Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Inoculums
2.2. Growth Media
2.3. Synthetic-Grape Must (SGM) and True-Grape Must (TGM) Fermentations Performed with B. bruxellensis in Single- and in Mixed-Culture with S. cerevisiae
2.4. Analysis of Growth
2.4.1. Culturability
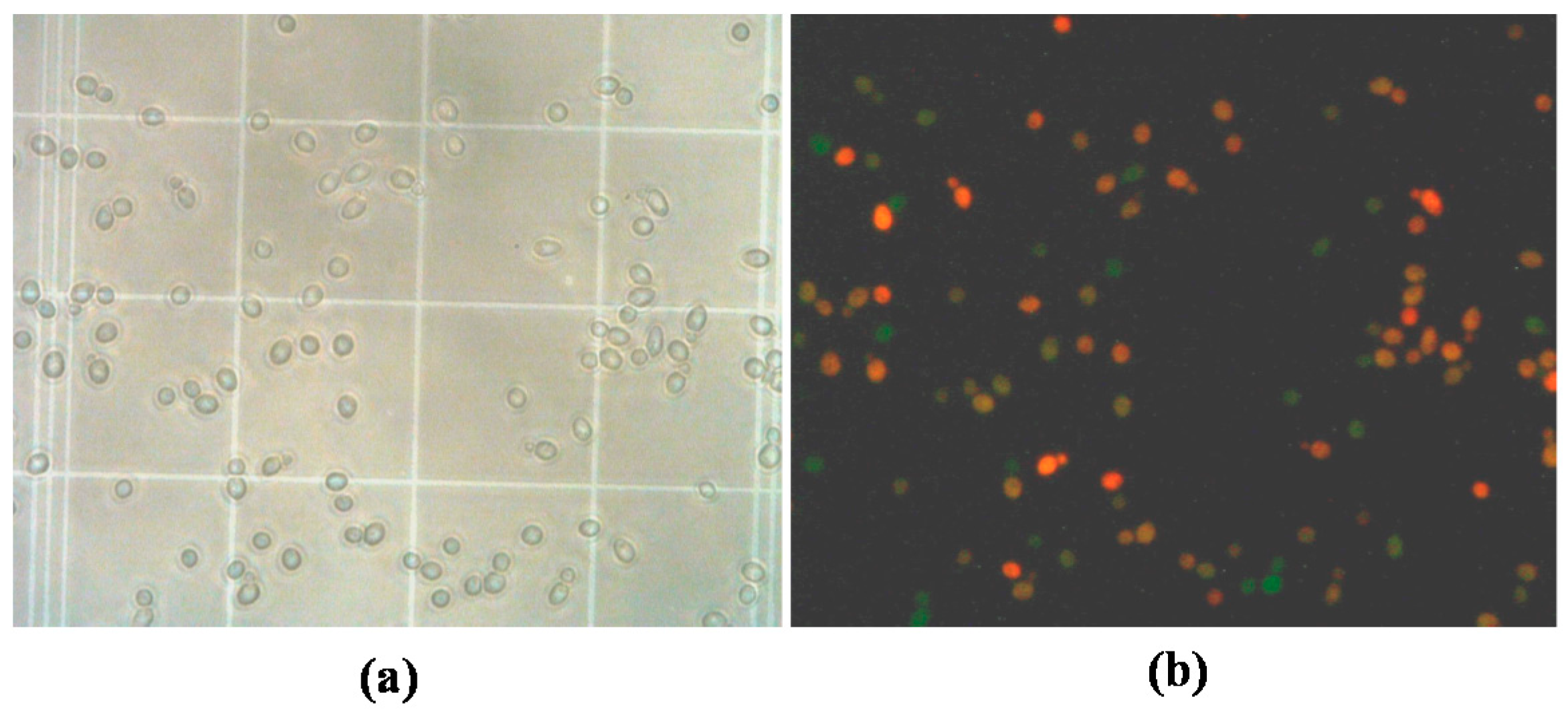
2.4.2. Viability
2.5. Quantification of Sugars and Ethanol by High Performance Liquid Chromatography
2.6. Quantification of 4-Ethylphenol by Gas-Chromatography
2.7. Production and Purification of Saccharomycin
2.8. Effectiveness of the Natural Biocide to Prevent B. bruxellensis Growth in Wine
2.9. Conjugated Effect of Saccharomycin with Sulfur Dioxide (SO2) on B. bruxellensis Culturability
2.10. Statistical Analyses
3. Results
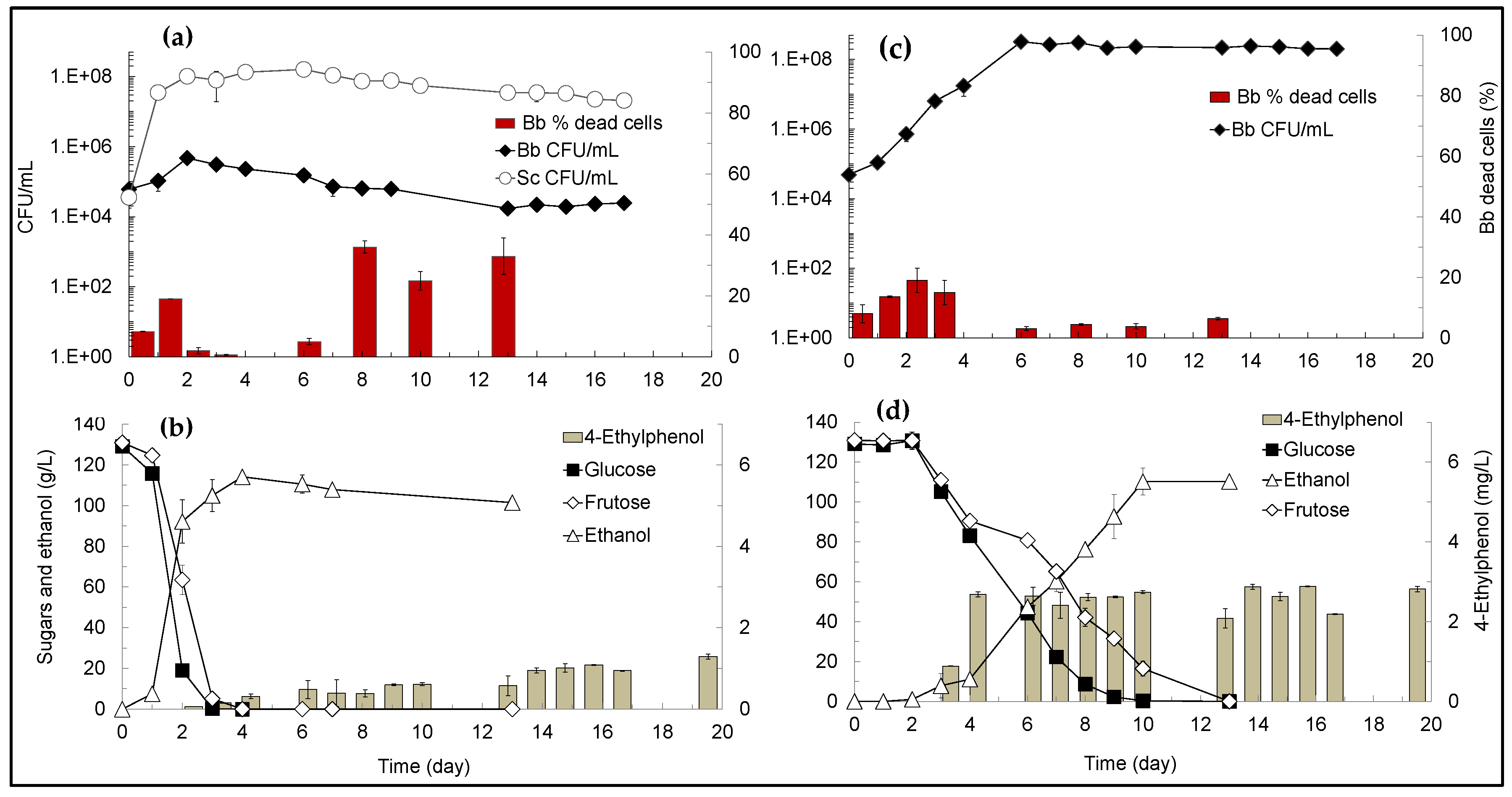
3.1. Synthetic-Grape Must (SGM) and True-Grape Must (TGM) Fermentations Performed with B. bruxellensis in Single- and in Mixed-Cultures with S. cerevisiae
3.2. Biopreservative Potential of Saccharomycin in Wine
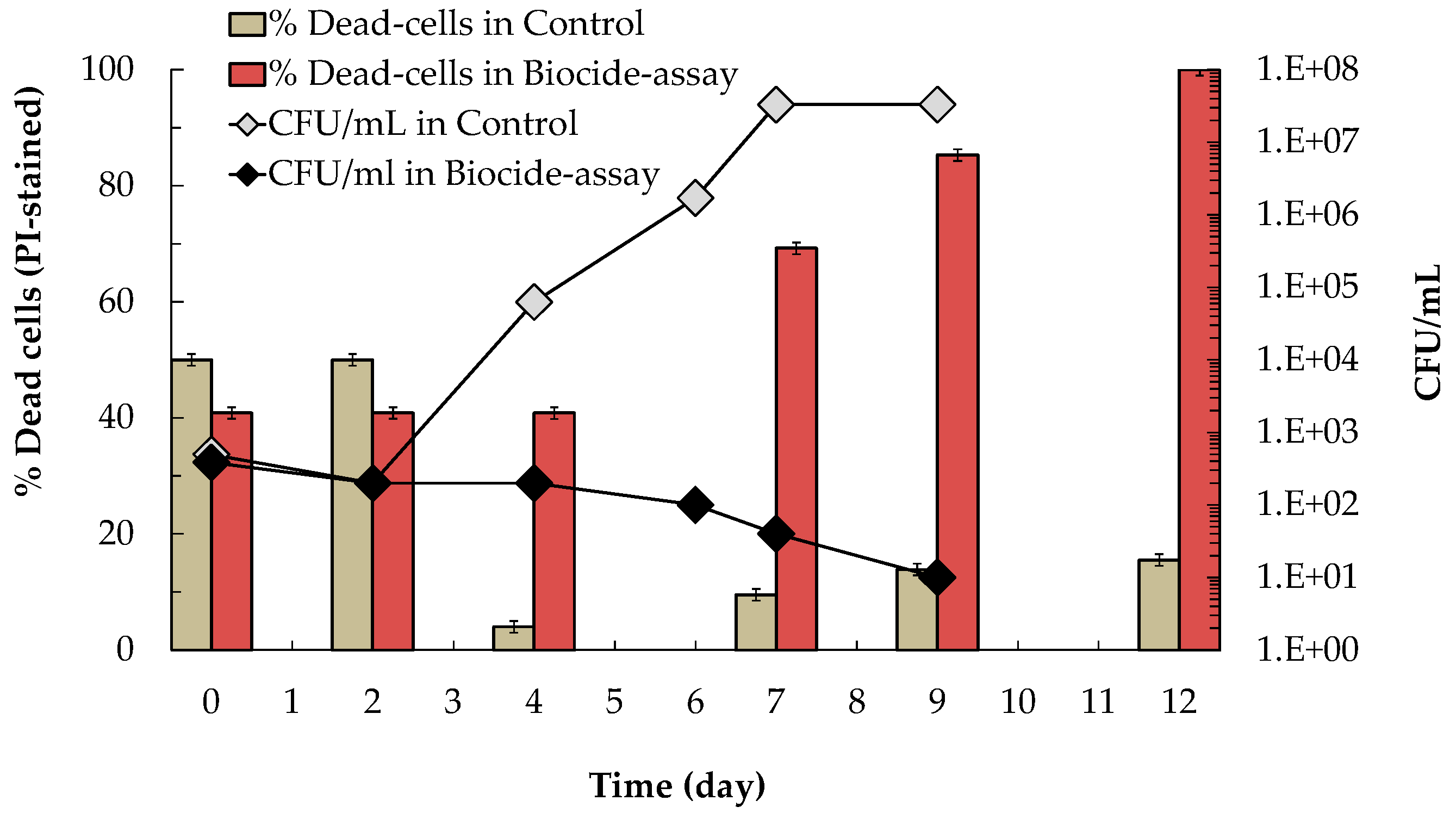
3.2.1. Effect of Saccharomycin on B. bruxellensis Culturability and Viability
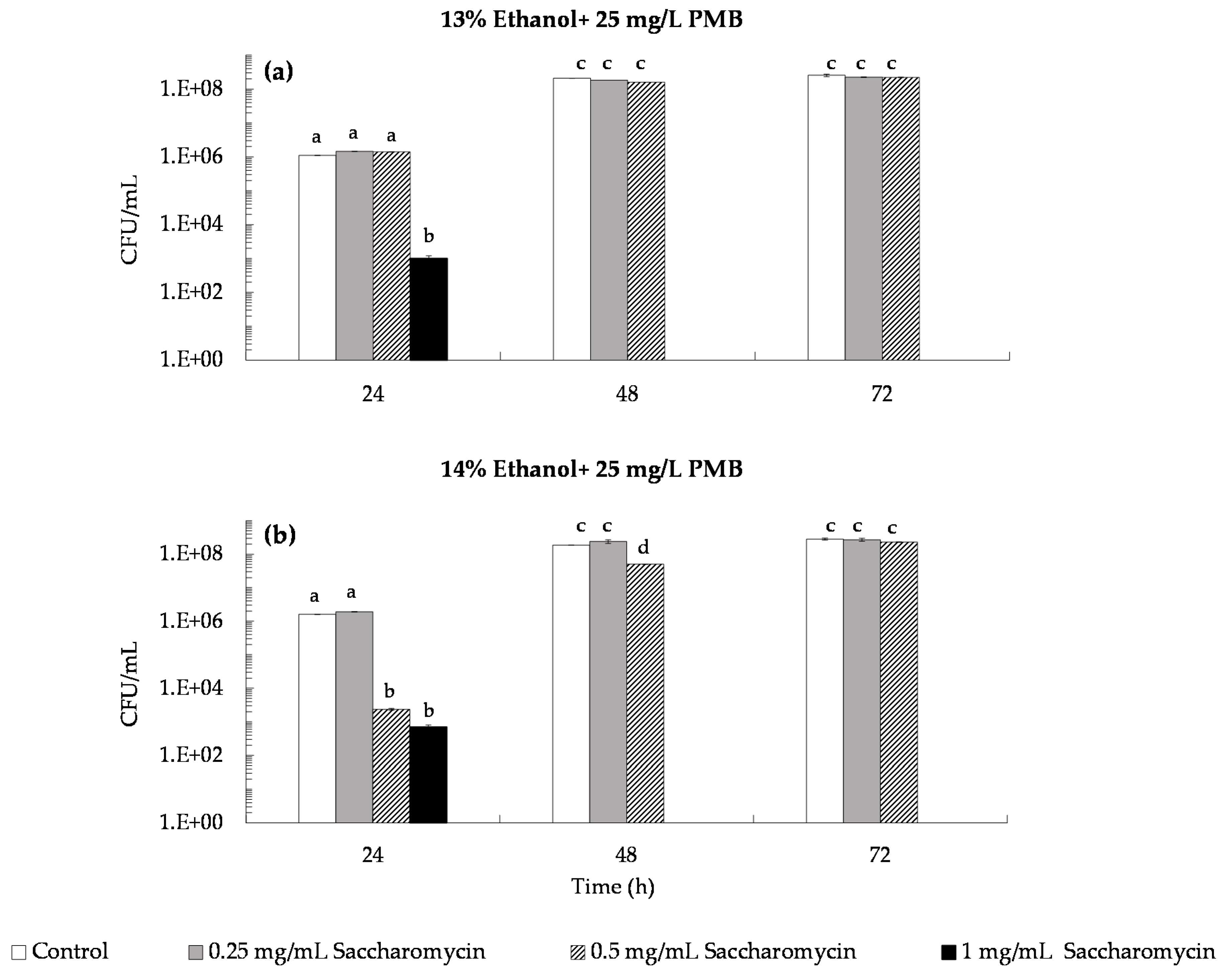
3.2.2. Conjugated Effect of Saccharomycin with Sulfur Dioxide (SO2)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fleet, G.H.; Heard, G.M. Yeast growth during fermentation. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Barnett, J.A.; Lichtenthaler, F.W. A history of research on yeasts 3: Emil Fischer Eduard Buchner and their contemporaries, 1880–1900. Yeast 2001, 18, 363–388. [Google Scholar] [CrossRef]
- Bauer, F.F.; Pretorius, I.S. Yeast stress response and fermentation efficiency: How to survive the making of wine—A review. S. Afr. J. Enol. Vitic. 2000, 21, 27–51. [Google Scholar] [CrossRef]
- Steensels, J.; Daenen, L.; Malcorps, P.; Derdelinckx, G.; Verachtert, H.; Verstrepen, K.J. Brettanomyces yeasts—From spoilage organisms to valuable contributors to industrial fermentations. Int. J. Food Microbiol. 2015, 206, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Albergaria, H.; Arneborg, N. Dominance of Saccharomyces cerevisiae in alcoholic fermentation processes: Role of physiological fitness and microbial interactions. Appl. Microbiol. Biotechnol. 2016, 100, 2035–2046. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Enrique, M.; Marcos, J.F.; Yuste, M.; Martínez, M.; Valles, S.; Manzanares, P. Antimicrobial action of synthetic peptides towards wine spoilage yeasts. Int. J. Food Microbiol. 2007, 118, 318–325. [Google Scholar] [CrossRef]
- Fugelsang, K.C. Yeasts. In Wine Microbiology; Fugelsang, K.C., Ed.; The Chapman and Hall Enology Library: New York, NY, USA, 1997; pp. 159–168. [Google Scholar]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Barata, A.; Caldeira, J.; Botelheiro, R.; Pagliara, D.; Malfeito-Ferreira, M.; Loureiro, V. Survival patterns of Dekkera bruxellensis in wines and inhibitory effect of sulfur dioxide. Int. J. Food Microbiol. 2008, 121, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Caruso, M.; Fiore, C.; Contursi, M.; Salzano, G.; Paparella, A.; Romano, P. Formation of biogenic amines as criteria for the selection of wine yeast. World J. Microbiol. Biotechnol. 2002, 18, 159–163. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. The Chemistry of Wine Stabilization and Treatments. In Handbook of Enology. The Microbiology of Wine and Vinifications; Ribéreau-Gayon, P., Glories, Y., Maujean, A., Dubourdieu, D., Eds.; John Wiley & Sons: New Jersey, NJ, USA, 2006; pp. 266–326. [Google Scholar]
- Romano, P.; Suzzi, G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Carrascón, V.; Vallverdú-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The kinetics of oxygen and SO2 consumption by red wines. What do they tell about oxidation mechanisms and about changes in wine composition? Food Chemist. 2018, 241, 206214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantí, M.; Reguant, C.; Poblet, M.; Zamora, F.; Mas, A.; Guillamón, J.M. Molecular analysis of yeast population dynamics: Effect of sulphur dioxide and inoculum on must fermentation. Int. J. Food Microbiol. 1998, 41, 169–175. [Google Scholar] [CrossRef]
- Albertin, W.; Miot-Sertier, C.; Bely, M.; Marullo, P.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Masneuf-Pomarede, I. Oenological prefermentation practices strongly impact yeast population dynamics and alcoholic fermentation kinetics in Chardonnay grape must. Int. J. Food Microbiol. 2014, 178, 87–97. [Google Scholar] [CrossRef]
- Timbo, B.; Koehler, K.M.; Wolyniak, C.; Klontz, K.C. Sulfites—A food and drug administration review of recalls and reported adverse events. J. Food Prot. 2004, 67, 1806–1811. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.A.; Madan, V. Clinical effects of sulphite additives. Clin. Exp. Allergy. 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- Curtin, C.; Kennedy, E.; Henschke, P.A. Genotype-dependent sulphite tolerance of Australian Dekkera (Brettanomyces) bruxellensis wine isolates. Lett. Appl. Microbiol. 2012, 55, 56–61. [Google Scholar] [CrossRef]
- Avramova, M.; Grbin, P.; Borneman, A.; Albertin, W.; Masneuf-Pomarède, I.; Cristian, V. Competition experiments between Brettanomyces bruxellensis strains reveal specific adaptation to sulfur dioxide and complex interactions at intraspecies level. Fems. Yeast Res. 2019, 19, foz010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelonnier-Magimel, E.; Windholtz, S.; Masneuf-Pomarède, I.; Barbe, J.C. Sensory characterisation of wines without added sulfites via specific and adapted sensory profile. OENO One 2020, 54, 671–685. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter cultures as biocontrol strategy to prevent Brettanomyces bruxellensis proliferation in wine. Appl. Microbiol. Biotechnol. 2018, 102, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef]
- Pinto, L.; Baruzzi, F.; Cocolin, L.; Malfeito-Ferreira, M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020, 99, 88–100. [Google Scholar] [CrossRef]
- Mehlomakulu, N.N.; Prior, K.J.; Setati, M.E.; Divol, B. Candida pyralidae killer toxin disrupts the cell wall of Brettanomyces bruxellensis in red grape juice. J. Appl. Microbiol. 2017, 122, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.L.; Mazzucco, M.B.; Lopes, C.A.; Ganga, M.A.; Sangorrín, M.P. Purification and characterization of Saccharomyces eubayanus killer toxin: Biocontrol effectiveness against wine spoilage yeasts. Int. J. Food Microbiol. 2020, 331, 108714. [Google Scholar] [CrossRef]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010, 86, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Albergaria, H.; Branco, P.; Francisco, D.; Coutinho, R.; Monteiro, M.; Malfeito-Ferreira, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J. Dominance of Saccharomyces cerevisiae in wine fermentations: Secretion of antimicrobial peptides and microbial interactions. In Proceedings of the 2nd International Conference on Microbial Diversity: Microbial Interactions in Complex Ecosystems, Turin, Italy, 23–25 October 2013; Gallego, J.B., Cardinalli, G., Casella, S., Cocolin, L., Neviani, E., Eds.; Società Italiana di Microbiologia Agraria-Alimentare e Ambientale: Firenze, Italy, 2013; pp. 98–101. [Google Scholar]
- Branco, P.; Francisco, D.; Monteiro, M.; Almeida, M.G.; Caldeira, J.; Arneborg, N.; Prista, C.; Albergaria, H. Antimicrobial properties and death-inducing mechanisms of saccharomycin, a biocide secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Nevado, F.; Albergaria, H.; Hogg, T.; Gírio, F. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006, 108, 336–345. [Google Scholar] [CrossRef]
- Branco, P.; Monteiro, M.; Moura, P.; Albergaria, H. Survival rate of wine-related yeasts during alcoholic fermentation assessed by direct live/dead staining combined with fluorescence in situ hybridization. Int. J. Food Microbiol. 2012, 158, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Roeder, C.; Koning, H.; Frohlich, J. Species-specific identification of Dekkera/Brettanomyces yeasts by fluorescently labelled DNA probes targeting the 26S rRNA. FEMS Yeast Res. 2007, 6, 1013–1026. [Google Scholar] [CrossRef] [Green Version]
- Dias, L.; Pereira-da-Silva, S.; Tavares, M.; Malfeito-Ferreira, M.; Loureiro, V. Factors affecting the production of 4-ethylphenol by the yeast Dekkera bruxellensis in enological conditions. Food Microbiol. 2003, 20, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Fry, J.C. One-way analysis of variance. In Biological Data Analysis: A Practical Approach; Fry, J.C., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 1–39. [Google Scholar]
- Sturm, M.E.; Arroyo-López, F.N.; Garrido-Fernández, A.; Querol, A.; Mercado, L.A.; Ramirez, M.L.M.; Combina, M. Probabilistic model for the spoilage wine yeast Dekkera bruxellensis as a function of pH, ethanol and free SO2 using time as a dummy variable. Int. J. Food Microbiol. 2014, 170, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Sabir, F.; Diniz, M.; Carvalho, L.; Albergaria, H.; Prista, C. Biocontrol of Brettanomyces/Dekkera bruxellensis in alcoholic fermentations using saccharomycin-overproducing Saccharomyces cerevisiae strains. Appl. Microbiol. Biotechnol. 2019, 103, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Avramova, M.; Cibrario, A.; Peltier, E.; Coton, M.; Coton, E.; Schacherer, J.; Spano, G.; Capozzi, V.; Blaiotta, G.; Salin, F.; et al. Brettanomyces bruxellensis population survey reveals a diploid-triploid complex structured according to substrate of isolation and geographical distribution. Sci. Rep. 2018, 8, 4136. [Google Scholar] [CrossRef] [PubMed]

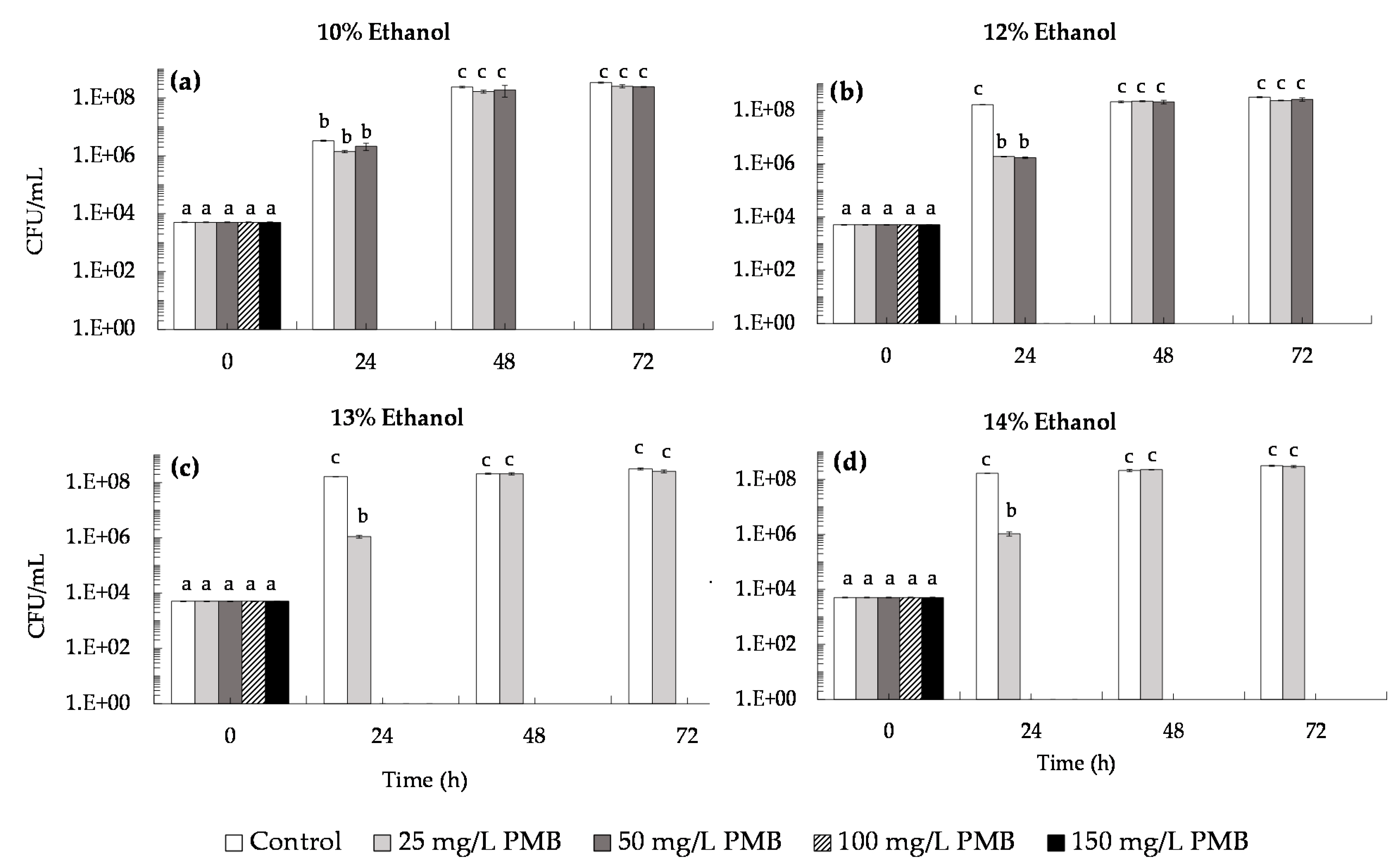

| B. bruxellensis Culturability (CFU/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time (h) | Control | Ethanol (% v/v) | PMB (mg/L) | ||||||
| - | 10 | 12 | 13 | 14 | 25 | 50 | 100 | 150 | |
| 0 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 | a (5.0 ± 0.2) × 103 |
| 24 | b (1.8 ± 0.2) × 108 | c (3.3 ± 0.1) × 106 | c (2.7 ± 0.5) × 106 | d (2.4 ± 0.1) × 106 | e (1.6 ± 0.1) × 106 | f (4.0 ± 0.1) × 105 | f (3.7 ± 0.1) × 105 | g (3.0 ± 0.1) × 104 | h (7.0 ± 1.2) × 103 |
| 48 | b (1.9 ± 0.2) × 108 | i (3.4 ± 0.2) × 108 | i (3.0 ± 0.1) × 108 | i (2.6 ± 0.1) × 108 | b (1.9 ± 0.2) × 108 | j (3.5 ± 0.1) × 107 | j (3.9 ± 0.1) × 107 | k (2.9 ± 0.5) × 107 | l (1.6 ± 0.6) × 107 |
| 72 | i (3.1 ± 0.1) × 108 | i (3.2 ± 0.2) × 108 | i (3.3 ± 0.1) × 108 | i (3.3 ± 0.3) × 108 | i (2.8 ± 0.2) × 108 | i (3.5 ± 0.3) × 108 | i (3.2 ± 0.1) × 108 | i (2.8 ± 0.5) × 108 | i (2.8 ± 0.1) × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, P.; Coutinho, R.; Malfeito-Ferreira, M.; Prista, C.; Albergaria, H. Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide. Microorganisms 2021, 9, 2528. https://doi.org/10.3390/microorganisms9122528
Branco P, Coutinho R, Malfeito-Ferreira M, Prista C, Albergaria H. Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide. Microorganisms. 2021; 9(12):2528. https://doi.org/10.3390/microorganisms9122528
Chicago/Turabian StyleBranco, Patrícia, Rute Coutinho, Manuel Malfeito-Ferreira, Catarina Prista, and Helena Albergaria. 2021. "Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide" Microorganisms 9, no. 12: 2528. https://doi.org/10.3390/microorganisms9122528
APA StyleBranco, P., Coutinho, R., Malfeito-Ferreira, M., Prista, C., & Albergaria, H. (2021). Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide. Microorganisms, 9(12), 2528. https://doi.org/10.3390/microorganisms9122528








