Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections
Abstract
1. Introduction
2. Mechanisms of Immunomodulatory Action
3. Therapeutic Indications in Rheumatology
4. Anti-Viral Aspects of IVIG
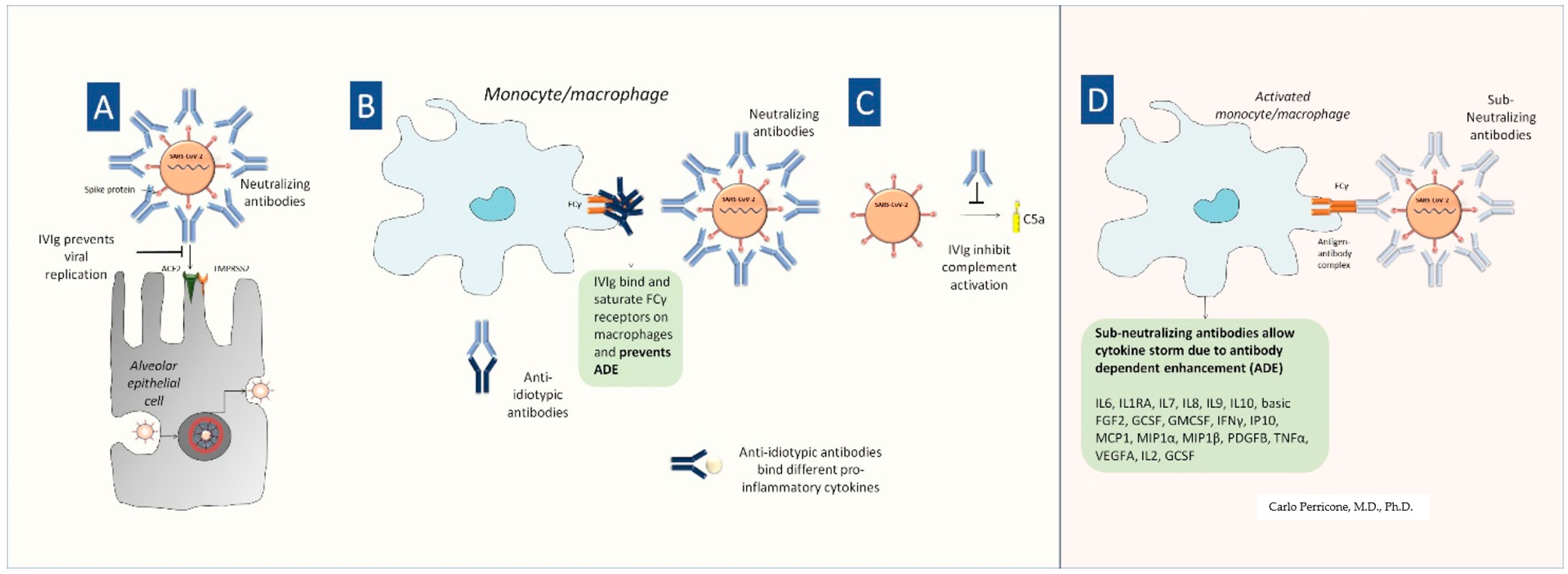
5. IVIG in COVID-19
6. Passive Immunotherapy and COVID-19: IVIG, Convalescent Plasma, Hyperimmune Immunoglobulins, and Monoclonal Antibodies
7. Conclusions
| Viruses | IVIG | Ref. | H-IG | Ref. | mAb | Ref. |
|---|---|---|---|---|---|---|
| Herpesviruses | ||||||
| HSV | X | [48,59] | ||||
| VZV | X | [48,57,58] | X | [4] | ||
| CMV | X | [50,52,53,54,55,56] | X | [4] | ||
| EBV | X | [48] | ||||
| Hepatitis A virus | X | [60,61] | ||||
| RSV | X | [63,64] | X | [4] | ||
| Measles | X | [48,60] | ||||
| Mumps | X | [48] | ||||
| Rubella | X | [48] | ||||
| Parvovirus B19 | X | [48,50,60] | ||||
| Polyomavirus BK | X | [49,50] |
| Passive Immunotherapy | Definition | Evidence in COVID-19 (Ref.) |
|---|---|---|
| IVIG | Purified IgG from a pool of thousands of donors | [42,75,76,77,78,79,80,81] |
| CP | Whole plasma from convalescent donors containing specific antibodies at titer ≥ 1:160 | [97,98,112,113,114,115,116,117,118,119,127,130,131] |
| H-IG | IVIG obtained from a pool of plasma with high titer of specific antibodies | [97,105] |
| mAbs | Fully human, neutralizing mAbs against SARS-CoV-2 spike protein | [42,133] |
| Advantages | Disadvantages |
|---|---|
| History of efficacy | Proven clinical utility (lack of RCTs) |
| High compliance and better disease control (repeated hospital access) | Less flexibility for patients and parents (infusion center/hospital) |
| Useful in critical patients | High costs (products, supply, nurses) |
| High dosage therapy | Larger volume |
| Safety profile (common) | Fatal systemic adverse events (rare) |
| Useful in patients with bleeding disorders | Risk of thrombosis (rare) |
| Useful in pregnancy | Need intravenous access |
| Rapid achievement of plasma levels and response (<72 h) | Transient response (<1 month) |
| Early empiric therapy (before the identification of pathogens) | Antibody-mediated enhancement (ADE) |
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Perez, E.E.; Orange, J.S.; Bonilla, F.; Chinen, J.; Chinn, I.K.; Dorsey, M.; El-Gamal, Y.; Harville, T.O.; Hossny, E.; Mazer, B.; et al. Update on the use of immunoglobulin in human disease: A review of evidence. J. Allergy Clin. Immunol. 2017, 139, S1–S46. [Google Scholar] [CrossRef] [PubMed]
- Schwab, I.; Nimmerjahn, F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat. Rev. Immunol. 2013, 13, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Ballow, M. Primary immunodeficiency disorders: Antibody deficiency. J. Allergy Clin. Immunol. 2002, 109, 581–591. [Google Scholar] [CrossRef]
- Salemi, S.; Markovic, M.; Martini, G.; D’Amelio, R. The expanding role of therapeutic antibodies. Int. Rev. Immunol. 2015, 34, 202–264. [Google Scholar] [CrossRef] [PubMed]
- Bayry, J.; Negi, V.S.; Kaveri, S.V. Intravenous immunoglobulin therapy in rheumatic diseases. Nat. Rev. Rheumatol. 2011, 7, 349–359. [Google Scholar] [CrossRef]
- Seite, J.F.; Shoenfeld, Y.; Youinou, P.; Hillion, S. What is the contents of the magic draft IVIg? Autoimmun. Rev. 2008, 7, 435–439. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Kaveri, S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef]
- Gelfand, E.W. Intravenous Immune Globulin in Autoimmune and Inflammatory Diseases. N. Engl. J. Med. 2012, 367, 2015–2025. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Aschermann, S.; Lux, A.; Baerenwaldt, A.; Biburger, M.; Nimmerjahn, F. The other side of immunoglobulin G: Suppressor of inflammation. Clin. Exp. Immunol. 2010, 160, 161–167. [Google Scholar] [CrossRef]
- Mitrevski, M.; Marrapodi, R.; Camponeschi, A.; Cavaliere, F.M.; Lazzeri, C.; Todi, L.; Visentini, M. Intravenous Immunoglobulin and Immunomodulation of B-Cell—In vitro and in vivo Effects. Front. Immunol. 2015, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.G.; Bjork, L.; Skansén- Saphir, U.; Andersson, J.P. Down-regulation of cytokine production and interleukin-2 receptor expression by pooled human IgG. Immunology 1993, 79, 211–216. [Google Scholar] [PubMed]
- Napoli, R.; Ruvolo, A.; Triggianese, P.; Prevete, N.; Schiattarella, G.G.; Nigro, C.; Miele, C.; Magliulo, F.; Grassi, S.; Pecoraro, A.; et al. Immunoglobulins G modulate endothelial function and affect insulin sensitivity in humans. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef]
- Yu, X.; Vasiljevic, S.; Mitchell, D.A.; Crispin, M.; Scanlan, C.N. Dissecting the molecular mechanisms of IVIg therapy: The interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J. Mol. Biol. 2013, 425, 1253–1258. [Google Scholar] [CrossRef]
- De Groot, A.S. Do Tregitopes have the potential to impact the current treatment landscape of autoimmune diseases? Expert. Rev. Clin. Immunol. 2013, 9, 1155–1157. [Google Scholar] [CrossRef]
- Crow, A.R.; Brinc, D.; Lazarus, A.H. New insight into the mechanism of action of IVIg: The role of dendritic cells. J. Thromb. Haemost. 2009, 7, 245–248. [Google Scholar] [CrossRef]
- Doran, M.F.; Crowson, C.S.; Pond, G.R.; O’Fallon, W.M.; Gabriel, S.E. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002, 46, 2287–2293. [Google Scholar] [CrossRef]
- Maddur, M.S.; Othy, S.; Hegde, P.; Vani, J.; Lacroix-Desmazes, S.; Bayry, J.; Kaveri, S.V. Immunomodulation by intravenous immunoglobulin: Role of regulatory T cells. J. Clin. Immunol. 2010, 30 (Suppl. 1), S4–S8. [Google Scholar] [CrossRef]
- Farcet, M.R.; Planitzer, C.B.; Stein, O.; Modrof, J.; Kreil, T.R. Hepatitis A virus antibodies in immunoglobulin preparations. J. Allergy Clin. Immunol. 2010, 125, 198–202. [Google Scholar] [CrossRef]
- Maddur, M.S.; Vani, J.; Hegde, P.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Inhibition of differentiation, amplification, and function of human T H17 cells by intravenous immunoglobulin. J. Allergy Clin. Immunol. 2011, 127, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Quinti, I.; Soresina, A.; Guerra, A.; Rondelli, R.; Spadaro, G.; Agostini, C.; Milito, C.; Trombetta, A.C.; Visentini, M.; Martini, H.; et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: Results from a multicenter prospective cohort study. J. Clin. Immunol. 2011, 31, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Imbach, P.; Barandun, S.; d’Apuzzo, V.; Baumgartner, C.; Hirt, A.; Morell, A.; Rossi, E.; Schöni, M.; Vest, M.; Wagner, H.P. High dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet 1981, 1, 1228–1232. [Google Scholar] [CrossRef]
- Conigliaro, P.; Triggianese, P.; Ballanti, E.; Perricone, C.; Perricone, R.; Chimenti, M.S. Complement, infection, and autoimmunity. Curr. Opin. Rheumatol. 2019, 31, 532–541. [Google Scholar] [CrossRef]
- Oates-Whitehead, R.M.; Baumer, J.H.; Haines, L.; Love, S.; Maconochie, I.K.; Gupta, A.; Roman, K.; Dua, J.S.; Flynn, I. Intravenous immunoglobulin for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 2003, 2003, CD004000. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, M.S.; Ballanti, E.; Triggianese, P.; Perricone, R. Vasculitides and the Complement System: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2015, 49, 333–346. [Google Scholar] [CrossRef]
- Martinez, V.; Cohen, P.; Pagnoux, C.; Vinzio, S.; Mahr, A.; Mouthon, L.; Sailler, L.; Delaunay, C.; Sadoun, A.; Guillevin, L.; et al. Intravenous immunoglobulins for relapses of systemic vasculitides associated with antineutrophil cytoplasmic autoantibodies: Results of a multicenter, prospective, open-label study of twenty-two patients. Arthritis Rheum. 2008, 58, 308–317. [Google Scholar] [CrossRef]
- Tsurikisawa, N.; Taniguchi, M.; Saito, H.; Himeno, H.; Ishibashi, A.; Suzuki, S.; Akiyama, K. Treatment of Churg-Strauss syndrome with high-dose intravenous immunoglobulin. Ann. Allergy Asthma Immunol. 2004, 92, 80–87. [Google Scholar] [CrossRef]
- Ballanti, E.; Di Muzio, G.; Novelli, L.; Perricone, C.; Perricone, R. Churg-Strauss syndrome with neurologic manifestations: Successful treatment with intravenous immunoglobulins. Isr. Med. Assoc. J. 2012, 14, 583–585. [Google Scholar]
- Zandman-Goddard, G.; Krauthammer, A.; Levy, Y.; Langevitz, P.; Shoenfeld, Y. Long-term therapy with intravenous immunoglobulin is beneficial in patients with autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 247–255. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Blank, M.; Shoenfeld, Y. Intravenous immunoglobulins in systemic lupus erytematosus. Lupus 2009, 18, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Mollnes, T.E.; Høgåsen, K.; De Carolis, C.; Vaquero, E.; Nielsen, E.W.; Fontana, L.; Perricone, R. High-dose intravenous immunoglobulin treatment activates complement in vivo. Scand. J. Immunol. 1998, 48, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Moghadam-Kia, S.; Oddis, C.V.; Aggarwal, R. Modern Therapies for Idiopathic Inflammatory Myopathies (IIMs): Role of Biologics. Clin. Rev. Allergy Immunol. 2017, 52, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Perricone, R.; De Carolis, C.; Kröegler, B.; Greco, E.; Giacomelli, R.; Cipriani, P.; Fontana, L.; Perricone, C. Intravenous immunoglobulin therapy in pregnant patients affected with systemic lupus erythematosus and recurrent spontaneous abortion. Rheumatology 2008, 47, 646–651. [Google Scholar] [CrossRef]
- Cervera Rodríguez-Pintó, I.; Colafrancesco, S.; Conti, F.; Valesini, G.; Rosário, C.; Agmon-Levin, N.; Shoenfeld, Y.; Ferrão, C.; Faria, R.; Vasconcelos, C.; et al. 14th International Congress on Antiphospholipid Antibodies Task Force Report on Catastrophic Antiphospholipid Syndrome. Autoimmun. Rev. 2014, 13, 699–707. [Google Scholar] [CrossRef]
- Valensise, H.; Vaquero, E.; De Carolis, C.; Stipa, E.; Perricone, R.; Arduini, D.; Romanini, C. Normal fetal growth in women with antiphospholipid syndrome treated with high-dose intravenous immunoglobulin (IVIG). Prenat. Diagn. 1995, 15, 509–517. [Google Scholar] [CrossRef]
- Triggianese, P.; Lattavo, G.; Chimenti, M.S.; Conigliaro, P.; Perricone, R.; Perricone, C.; De Carolis, C. Reproductive outcomes 20 years after the intravenous immunoglobulin treatment in women with recurrent pregnancy losses. Am. J. Reprod. Immunol. 2020, 83, e13224. [Google Scholar] [CrossRef]
- Cervera, R. Update on the diagnosis, treatment and prognosis of the catastrophic antiphospholipid syndrome. Curr. Rheumatol. Rep. 2010, 12, 70–76. [Google Scholar] [CrossRef]
- Kivity, S.; Katz, U.; Daniel, N.; Nussinovitch, U.; Papageorgiou, N.; Shoenfeld, Y. Evidence for the use of intravenous immunoglobulins—A review of the literature. Clin. Rev. Allergy Immunol. 2010, 38, 201–269. [Google Scholar] [CrossRef]
- Ephrem, A.; Misra, N.; Hassan, G.; Dasgupta, S.; Delignat, S.; Duong Van Huyen, J.P.; Chamat, S.; Prost, F.; Lacroix-Desmazes, S.; Kavery, S.V.; et al. Immunomodulation of autoimmune and inflammatory diseases with intravenous immunoglobulin. Clin. Exp. Med. 2005, 5, 135–140. [Google Scholar] [CrossRef]
- Galeotti, C.; Kaveri, S.V.; Bayry, J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 2017, 29, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Triggianese, P.; Bartoloni, E.; Cafaro, G.; Bonifacio, A.F.; Bursi, R.; Perricone, R.; Gerli, R. The anti-viral facet of anti-rheumatic drugs: Lessons from COVID-19. J. Autoimmun. 2020, 111, 102468. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, L.; Guillevin, L.; Tellier, Z. Intravenous immunoglobulins in autoimmune- or parvovirus B19-mediated pure red-cell aplasia. Autoimmun. Rev. 2005, 4, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Mouthon, L.; Lortholary, O. Intravenous immunoglobulins in infectious diseases: Where do we stand? Clin. Microbiol. Infect. 2003, 9, 333–338. [Google Scholar] [CrossRef]
- Manaresi, E.; Gallinella, G. Advances in the development of antiviral strategies against parvovirus B19. Viruses 2019, 11, 659. [Google Scholar] [CrossRef]
- Planitzer, C.B.; Farcet, M.R.; Schiff, R.I.; Ochs, H.D.; Kreil, T.R. Neutralization of different echovirus serotypes by individual lots of intravenous immunoglobulin. J. Med. Virol. 2011, 83, 305–310. [Google Scholar] [CrossRef]
- Siegel, J. The product: All intravenous immunoglobulins are not equivalent. Pharmacotherapy 2005, 25, 78S–84S. [Google Scholar] [CrossRef]
- Krause, I.; Wu, R.; Sherer, Y.; Patanik, M.; Peter, J.B.; Shoenfeld, Y. In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations—A potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfus. Med. 2002, 12, 133–139. [Google Scholar] [CrossRef]
- Randhawa, P.; Pastrana, D.V.; Zeng, G.; Huang, Y.; Shapiro, R.; Sood, P.; Puttarajappa, C.; Berger, M.; Hariharan, S.; Buck, C.B. Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK. Am. J. Transplant. 2015, 15, 1014–1020. [Google Scholar] [CrossRef]
- Jordan, S.C.; Toyoda, M.; Kahwaji, J.; Vo, A.A. Clinical aspects of intravenous immunoglobulin use in solid organ transplant recipients. Am. J. Transplant. 2011, 11, 192–202. [Google Scholar] [CrossRef]
- Jordan, S.C.; Toyoda, M.; Vo, A.A. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation 2009, 88, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reed, E.C.; Bowden, R.A.; Dandliker, P.S.; Lilleby, K.E.; Meyers, J.D. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann. Intern. Med. 1988, 109, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, B.I.; Gora-Harper, M.L.; McCraney, S.A.; Gosland, M. Studies evaluating high-dose acyclovir, intravenous immune globulin, and cytomegalovirus hyperemmunoglobulin for prophylaxis against cytomegalovirus in kidney transplant recipients. Ann. Pharmacother. 1996, 30, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Emanuel, D.; Cunningham, I.; Jules-Elysee, K.; Brochstein, J.A.; Kernan, N.A.; Laver, J.; Stover, D.; White, D.A.; Fels, A.; Polsky, B.; et al. Cytomegalovirus pneumonia after bone marrow transplantation successfully treated with the combination of ganciclovir and high-dose intravenous immune globulin. Ann. Intern. Med. 1988, 109, 777–782. [Google Scholar] [CrossRef]
- Carbone, J. The immunology of posttransplant CMV infection: Potential effect of CMV immunoglobulins on distinct components of the immune response to CMV. Transplantation 2016, 100, S11–S18. [Google Scholar] [CrossRef]
- Ljungman, P.; Cordonnier, C.; Einsele, H.; Bender-Götze, C.; Bosi, A.; Dekker, A.; De La Camara, R.; Gmür, J.; Newland, A.C.; Prentice, H.G.; et al. Use of intravenous immune globulin in addition to antiviral therapy in the treatment of CMV gastrointestinal disease in allogeneic bone marrow transplant patients: A report from the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 1998, 21, 473–476. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.C.; Fan, H.C.; Wang, C.C.; Cheng, S.N. Concomitant use of acyclovir and intravenous immunoglobulin rescues an immunocompromised child with disseminated varicella caused multiple organ failure. J. Pediatr. Hematol. Oncol. 2011, 33, e350–e351. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, T.Y.; Lin, Y.J.; Lien, R.I.; Chou, Y.H. Prophylaxis of intravenous immunoglobulin and acyclovir in perinatal varicella. Eur. J. Pediatr. 2001, 160, 91–94. [Google Scholar] [CrossRef]
- Masci, S.; De Simone, C.; Famularo, G.; Gravante, M.; Ciancarelli, M.; Andreassi, M.; Amerio, P.; Santini, G. Intravenous immunoglobulins suppress the recurrences of genital herpes simplex virus: A clinical and immunological study. Immunopharmacol. Immunotoxicol. 1995, 17, 33–47. [Google Scholar] [CrossRef]
- Berger, E.; Melnick, J.L. Progress in Medical Virology; S. Karger: Basle, Switzerland; New York, NY, USA, 1962; Volume 4, pp. 87–118. [Google Scholar]
- DesJardin, J.A.; Snydman, D.R. Antiviral immunotherapy: A review of current status. BioDrugs 1998, 9, 487–507. [Google Scholar] [CrossRef]
- Victor, J.C.; Monto, A.S.; Surdina, T.Y.; Suleimenova, S.Z.; Vaughan, G.; Nainan, O.V.; Favorov, M.O.; Margolis, H.S.; Bell, B.P. Hepatitis A vaccine versus immune globulin for postexposure prophylaxis. N. Engl. J. Med. 2007, 357, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Champlin, R.E.; Englund, J.; Giralt, S.A.; Rolston, K.; Raad, I.; Jacobson, K.; Neumann, J.; Ippoliti, C.; Mallik, S.; et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: Combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. 2000, 25, 751–755. [Google Scholar] [CrossRef] [PubMed]
- DeVincenzo, J.P.; Hirsch, R.L.; Fuentes, R.J.; Top, F.H. Respiratory syncytial virus immune globulin treatment of lower respiratory tract infection in pediatric patients undergoing bone marrow transplantation—A compassionate use experience. Bone Marrow Transplant. 2000, 25, 161–165. [Google Scholar] [CrossRef] [PubMed]
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998, 102, 531–537. [Google Scholar] [CrossRef]
- Feltes, T.F.; Cabalka, A.K.; Meissner, H.C.; Piazza, F.M.; Carlin, D.A.; Top, F.H., Jr.; Connor, E.M.; Sondheimer, H.M.; Cardiac Synagis Study Group. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J. Pediatr. 2003, 143, 532–540. [Google Scholar] [CrossRef]
- Hu, J.; Robinson, J.L. Treatment of respiratory syncytial virus with palivizumab: A systematic review. World J. Pediatr. 2010, 6, 296–300. [Google Scholar] [CrossRef]
- Green, M.; Reyes, J.; Webber, S.; Rowe, D. The role of antiviral and immunoglobulin therapy in the prevention of Epstein-Barr virus infection and post-transplant lymphoproliferative disease following solid organ transplantation. Transpl. Infect. Dis. 2001, 3, 97–103. [Google Scholar] [CrossRef]
- Imashuku, S.J. Treatment of Epstein-Barr virus-related hemophagocytic lymphohistiocytosis (EBV-HLH); update 2010. Pediatr. Hematol. Oncol. 2011, 33, 35–39. [Google Scholar] [CrossRef]
- Restrepo-Jiménez, P.; Rodríguez, Y.; González, P.; Chang, C.; Gershwin, M.E.; Anaya, J.M. The immunotherapy of Guillain-Barré syndrome. Expert Opin. Biol. Ther. 2018, 18, 619–631. [Google Scholar] [CrossRef]
- Tetro, J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020, 22, 72–73. [Google Scholar] [CrossRef]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.N.; To, K.K.W.; Lee, C.K.; Lee, K.L.; Yan, W.W.; Chan, K.; Chan, W.M.; Ngai, C.W.; Law, K.I.; Chow, F.L.; et al. Hyperimmune IV immunoglobulin treatment: A multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 2013, 144, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Díez, J.M.; Romero, C.; Gajardo, R. Currently available intravenous immunoglobulin contains antibodies reacting against severe acute respiratory syndrome coronavirus 2 antigens. Immunotherapy 2020, 12, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Carannante, N.; Fiorentino, G.; Corcione, A.; Di Sarno, R.; Spatarella, M.; Maturo, N.; Fragranza, F.; Di Micco, P. Administration of Immunoglobulins in SARS-CoV-2-Positive Patient Is Associated with Fast Clinical and Radiological Healing: Case Report. Front. Med. 2020, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, N.; Ghaysouri, A.; Shirazi, S.; Shafiee, E.S.A.; Bastani, E.; Kokhazadeh, T.; Tavan, H. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: A case series. Virology 2020, 548, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Cadena, A.D.; Negreira Caamaño, M.; Pérez Serrano, R.; Porras Leal, M.L. Intravenous immunoglobulins: A therapeutic alternative to consider in kidney transplant patients with COVID-19. Nefrologia 2020, S0211-6995, 30065-5. [Google Scholar]
- Lanza, M.; Polistina, G.E.; Imitazione, P.; Annunziata, A.; Di Spirito, V.; Novella, C.; Fiorentino, G. Successful intravenous immunoglobulin treatment in severe COVID-19 pneumonia. IDCases 2020, 21, e00794. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, S.; Dong, H.; Li, Q.; Chen, E.; Zhang, W.; Yang, L.; Fu, S.; Wang, R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020, 81, 318–356. [Google Scholar] [CrossRef]
- Liu, X.; Cao, W.; Li, T. High-Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects. Front. Immunol. 2020, 11, 1660. [Google Scholar] [CrossRef]
- El-Zein, R.S.; Cardinali, S.; Murphy, C.; Keeling, T. COVID-19-associated meningoencephalitis treated with intravenous immunoglobulin. BMJ Case Rep. 2020, 13, 12–14. [Google Scholar] [CrossRef]
- Parsons, T.; Banks, S.; Bae, C.; Gelber, J.; Alahmadi, H.; Tichauer, M. COVID-19-associated acute disseminated encephalomyelitis (ADEM). J. Neurol. 2020, 267, 2799–2802. [Google Scholar] [CrossRef] [PubMed]
- Assini, A.; Benedetti, L.; Di Maio, S.; Schirinzi, E.; Del Sette, M. Correction to: New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: Two Italian cases. Neurol. Sci. 2020, 41, 2307. [Google Scholar] [CrossRef] [PubMed]
- Bigaut, K.; Mallaret, M.; Baloglu, S.; Nemoz, B.; Morand, P.; Baicry, F.; Godon, A.; Voulleminot, P.; Kremer, L.; Chanson, J.B.; et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e785. [Google Scholar] [CrossRef] [PubMed]
- Scheidl, E.; Canseco, D.D.; Hadji-Naumov, A.; Bereznai, B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J. Peripher. Nerv. Syst. 2020, 25, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Nguyen, C.B.; Yeung, Z.; Sanchez, K.; Rosen, D.; Bushan, S. Evans syndrome in a patient with COVID-19. Br. J. Haematol. 2020, 190, e59–e61. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Brown, C.; Rouse, M.; Hoffmann, M.; Ye, Z. Immune Thrombocytopenia Purpura Secondary to COVID-19. Cureus 2020, 12, e9083. [Google Scholar]
- Artru, F.; Alberio, L.; Moradpour, D.; Stalder, G. Acute immune thrombocytopaenic purpura in a patient with COVID-19 and decompensated cirrhosis. BMJ Case Rep. 2020, 13, 1–2. [Google Scholar] [CrossRef]
- Pouletty, M.; Borocco, C.; Ouldali, N.; Caseris, M.; Basmaci, R.; Lachaume, N.; Bensaid, P.; Pichard, S.; Kouider, H.; Morelle, G.; et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 2020, 79, 999–1006. [Google Scholar] [CrossRef]
- Novi, G.; Rossi, T.; Pedemonte, E.; Saitta, L.; Rolla, C.; Roccatagliata, L.; Inglese, M.; Farinini, D. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e797. [Google Scholar] [CrossRef]
- Daneshpazhooh, M.; Soori, T.; Isazade, A.; Noormohammadpour, P. Mucous membrane pemphigoid and COVID-19 treated with high-dose intravenous immunoglobulins: A case report. J. Dermatol. Treat. 2020, 31, 446–447. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Yang, N.; Ma, Y.; Zhou, Q.; Li, W.; Wang, X.; Huang, L.; Luo, X.; Fukuoka, T.; et al. Effectiveness of intravenous immunoglobulin for children with severe COVID-19: A rapid review. Ann. Transl. Med. 2020, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Tseng, S.P.; Yen, C.H.; Yang, J.Y.; Tsao, C.H.; Shen, C.W.; Chen, K.H.; Liu, F.T.; Liu, W.T.; Chen, Y.M.A.; et al. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem. Biophys. Res. Commun. 2014, 451, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-reig, J.; Esteve-valverde, E.; Belizna, C. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, C.; He, P.; Huang, S.; Duan, Y.; Wang, X.; Lin, K.; Zhou, C.; Zhang, X.; Zha, Y. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int. J. Antimicrob. Agents 2020, 56, 105974. [Google Scholar] [CrossRef]
- Halstead, S.B.; Akkina, R. COVID-19 and SARS Coronavirus 2: Antibodies for the Immediate Rescue and Recovery Phase. Front. Immunol. 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Chai, K.L.; Valk, S.J.; Piechotta, V.; Kimber, C.; Monsef, I.; Doree, C.; Wood, E.M.; Lamikanra, A.A.; Roberts, D.J.; McQuilten, Z.; et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2020, 10, CD013600. [Google Scholar]
- Zeng, H.; Wang, D.; Nie, J.; Liang, H.; Gu, J.; Zhao, A.; Xu, L.; Lang, C.; Cui, X.; Guo, X.; et al. The efficacy assessment of convalescent plasma therapy for COVID-19 patients: A multi-center case series. Signal Transduct. Target Ther. 2020, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- AminJafari, A.; Ghasemi, S. The possible of immunotherapy for COVID-19: A systematic review. Int. Immunopharmacol. 2020, 83, 106455. [Google Scholar] [CrossRef] [PubMed]
- Walker Laura, M.; Burton Dennis, R. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018, 18, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020, 130, 1545–1548. [Google Scholar] [CrossRef]
- Abraham, J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020, 20, 401–403. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Morabito, C.J.; Gangadharan, B. Active Therapy with Passive Immunotherapy May Be Effective in the Fight against COVID-19. Clin. Transl. Sci. 2020, 13, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Petrungaro, A.; Quartarone, E.; Sciarrone, P. Anti-SARS-CoV-2 Hyperimmune Plasma Workflow. Transfus. Apher. Sci. 2020, 59, 102850. [Google Scholar]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody response to SARS-CoV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yuan, Q.; Wang, H.; Liu, W.; Liao, X.; Su, Y.; Wang, X.; Yuan, J.; Li, T.; Li, J. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020, 71, 2027–2034. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Ko, J.H.; Muller, M.A.; Seok, H. Serologic responses of 42 MERS-coronavirus- infected patients according to the disease severity. Diagn. Microbiol. Infect. Dis. 2017, 89, 106–111. [Google Scholar] [CrossRef]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Nguyen-Van-Tam, J.S.; Beck, C.R.; et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: A systemic review and exploratory meta-analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef]
- Lai, S.T. Treatment of severe acute respiratory syndrome. Eur. J. Clin. Microbiol. Dis. 2005, 24, 583–591. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xiong, J.; Bao, L.; Shi, Y. Convalescent plasma as a potential therapy for COVID19. Lancet Infect. Dis. 2020, 20, 398–400. [Google Scholar] [CrossRef]
- Bloch, E.M.; Shoham, S.; Casadevall, A.; Sachais, B.S.; Shaz, B.; Winters, J.L.; van Buskirk, C.; Grossman, B.J.; Joyner, M.; Henderson, J.P.; et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Investig. 2020, 130, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Kaveri, S.V.; Sakuntabhai, A.; Gilardin, L.; Bayry, J. Adjunct Immunotherapies for the Management of Severely Ill COVID-19 Patients. Cell Rep. Med. 2020, 1, 100016. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.L.; Yu, Z.J.; Gou, J.J.; Li, G.M.; Ma, S.H.; Zhang, G.F.; Xu, J.H.; Lin, W.B.; Cui, G.L.; Zhang, M.M.; et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J. Infect. Dis. 2020, 222, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Chan, P.K.; Ip, M.; Wong, E.; Ho, J.; Ho, C.; Cockram, C.S.; Hui, D.S. Anti- SARS- CoV IgG response in relation to disease severity of severe acute respiratory syndrome. J. Clin. Virol. 2006, 35, 179–184. [Google Scholar] [CrossRef]
- Li, L.; Yang, R.; Wang, J.; Lv, Q.; Ren, M.; Zhao, L.; Chen, H.; Xu, H.; Xie, S.; Xie, J.; et al. Feasibility of a pilot program for COVID-19 convalescent plasma collection in Wuhan, China. Transfusion 2020, 60, 1773–1777. [Google Scholar] [CrossRef]
- Pau, A.K.; Aberg, J.; Baker, J.; Belperio, P.S.; Coopersmith, C.; Crew, P.; Glidden, D.V.; Grund, B.; Gulick, R.M.; Harrison, C.; et al. Convalescent Plasma for the Treatment of COVID-19: Perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann. Intern. Med. 2020, M20, 6448. [Google Scholar]
- US Food & Drug Administration Development & Approval Process (CBER) Investigational COVID-19 Convalescent Plasma-Emergency INDs. 2020. Available online: https://www.fda.gov/vaccines-blood-biologics/investigational-newdrug-ind-or-device-exemption-ide-process-cber/recommendations-investigationalcovid-19-convalescent-plasma (accessed on 24 March 2020).
- FDA. Recommendations for Investigational COVID-19 Convalescent Plasma. 2020. Available online: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma (accessed on 1 May 2020).
- Lan, L.; Xu, D.; Ye, G.; Xia, C.; Wang, S.; Li, Y.; Xu, H. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 2020, 323, 1502–1503. [Google Scholar] [CrossRef]
- Tiberghien, P.; de Lambalerie, X.; Morel, P.; Gallian, P.; Lacombe, K.; Yazdanpanah, Y. Collecting and evaluating convalescent plasma forCOVID-19 treatment: Why and how. Vox Sang 2020, 115, 488–494. [Google Scholar] [CrossRef]
- Cao, W.C.; Liu, W.; Zhang, P.H.; Zhang, F.; Richardus, J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007, 357, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Fierz, W.; Walz, B. Antibody Dependent Enhancement Due to Original Antigenic Sin and the Development of SARS. Front. Immunol. 2020, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.B. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody dependent Enhancement. J. Clin. Virol. 2020, 127, 104388. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.T.H.; Lin, H.M.; Baine, I.; Wajnberg, A.; Gumprecht, J.P.; Rahman, F.; Rodriguez, D.; Tandon, P.; Bassily-Marcus, A.; Bander, J.; et al. Convalescent plasma treatment of severe COVID-19: A propensity score-matched control study. Nat. Med. 2020, 26, 1708–1713. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wright, R.S.; Fairweather, D.; Senefeld, J.W.; Bruno, K.A.; Klassen, S.A.; Carter, R.E.; Klompas, A.M.; Wiggins, C.C.; Shepherd, J.R.; et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J. Clin. Investig. 2020, 130, 4791–4797. [Google Scholar] [CrossRef]
- Chowdhury, F.R.; Hoque, A.; Chowdhury, F.U.H.; Amin, M.R.; Rahim, A.; Rahman, M.M.; Yasmin, R.; Amin, M.R.; Miah, M.T.; Kalam, M.A.; et al. Convalescent plasma transfusion therapy in severe COVID-19 patients—A safety, efficacy and dose response study: A structured summary of a study protocol of a phase II randomized controlled trial. Trials 2020, 21, 883. [Google Scholar] [CrossRef]
- Simonovich, V.A.; Burgos Pratx, L.D.; Scibona, P.; Beruto, M.V.; Vallone, M.G.; Vázquez, C.; Savoy, N.; Giunta, D.H.; Pérez, L.G.; Sánchez, M.d.L.; et al. A Randomized Trial of Convalescent Plasma in Covid-19 Severe Pneumonia. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Iqbal Yatoo, M.; Kumar Patel, S.; Natesan, S.; Dhama, J.; Malik, Y.S.; Harapan, H.; Singh, R.K.; Dhama, K. Antibody-based immunotherapeutics and use of convalescent plasma to counter COVID-19: Advances and prospects. Expert Opin. Biol. Ther. 2020, 20, 1033–1046. [Google Scholar] [CrossRef]
- Cherin, P.; Marie, I.; Michallet, M.; Pelus, E.; Dantal, J.; Crave, J.C.; Delain, J.C.; Viallard, J.F. Management of adverse events in the treatment of patients with immunoglobulin therapy: A review of evidence. Autoimmun. Rev. 2016, 15, 71–81. [Google Scholar] [CrossRef]
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Verkerke, H.P.; Meier, C.L. Towards characterized convalescent plasma for COVID-19: The dose matters. EClinicalMedicine 2020, 26, 100545. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perricone, C.; Triggianese, P.; Bursi, R.; Cafaro, G.; Bartoloni, E.; Chimenti, M.S.; Gerli, R.; Perricone, R. Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections. Microorganisms 2021, 9, 121. https://doi.org/10.3390/microorganisms9010121
Perricone C, Triggianese P, Bursi R, Cafaro G, Bartoloni E, Chimenti MS, Gerli R, Perricone R. Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections. Microorganisms. 2021; 9(1):121. https://doi.org/10.3390/microorganisms9010121
Chicago/Turabian StylePerricone, Carlo, Paola Triggianese, Roberto Bursi, Giacomo Cafaro, Elena Bartoloni, Maria Sole Chimenti, Roberto Gerli, and Roberto Perricone. 2021. "Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections" Microorganisms 9, no. 1: 121. https://doi.org/10.3390/microorganisms9010121
APA StylePerricone, C., Triggianese, P., Bursi, R., Cafaro, G., Bartoloni, E., Chimenti, M. S., Gerli, R., & Perricone, R. (2021). Intravenous Immunoglobulins at the Crossroad of Autoimmunity and Viral Infections. Microorganisms, 9(1), 121. https://doi.org/10.3390/microorganisms9010121





