaling peptides [16]. A BLASTP search of the National Center for Biotechnology Information (NCBI) database using SpyM3_0132 as a query identified 1982 similar sequences among GAS isolates. We performed signal peptide cleavage site predictions for SpoV using SignalP 5.0 [16]. The software predicted that, in isolate MGAS315, SpoV contains a typical bacterial signal peptide of 31 amino acids followed by a secreted 20 amino acid extracellular peptide [16]. The extracellular 20 amino acid SpoV peptide (NDASFYGHTGPDSWLLYTVW) is found among 7% of sequenced GAS isolates, and there is no amino acid sequence variation among GAS isolates that encode the 20 amino acid extracellular SpoV [16]. The majority (93%) of GAS isolates encode a 55 amino acid peptide, which is processed to an extracellular 24 amino acid SpoV peptide [16]. Thirteen different amino acid sequence variations of the 24 amino acid SpoV peptide occur among the 1982 GAS isolates identified in our BLASTP search [16]. The main difference between the 20 and 24 amino acid extracellular SpoV peptides is the presence or absence of amino acids tyrosine, serine, asparagine, and glycine (YSNG) near the N terminus. While our analysis was limited, gene expression was equally affected following the addition of either the 20 or 24 amino acid peptides, indicating that both peptide variants have the same effect on GAS gene expression [16].
The expression of spoV varies among GAS isolates due to allelic variation in rocA (regulator of CovS), which is a component of the control of virulence (CovRS) regulatory system [16]. Mutations to covS can naturally occur during infection, which alters the transcription of CovR regulated genes such as slo and results in more invasive GAS diseases [17,18]. SpoV is also important for the expression of several CovRS regulated genes, including slo, sagA (streptolysin S; SLS), and speB (streptococcal exotoxin B); however, the direct mechanisms involved in the SpoV-mediated gene regulation of CovRS-regulated genes are unknown [16]. One way in which pore-forming toxins SLO and SLS are associated with iGAS disease is by forming large pores in host cell membranes, which disrupts their integrity [19,20]. The virulence of SpeB throughout infection is complex. SpeB cleaves multiple host proteins, including extracellular matrix proteins, immunoglobulins, and antimicrobial peptides [21,22], which interferes with host immune functions. Additionally, SpeB cleaves several GAS proteins, including the M protein [23], superantigens [24,25], and streptokinase [26], which interferes their functions. Changes in virulence gene expression suggest that SpoV is likely to be important for GAS virulence.
SpoV is not encoded in the genomes of any other bacterial species, but orthologs are present in the genomes of all GAS isolates. In all GAS isolates, SpoV is encoded proximal to the slo gene, which encodes the SLO cytolysin. The deletion of spoV decreased SLO-specific hemolytic activity and resistance to murine immune effector cells [16]. Further, the deletion of spoV and subsequent addition of synthesized SpoV peptides increased slo expression [16]. Because peptide signaling plays an important regulatory role during disease progression, and SpoV affects virulence gene expression, we hypothesized that SpoV may contribute to GAS virulence. In this study, the contribution of SpoV to GAS virulence, and the efficacy of anti-SpoV immunotherapy are evaluated.
2. Materials and Methods
2.1. Strain and Culture Conditions
Frozen stocks of GAS isolate MGAS315 (serotype M3) (American Type Culture Collection; ATCC; Manassas, VA, USA) were stored in 50% glycerol at −80 °C until use. Frozen stocks were streaked onto Todd-Hewitt (THY) (Becton Dickinson; Sparks, MD, USA) agar and incubated overnight at 37 °C in a 5% CO2 atmosphere. Then, 10 mL of THY broth containing 0.2% (w/v) yeast extract (Becton Dickinson) was used to suspend GAS in liquid media without shaking. The culture was then grown at 37 °C in a 5% CO2 atmosphere. Escherichia coli was grown with a Luria–Bertani (LB) (Fisher Scientific; Waltham, MA, USA) medium at 37 °C with agitation or on LB agar plates. When appropriate, spectinomycin (Spec; 100 µg/mL for both S. pyogenes and E. coli), or chloramphenicol (Cm; 10 µg/mL for S. pyogenes and 50 µg/mL for E. coli) was added to the media. Antibiotics were purchased from Fisher Scientific; Waltham, MA, USA.
2.2. DNA Manipulation
Standard protocols or manufacturer’s instructions were used to isolate plasmid DNA, and conduct restriction endonuclease, DNA ligase, PCR, and other enzymatic treatments of plasmids and DNA fragments. Enzymes were purchased from New England Biolabs, Inc. (NEB; Ipswich, MA, USA). Phusion DNA polymerase (NEB; Ipswich, MA, USA) was used to amplify specific regions of the MGAS315 chromosome. Oligonucleotides were purchased from Eurofins Genomics (Louisville, KY, USA).
2.3. Construction of spoV Mutant
The MGAS315 spoV deletion mutant was constructed by double-crossover recombination using a plasmid (pAH3) that contained a spectinomycin-resistance gene (SpecR; aad9) cloned between DNA identical to DNA sequences located upstream and downstream of spoV [16].
2.4. Construction of spoV Complemented Mutant
We complemented the MGAS315 spoV mutant by using a derivative of the pAM401 shuttle vector with spoV expression controlled by the native spoV promoter. The spoV ORF and the nucleotide sequence beginning 183 bases upstream of the spoV start codon were amplified from the MGAS315 genome with PCR, and cloned into the pAM401 vector using restriction sites SalI and BamHI, which were present within the primers. Recombinant plasmid pAH5 was then used to transform the MGAS315 spoV mutant by electroporation, and transformants were selected on agar plates containing chloramphenicol.
2.5. Generation of Anti-SpoV Antibodies
Four rabbits were each immunized with a total of 4 mg of a synthesized SpoV (ANDASFYGQNAPDSWLLYTV) peptide (Biomatik; Cambridge, Ontario, Canada). The 1st and 2nd immunizations (350 µg antigen/rabbit) used complete Freund’s adjuvant and were administered on days 0 and 14. The 3rd–8th immunizations (150 µg antigen/rabbit) used incomplete Freund’s adjuvant and were administered on days 28, 35, 42, 49, 56, and 64. Blood was harvested on day 72, and anti-SpoV antibodies were antigen-specific affinity-purified.
2.6. Ex Vivo Human Model of Virulence
MGAS315 wt, the spoV mutant, or the spoV complemented strain were grown with THY broth at 37 °C to an A600 of 0.6–0.8, centrifuged, suspended in PBS, aliquoted, and stored at −80 °C as frozen stocks. The number of viable GAS cells in the stock preparation was enumerated by dilution plating with THY agar plates. A human blood sample (BioIVT; Baltimore, MD, USA) from pooled donors (male and female) was pretreated with IdeS (100 U/mL) (Sigma-Aldrich; St. Louis, MO, USA) for 1 h at 37 °C [27]. Then, GAS stocks were thawed on ice, and approximately 1000 CFUs (diluted in 15 µL PBS) were added to 270 µL of whole human blood and incubated at 37 °C with rotation. Diluted blood samples were plated onto agar plates after 3 h to enumerate the surviving bacteria.
In some experiments, MGAS315 wt was incubated with 6 µg of anti-SpoV, normal rabbit IgG (rb-IgG) (Sigma-Aldrich; St. Louis, MO, USA), or PBS for 15 min at room temperature. After exposure to anti-SpoV or controls, bacteria were mixed with 270 µL human blood (not pretreated with IdeS) and incubated at 37 °C for 3 h. The survival rate was calculated as (CFU (at a given time point)/CFU (at the start)) × 100.
2.7. Mice
Female Balb/c mice were purchased from Jackson Laboratories (Indianapolis, IN, USA) and used in experiments when they were between 6 and 8 weeks of age. They were housed in cages and given 24 h access to food and water. All animal experiments were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and according to the guidelines of the local Institutional Animal Care and Use Committee of the University of South Dakota. Protocol number 10-06-18-21E was approved on 25 July 2018. Victor Huber and Ruth Bakker provided all animal care and handling training. All efforts were made to minimize suffering, ensure the highest ethical standard, and adhere to the 3R principle (reduction, refinement, and replacement).
2.8. Murine Model of iGAS Disease
GAS was harvested during the mid-exponential phase of growth and diluted 1 × 105 CFUs/500 μL. Mice were lightly anesthetized with 2.5% isoflurane, and GAS was given intraperitoneally (i.p.) using a 25-gauge needle, which was inserted at approximately 45° into the side of the abdominal wall. LD50 was determined by the method of Reed and Muench [28]. Animal health and behavior was monitored at least three times a day over the 14 day course of the experiment. Body weight was recorded daily. Endpoint criteria included extreme clinical signs of infection (huddling, hunched posture, ruffled fur, tachypnea), severe hypothermia as indicated by a temperature of 34 °C (~4.5 °C below normal), and weight loss equal to or greater than 20% of starting weight. Mice with one or more of these symptoms were immediately euthanized, and the infection was considered to be lethal.
2.9. Passive Immunotherapy with Anti-SpoV Antibodies
In some experiments, 0.5 mg of anti-SpoV, rb-IgG, or PBS (500 μL) was administered two hours prior to GAS infection. Antibodies were administered i.p.
2.10. GAS Quantification in Mouse Tissues
Mouse tissue samples were collected 24 h after GAS inoculation. Blood was collected from the submandibular vein of each animal, and immediately after they had been euthanized by CO2 inhalation, the lungs and spleens were removed and homogenized in sterile PBS. GAS were enumerated by dilution plating with THY or blood agar plates as previously described [29].
2.11. Statistics
All quantification and statistical data analyses were conducted with GraphPad Prism 8 software (San Diego, CA, USA). Unless otherwise stated, error bars represent standard error of the mean (SEM), and p values were calculated using either one-way analysis of variance (ANOVA) with a Tukey multiple-comparison post hoc test or a Student’s t-test. For mouse survival studies, results were graphed as Kaplan–Meier curves, and data were analyzed using the log-rank test. Values were accepted as significant if the p value was less than 0.05.
3. Results
3.1. Assessment of Virulence in a Murine Model of Invasive GAS Disease
To determine if SpoV is important for GAS virulence, we compared morbidity and mortality in a murine model of iGAS disease. Groups of mice were inoculated intraperitoneally (i.p) with the MGAS315 wt strain, the isogenic spoV mutant, or the spoV complemented mutant, and mortality was assessed for 14 days after infection (Figure 1). Of mice infected with the spoV mutant, 100% (12/12) survived, whereas only 17% (2/12) mice infected with MGAS315 wt survived the infection. Overall, mortality was significantly greater in mice inoculated with the parental MGAS315 strain compared to mice inoculated with the spoV mutant (p < 0.0001). There was also significantly greater morbidity (weight loss) with MGAS315 wt compared to the spoV mutant (Figure S1). We complemented the spoV mutant by transforming it with a shuttle plasmid that expressed the spoV open reading frame adjacent to its promotor. Complementation decreased survival compared to the spoV mutant, and survival was like that observed with wild-type infection (p < 0.0001). Overall, results showed that SpoV is essential for virulence in a mouse model of invasive infection.
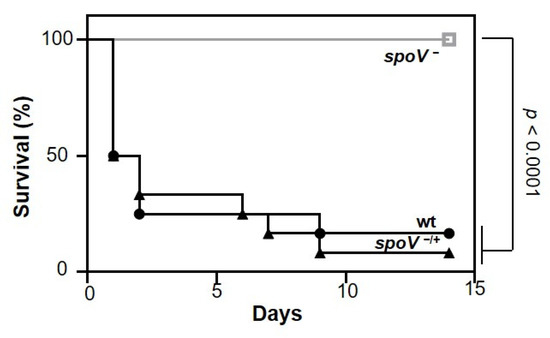
Figure 1.
SpoV is essential for virulence in a murine model of iGAS disease. Kaplan–Meier survival analysis shown for mice following intraperitoneal infection (1 × 105 CFU) with MGAS315 wt, spoV mutant (spoV−), or spoV complemented mutant (spoV−/+). Mortality was assessed for 14 days after infection (n = 12) (p < 0.0001 by log-rank test).
3.2. SpoV Enhanced GAS Survival in an Ex Vivo Human Model of Virulence
Invasive GAS diseases are often associated with bacteremia. Therefore, the ability of GAS to survive and grow in whole human blood was tested using an ex vivo human model of virulence (Lancefield assay). Blood was pretreated with IdeS to degrade IgG that might be specific to MGAS315 [30]. Then, MGAS315 wt, the isogenic spoV mutant, or the spoV complemented mutant was added and incubated at 37 °C for 3 h prior to determining the number of viable bacteria by dilution plating. Fewer CFUs were recovered from blood containing the MGAS315 spoV mutant compared to blood containing either the MGAS315 wt strain or the complemented mutant strain (Figure 2; p < 0.05). Overall, results showed that SpoV enhances GAS virulence in an ex vivo human model of virulence.
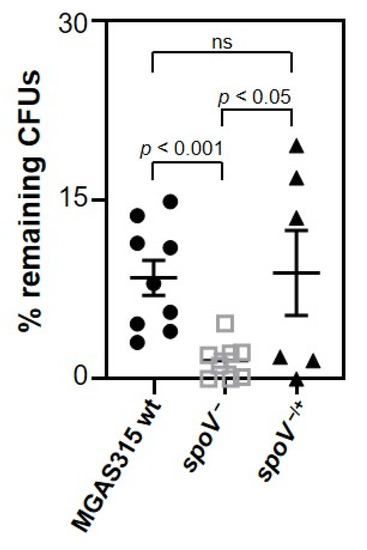
Figure 2.
SpoV is essential for virulence in an ex vivo human model of virulence. Human blood from pooled donors (270 µL) was treated with 100 U/mL IdeS. Approximately 1000 CFUs of each bacterial strain were added and incubated at 37 °C. After 3 h, diluted samples were plated onto THY agar plates for enumeration. Three different human blood collections, each consisting of one female and one male donor, were used for independent experiments that were completed in triplicate or duplicate. Lines represent the mean and SEM value in each group. Statistical significance was determined by using one-way ANOVA with Tukey’s multiple-comparison test. p values determined by comparison among the indicated strains.
3.3. SpoV Decreased GAS Dissemination in a Mouse Model of Systemic Infection
Growth in blood mimics a key feature of invasive GAS diseases, which is the ability to disseminate and potentially colonize multiple organs. To investigate whether SpoV could affect GAS dissemination, and hence contribute to virulence, we compared the number of viable GAS recovered from the blood, spleen, and lungs of mice 24 h after i.p. inoculation with MGAS315 wt, the spoV mutant, or the spoV complemented strain (Figure 3). There were fewer GAS recovered from the blood of mice infected with the spoV mutant compared to mice infected with the wt strain (p < 0.05). Specifically, we did not culture any GAS from the blood of mice inoculated with the spoV mutant. Compared to the spoV mutant, a greater number (103 CFU/mL) of CFUs were recovered in the blood following infection with the spoV complemented mutant; however, the difference was not statistically significant.
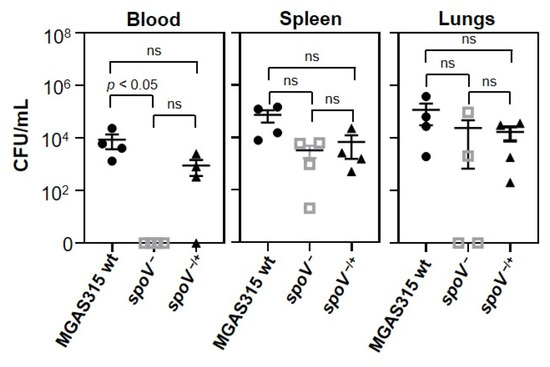
Figure 3.
Deletion of spoV decreased GAS dissemination in a murine model of iGAS disease. Dissemination of GAS determined 24 h following intraperitoneal infection (1 × 105 CFU) with MGAS315 wt, the spoV mutant, or the spoV complemented mutant (n = 4). Mice were euthanized 24 h after GAS infection, and total number of CFUs in blood, spleen, and lungs was determined for each mouse. Lines represent the mean and SEM value in each group. Significance determined by using one-way ANOVA with Tukey’s multiple-comparison test (ns, not significant).
GAS enumeration in the spleen showed that there was a 1.4 log decrease in the number of CFUs among mice inoculated with the spoV mutant compared to mice infected with the MGAS315 wt strain, and complementation of the mutant partially increased the number of GAS recovered in the spleen, but differences were not statistically significant. Enumeration of GAS in the lungs showed that 50% (2/4) of the mice injected with the spoV mutant did not have recoverable GAS in their lungs, whereas 100% (4/4) of mice infected with MGAS315 wt or the complemented spoV mutant did, although results were again not statistically significant. Overall, deletion of spoV decreased the number of bacteria in the blood, and while the results were not statistically significant, this also resulted in fewer CFUs recovered from the spleen and lungs following i.p infection.
3.4. Administration of Anti-SpoV Decreased GAS Survival in an Ex Vivo Human Model of Virulence
To determine if antigen-purified antibodies specific to SpoV (anti-SpoV) could decrease GAS survival in human blood, approximately 1000 CFUs of MGAS315 wt, the spoV mutant, or the spoV complemented strains were preincubated with anti-SpoV, a rabbit control IgG (rb-IgG), or PBS and mixed with human blood at 37 °C. After 3 h, diluted samples were plated onto THY agar plates for enumeration (Figure 4). In this experiment, the human blood was not pretreated with IdeS to ensure that neither anti-SpoV nor rb-IgG were degraded. Treatment with anti-SpoV significantly enhanced the killing of MGAS315 wt (red circles) in human blood compared to the addition of rb-IgG (gray circles) or PBS (black circles). Treatment with anti-SpoV had no effect on the killing of spoV mutant (red squares) compared to the addition of rb-IgG (gray squares; p < 0.05) or PBS (black squares; p < 0.05). Treatment with anti-SpoV had no effect on the killing of spoV mutant (red squares) compared to the addition of rb-IgG (gray squares) but enhanced GAS killing when samples were treated with PBS (black squares; p < 0.05). The spoV complemented strain (red triangles) had significantly increased resistance to killing in whole blood compared to the spoV mutant (red squares; p < 0.05), indicating a specificity of anti-SpoV to SpoV.
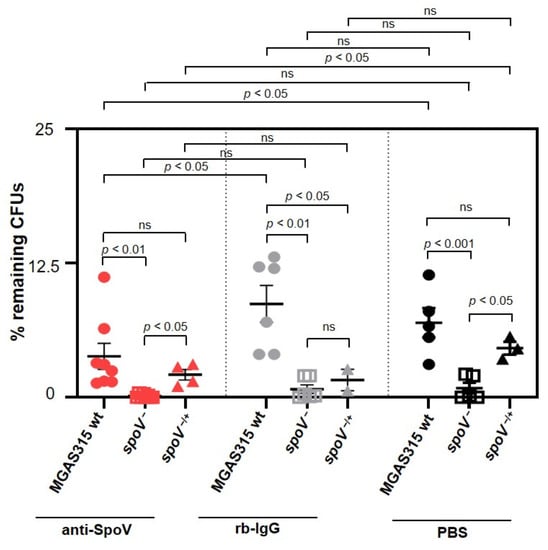
Figure 4.
Anti-SpoV decreased MGAS315 wt survival in an ex vivo model of virulence. MGAS315 wt (circles), the spoV mutant (squares), or spoV complemented (triangle) strains incubated with anti-SpoV (red), control rabbit IgG (rb-IgG) (gray), or PBS (black) for 15 min at room temperature; then, 270 µL of pooled whole human blood added and incubated at 37 °C. After 3 h, viable CFUs were enumerated by dilution plating. Three different human blood collections, each consisting of one female and one male donor, were used for independent experiments that were completed in triplicate or duplicate. Lines represent the mean and SEM value in each group. Significance assessed by using one-way ANOVA with Tukey’s multiple-comparison test (ns, not significant).
3.5. Prophylactic Protection following Addition of Anti-spoV in a Murine Model of iGAS Disease
Next, we tested the protective efficacy of anti-SpoV in a mouse model of iGAS disease. Mice were inoculated i.p. with 0.5 mg anti-SpoV, rb-IgG, or PBS. Two hours later, mice were inoculated with MGAS315 wt. Two independent experiments were completed. After 14 days, 28% of the animals that had received PBS and 44% of mice that had received rb-IgG survived the GAS infection (Figure 5a). In contrast, 60% of the mice that had received anti-SpoV survived iGAS infection. Differences in survival among the groups were not statistically different.
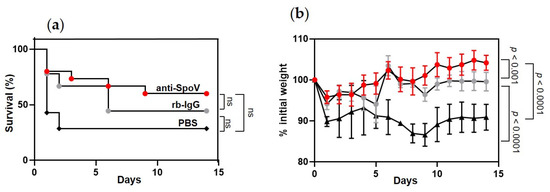
Figure 5.
Prophylactic administration of anti-SpoV increased GAS survival against infection with MGAS315 wt. (a) Kaplan–Meier survival analysis for groups of mice injected i.p. with 0.5 mg of anti-SpoV, control rabbit IgG (rb-IgG), or PBS 2 h prior to i.p. infection of MGAS315 wt (1 × 105 CFUs). Mortality assessed for 14 days after infection from two independent experiments (n = 15). (b) Morbidity (weight loss) assessed for 14 days after infection from two independent experiments (n = 15; ns, not significant).
Overall, mice showed the greatest survival against iGAS when they were prophylactically treated with anti-SpoV; however, nonimmune rabbit IgG (rb-IgG) also increased survival compared to mice that received no treatment (PBS). Anti-SpoV treatment also prolonged death from iGAS disease compared to both control-treated groups of mice. Specifically, of the mice that succumbed to infection, the last death was recorded 9 days after treatment with anti-SpoV, compared to 6 or 2 days after mice had been treated with rb-IgG or PBS, respectively. Additionally, morbidity was significantly decreased in mice that had received anti-SpoV treatment compared to mice that had received PBS (p < 0.0001) or rb-IgG (p < 0.001) (Figure 5b). Mice that had received PBS never regained their initial starting weight, whereas mice that had received anti-SpoV recovered to 100% of their initial starting weight approximately 5 days after infection, which is a striking effect on morbidity.
4. Discussion
GAS secretes peptides that can function as signaling molecules to influence the production of extracellular virulence factors [9,10,13,14] and biofilm production [11,12]. Additionally, gene expression within the competence regulon is activated by a peptide [15]. GAS peptide SpoV is important for the expression of several genes associated with virulence, including slo, sagA (streptolysin S), and speB [16]. In this study, we used a spoV mutant to assess the contribution of SpoV to GAS virulence by using a murine model of invasive disease and an ex vivo human model (Lancefield assay). We then used anti-SpoV antibodies in both models to evaluate their therapeutic potential. Results showed that SpoV is essential for GAS virulence, and targeting the peptide could mitigate severe GAS disease.
Prophylactic treatment of mice with anti-SpoV resulted in increased survival compared to treatment with either rb-IgG or PBS. However, treatment with rb-IgG also increased survival compared to in untreated mice (PBS) (Figure 5a). GAS expresses several surface-bound proteins that bind to nonimmune immunoglobulins (Igs) via the Fc region, which interferes with Fc-mediated phagocytosis by immune cells. In addition to human IgG, GAS binds nonspecifically bind IgG from several animal species, including rabbits [31]. The M protein and M-like proteins bind to both IgG and IgA via the Fc region, which inhibits phagocytosis and increases GAS survival in blood [31,32,33]. SfbI binds to the Fc region of IgG, which prevents antibody-dependent cell cytotoxicity (ADCC) by macrophages [34]. Protein H interacts with Fc region IgG and inhibits IgG-dependent complement activation [35]. Overall, nonimmune IgG binding may contribute to some increased protection compared to mice treated with PBS (Figure 5).
Small peptide antagonists were examined in GAS as a way of disrupting peptide-signaling systems [36,37]. In GAS, short hydrophobic peptides 2/3 (Shp2 and Shp3) induce biofilm production by interacting directly with the transcriptional regulators Rgg2 and Rgg3, respectively [12]. Aggarwal et al. used a high-throughput screen to identify compounds that specifically disrupted Shp–Rgg3 complexes and thereby interfered with Rgg3-regulated pathways, including biofilm development [36]. The addition of peptide antagonists blocked Shp-dependent biofilm formation, indicating that they can disrupt GAS peptide signaling [36]. Thus, peptide antagonists have shown promise at disrupting peptide signaling in GAS; however, the small compounds must be imported into the cytoplasm and compete for binding to the transcriptional regulator in order to exert their function.
To date, there are no reports of antibodies targeting GAS signaling peptides to disrupt their function. In Staphylococcus aureus, the production of several virulence factors, including pore-forming toxin α-hemolysin, are controlled by the accessory gene regulator (agr) quorum-sensing (QS) system [38]. Similar to GAS peptide signaling systems that use linear peptides, the agr system utilizes small cyclic peptides, termed autoinducing peptides (AIPs), as its signaling molecules [39]. Park et al. generated a monoclonal antibody (AP4-24H11) against AIP-4 and showed that AP4-24H11 reduced α-hemolysin expression and activity when supernatants were treated with AP4-24H11 [40]. Additionally, a simultaneous injection of 108 S. aureus with 0.6 mg AP4-24H11 prevented abscess formation in a subcutaneous infection model, compared to controls [40]. In our study, we tested a single 0.5 mg i.p. injection of polyclonal antibodies to SpoV two hours prior to a lethal GAS infection, which significantly decreased morbidity and moderately decreased mortality compared to the controls (Figure 5). Park et al. also used an infection model similar to our study: a single 1 mg i.p. injection of AP4-24H1 given two hours prior to a lethal S. aureus infection. Prophylactic treatment with AP4-24H11 resulted in 100% protection compared to control-treated mice [40]. Thus, targeting an AIP with an antibody was sufficient to suppress peptide signaling and virulence in S. aureus.
SpoV signaling directly contributes to GAS pathogenesis by controlling the expression of several important virulence determinants, such as toxins, proteases, and other immune-evading factors [16]. Peptide signaling relies on coordinating virulence gene expression among a bacterial population. Thus, blocking SpoV-mediated signaling with anti-SpoV presumably causes GAS to lose the ability express critical virulence factors necessary to mount an organized defense against the host immune system. Because SpoV is found in all sequenced GAS isolates, and no orthologs have been detected among other species, including other streptococcal species, SpoV appears to be unique to GAS [16]. Therefore, anti-SpoV is unlikely to disrupt the normal flora. Overall, the development of GAS-specific antibodies, such as those targeting SpoV, may be useful as an adjunct treatment to decrease the morbidity and mortality of iGAS diseases.
Supplementary Materials
The following is available online at https://www.mdpi.com/article/10.3390/microorganisms9112321/s1, Figure S1, SpoV decreased morbidity in a murine model of iGAS disease.
Author Contributions
Conceptualization, M.S.C. and A.L.H.; methodology, A.L.H.; software, A.L.H.; validation, A.L.H.; formal analysis, M.S.C. and A.L.H.; investigation, A.L.H.; resources, M.S.C.; data curation, A.L.H.; writing—original draft preparation, A.L.H.; writing—review and editing, M.S.C.; visualization, M.S.C. and A.L.H.; supervision, A.L.H.; project administration, M.S.C.; funding acquisition, M.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the South Dakota Center for Biologics Research and Commercialization (SD-CBRC) from the SD GOED Governors Research Center.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The University of South Dakota (10-06-18-21E and was approved on 25 July 2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We appreciate the technical assistance of Mariah Volesky and Jordan Grosdidier.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steer, A.C.; Lamagni, T.; Curtis, N.; Carapetis, J.R. Invasive group a streptococcal disease: Epidemiology, pathogenesis and management. Drugs 2012, 72, 1213–1227. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef]
- Musser, J.M.; Shelburne, S.A., 3rd. A decade of molecular pathogenomic analysis of group A Streptococcus. J. Clin. Investig. 2009, 119, 2455–2463. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.C.; Federle, M.J. Quorum sensing in group A Streptococcus. Front. Cell. Infect. Microbiol. 2014, 4, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Declerck, N.; Bouillaut, L.; Chaix, D.; Rugani, N.; Slamti, L.; Hoh, F.; Lereclus, D.; Arold, S.T. Structure of PlcR: Insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 18490–18495. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Estrada, J.; Aceves-Diez, A.E.; Guarneros, G.; de la Torre, M. The RNPP family of quorum-sensing proteins in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2010, 87, 913–923. [Google Scholar] [CrossRef]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- Do, H.; Makthal, N.; VanderWal, A.R.; Rettel, M.; Savitski, M.M.; Peschek, N.; Papenfort, K.; Olsen, R.J.; Musser, J.M.; Kumaraswami, M. Leaderless secreted peptide signaling molecule alters global gene expression and increases virulence of a human bacterial pathogen. Proc. Natl. Acad. Sci. USA 2017, 114, E8498–E8507. [Google Scholar] [CrossRef] [Green Version]
- Do, H.; Makthal, N.; VanderWal, A.R.; Saavedra, M.O.; Olsen, R.J.; Musser, J.M.; Kumaraswami, M. Environmental pH and peptide signaling control virulence of Streptococcus pyogenes via a quorum-sensing pathway. Nat. Commun. 2019, 10, 2586. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, C.; Jimenez, J.C.; Nanavati, D.; Federle, M.J. Multiple length peptide-pheromone variants produced by Streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation. J. Biol. Chem. 2014, 289, 22427–22436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.C.; LaSarre, B.; Jimenez, J.C.; Aggarwal, C.; Federle, M.J. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog. 2011, 7, e1002190. [Google Scholar] [CrossRef]
- Ma, Y.; Bryant, A.E.; Salmi, D.B.; McIndoo, E.; Stevens, D.L. vfr, a novel locus affecting cysteine protease production in Streptococcus pyogenes. J. Bacteriol. 2009, 191, 3189–3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelburne, S.A., 3rd; Olsen, R.J.; Makthal, N.; Brown, N.G.; Sahasrabhojane, P.; Watkins, E.M.; Palzkill, T.; Musser, J.M.; Kumaraswami, M. An amino-terminal signal peptide of Vfr protein negatively influences RopB-dependent SpeB expression and attenuates virulence in Streptococcus pyogenes. Mol. Microbiol. 2011, 82, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Mashburn-Warren, L.; Morrison, D.A.; Federle, M.J. The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J. Bacteriol. 2012, 194, 4589–4600. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.L.; Callegari, E.A.; Chaussee, M.S. The Streptococcus pyogenes signaling peptide SpoV regulates streptolysin O and enhances survival in murine blood. J. Bacteriol. 2021, 203, e00586-20. [Google Scholar] [CrossRef]
- Horstmann, N.; Sahasrabhojane, P.; Suber, B.; Kumaraswami, M.; Olsen, R.J.; Flores, A.; Musser, J.M.; Brennan, R.G.; Shelburne, S.A., 3rd. Distinct single amino acid replacements in the control of virulence regulator protein differentially impact streptococcal pathogenesis. PLoS Pathog. 2011, 7, e1002311. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Zhang, Y.; Agrahari, G.; Chandrahas, V.; Glinton, K.; Donahue, D.L.; Balsara, R.D.; Ploplis, V.A.; Castellino, F.J. A natural inactivating mutation in the CovS component of the CovRS regulatory operon in a pattern D Streptococcal pyogenes strain influences virulence-associated genes. J. Biol. Chem. 2013, 288, 6561–6573. [Google Scholar] [CrossRef] [Green Version]
- Alouf, J.E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol. Ther. 1980, 11, 661–717. [Google Scholar] [CrossRef]
- Ginsburg, I. Mechanisms of cell and tissue injury induced by group A streptococci: Relation to poststreptococcal sequelae. J. Infect. Dis. 1972, 126, 294–340. [Google Scholar] [CrossRef]
- Cunningham, M.W. Pathogenesis of group A streptococcal infections and their sequelae. Adv. Exp. Med. Biol. 2008, 609, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Hynes, W. Virulence factors of the group A streptococci and genes that regulate their expression. Front. Biosci. A J. Virtual Libr. 2004, 9, 3399–3433. [Google Scholar] [CrossRef] [Green Version]
- Raeder, R.; Woischnik, M.; Podbielski, A.; Boyle, M.D. A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin binding properties. Res. Microbiol. 1998, 149, 539–548. [Google Scholar] [CrossRef]
- Kansal, R.G.; Nizet, V.; Jeng, A.; Chuang, W.J.; Kotb, M. Selective modulation of superantigen-induced responses by streptococcal cysteine protease. J. Infect. Dis. 2003, 187, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Aziz, R.K.; Pabst, M.J.; Jeng, A.; Kansal, R.; Low, D.E.; Nizet, V.; Kotb, M. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol. Microbiol. 2004, 51, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezcallah, M.S.; Boyle, M.D.P.; Sledjeski, D.D. Mouse skin passage of Streptococcus pyogenes results in increased streptokinase expression and activity. Microbiology 2004, 150, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Reglinski, M.; Lynskey, N.N.; Sriskandan, S. Modification of the classical Lancefield assay of group A streptococcal killing to reduce inter-donor variation. J. Microbiol. Methods 2016, 124, 69–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed LJ., M.H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Chaussee, M.S.; Sandbulte, H.R.; Schuneman, M.J.; Depaula, F.P.; Addengast, L.A.; Schlenker, E.H.; Huber, V.C. Inactivated and live, attenuated influenza vaccines protect mice against influenza: Streptococcus pyogenes super-infections. Vaccine 2011, 29, 3773–3781. [Google Scholar] [CrossRef] [Green Version]
- Reglinski, M.; Gierula, M.; Lynskey, N.N.; Edwards, R.J.; Sriskandan, S. Identification of the Streptococcus pyogenes surface antigens recognised by pooled human immunoglobulin. Sci. Rep. 2015, 5, 15825. [Google Scholar] [CrossRef] [Green Version]
- Courtney, H.S.; Li, Y. Non-immune binding of human IgG to M-related proteins confers resistance to phagocytosis of group A streptococci in blood. PLoS ONE 2013, 8, e78719. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, L.; O’Toole, P.; Lindahl, G. Many group A streptococcal strains express two different immunoglobulin-binding proteins, encoded by closely linked genes: Characterization of the proteins expressed by four strains of different M-type. Mol. Microbiol. 1992, 6, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.O.; Ghosh, P. Nonimmune antibody interactions of Group A Streptococcus M and M-like proteins. PLoS Pathog. 2021, 17, e1009248. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Molinari, G.; Rohde, M.; Haase, B.; Chhatwal, G.S.; Guzman, C.A. Fc-mediated nonspecific binding between fibronectin-binding protein I of Streptococcus pyogenes and human immunoglobulins. J. Immunol. 1999, 163, 3396–3402. [Google Scholar] [PubMed]
- Berge, A.; Kihlberg, B.M.; Sjöholm, A.G.; Björck, L. Streptococcal protein H forms soluble complement-activating complexes with IgG, but inhibits complement activation by IgG-coated targets. J. Biol. Chem. 1997, 272, 20774–20781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, C.; Jimenez, J.C.; Lee, H.; Chlipala, G.E.; Ratia, K.; Federle, M.J. Identification of Quorum-Sensing Inhibitors Disrupting Signaling between Rgg and Short Hydrophobic Peptides in Streptococci. mBio 2015, 6, e00393-15. [Google Scholar] [CrossRef] [Green Version]
- Parashar, V.; Aggarwal, C.; Federle, M.J.; Neiditch, M.B. Rgg protein structure-function and inhibition by cyclic peptide compounds. Proc. Natl. Acad. Sci. USA 2015, 112, 5177–5182. [Google Scholar] [CrossRef] [Green Version]
- George, E.A.; Muir, T.W. Molecular mechanisms of agr quorum sensing in virulent staphylococci. ChemBioChem A Eur. J. Chem. Biol. 2007, 8, 847–855. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751. [Google Scholar] [CrossRef]
- Park, J.; Jagasia, R.; Kaufmann, G.F.; Mathison, J.C.; Ruiz, D.I.; Moss, J.A.; Meijler, M.M.; Ulevitch, R.J.; Janda, K.D. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 2007, 14, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).