Central Asian Rodents as Model Animals for Leishmania major and Leishmania donovani Research
Abstract
1. Introduction
2. Materials and Methods
2.1. Sand Flies, Parasites, and Rodents
2.2. Experimental Infections of Sand Flies
2.3. Infections of Rodents
2.4. Xenodiagnosis
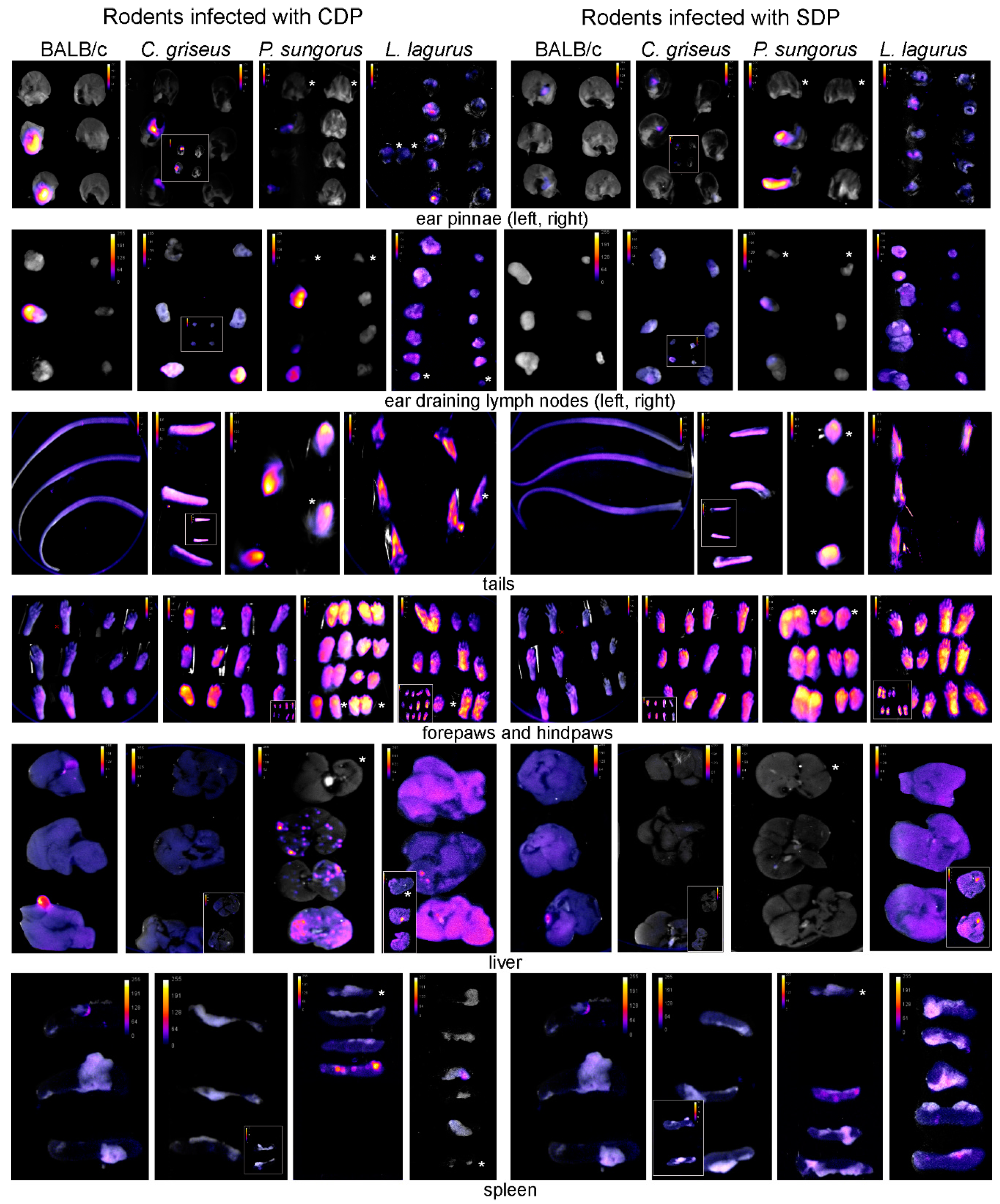
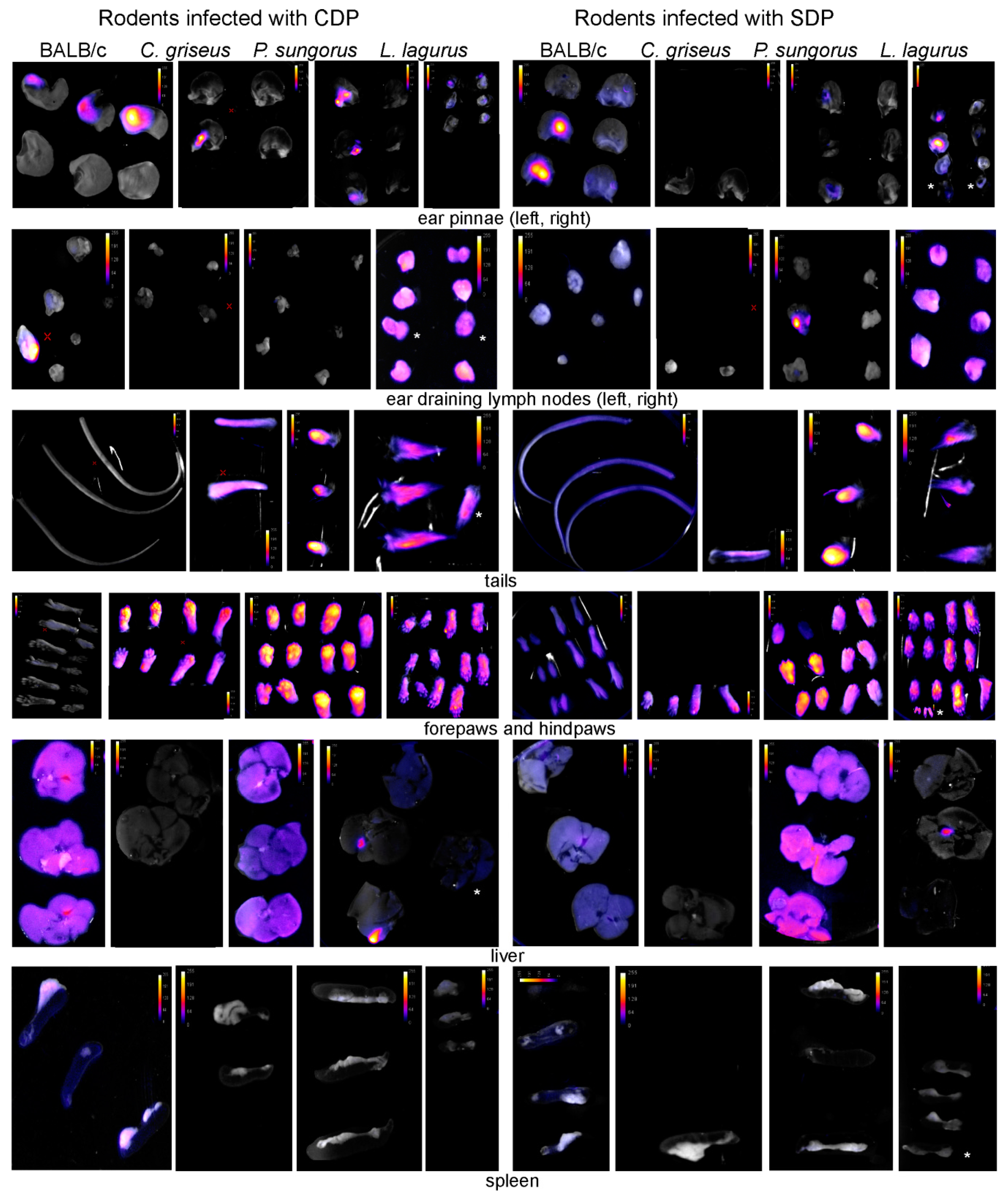
2.5. Tissue Sampling and Fluorescence Detection of Leishmania Post-Mortem
2.6. Flow Cytometry and qPCR
2.7. Statistical Analysis
2.8. Animal Experimentation Guidelines
3. Results
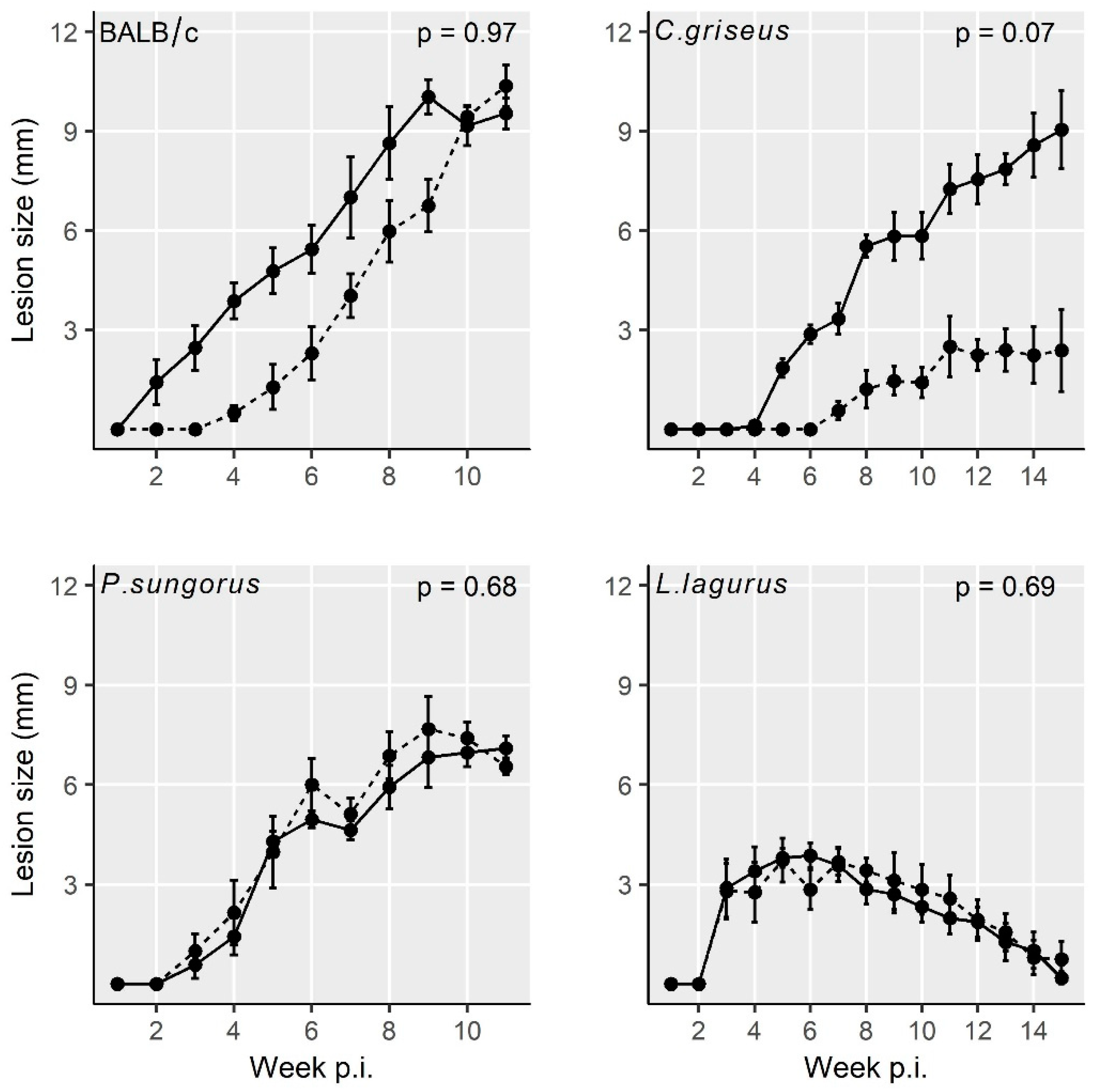
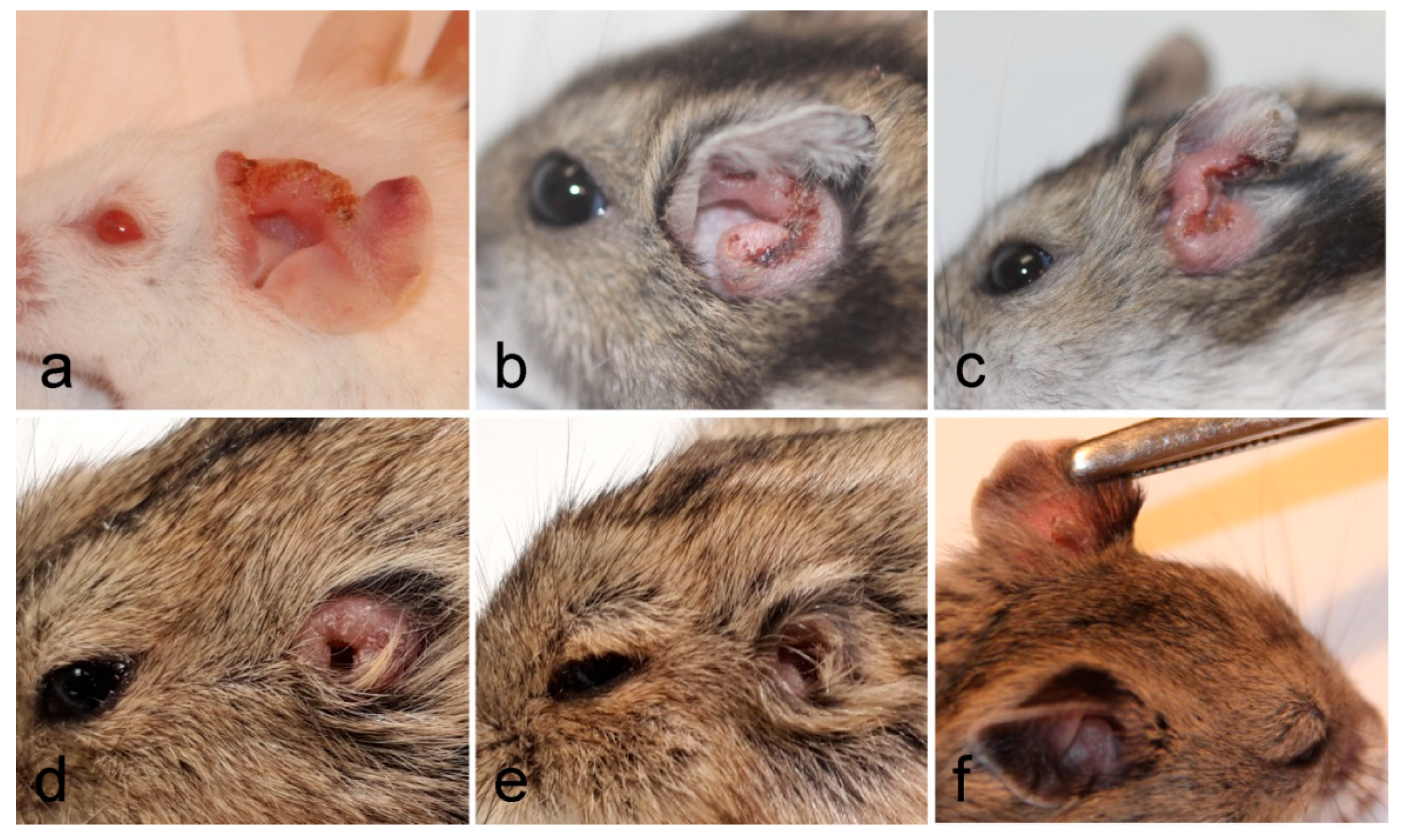
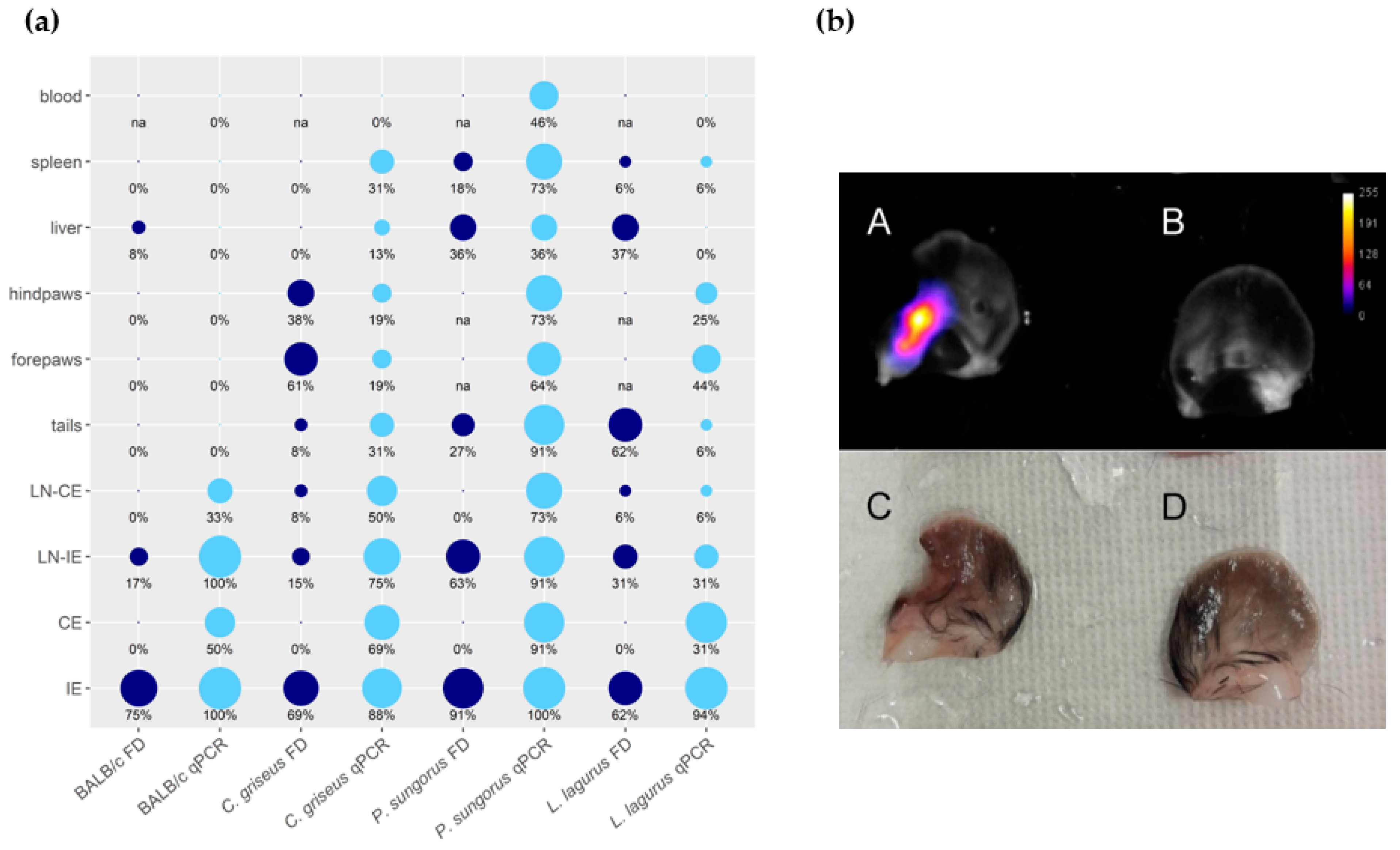
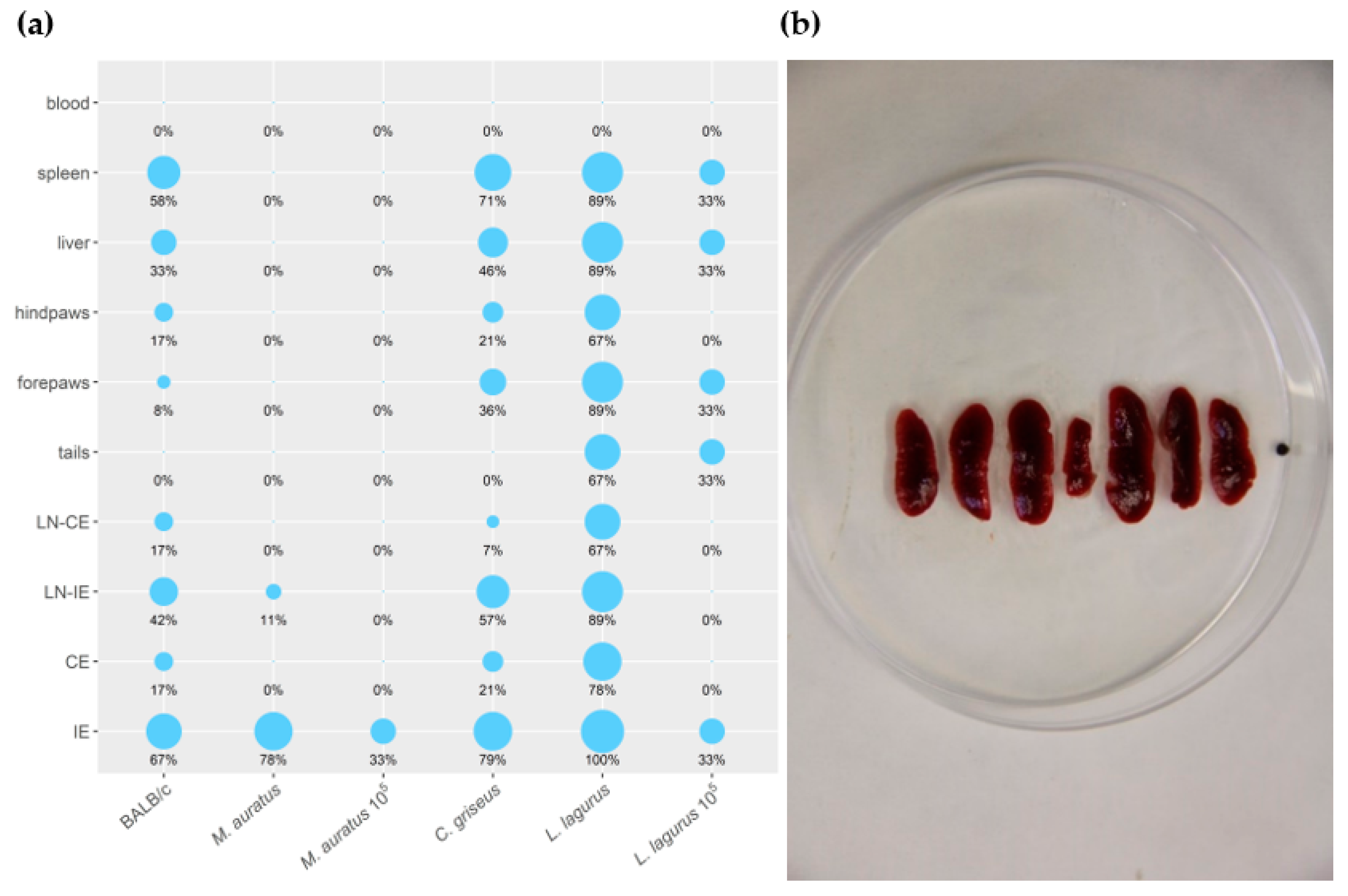
3.1. Development of L. major in Four Rodent Species
3.2. Development of L. donovani in Four Rodent Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.; Maguire, J.H.; Alvar, J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl. Trop. Dis. 2008, 2, e313. [Google Scholar] [CrossRef]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef]
- Akilov, O.E.; Khachemoune, A.; Hasan, T. Clinical manifestations and classification of Old World cutaneous leishmaniasis. Int. J. Dermatol. 2007, 46, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Leishmaniasis: Public health aspects and control. Clin. Dermatol. 1996, 14, 417–423. [Google Scholar] [CrossRef]
- Loría-Cervera, N.E.; Andrade-Narváez, J.F. Animal models for the study of leishmaniasis immunology. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guenet, J.-L.; Bonhomme, F. Wild mice: An ever-increasing contribution to a popular mammalian model. Trends Genet. 2003, 19, 24–31. [Google Scholar] [CrossRef]
- Hommel, M.; Jaffe, C.L.; Travi, B.; Milon, G. Experimental models for leishmaniasis and for testing anti-leishmanial vaccines. Ann. Trop. Med. Parasitol. 1995, 89, 55–73. [Google Scholar] [CrossRef]
- Roque, A.L.R.; Cupolillo, E.; Marchevsky, R.S.; Jansen, A.M. Thrichomys laurentius (Rodentia; Echimyidae) as a putative reservoir of Leishmania infantum and L. braziliensis: Patterns of experimental infection. PLoS Negl. Trop. Dis. 2010, 4, e589. [Google Scholar] [CrossRef]
- Sosa-Bibiano, E.I.; Van Wynsberghe, N.R.; Canto-Lara, S.B.; Andrade-Narvaez, F.J. Preliminary study towards a novel experimental model to study localized cutaneous leishmaniasis caused by Leishmania (Leishmania) mexicana. Rev. Inst. Med. Trop. Sao Paulo 2012, 54, 165–170. [Google Scholar] [CrossRef]
- Smyly, H.J.; Young, C.W. The experimental transmission of leishmaniasis to animals. Proc. Soc. Exp. Biol. Med. 1924, 21, 354–356. [Google Scholar] [CrossRef]
- Young, C.W.; Smyly, H.J.; Brown, C. Experimental kala azar in a hamster, Cricetulus griseus M.Edw. Am. J. Hyg. 1926, 6, 254–275. [Google Scholar]
- Hindle, E.; Patton, W.S. Reports from the Royal Society’s Kala Azar Commission in China. No. 2.—Experiments Bearing on the Susceptibility of the Striped Hamster (Cricetulus griseus) to Leishmania of Chinese Kala Azar. Proc. R. Soc. B Biol. Sci. 1926, 374–379. [Google Scholar]
- Meleney, H.E. The Histopathology of Kala-Azar in the Hamster, Monkey, and Man. Am. J. Pathol. 1925, 1, 147–174. [Google Scholar]
- Lun, Z.R.; Wu, M.-S.; Chen, Y.-F.; Wang, J.-Y.; Zhou, X.-N.; Liao, L.-F.; Chen, J.-P.; Chow, L.M.C.; Chang, K.P. Visceral leishmaniasis in China: An endemic disease under control. Clin. Microbiol. Rev. 2015, 28, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Volf, P.; Volfova, V. Establishment and maintenance of sand fly colonies. J. Vector Ecol. 2011, 36, 1–9. [Google Scholar] [CrossRef]
- Sadlova, J.; Yeo, M.; Seblova, V.; Lewis, M.D.; Mauricio, I.; Volf, P.; Miles, M. Visualisation of Leishmania donovani fluorescent hybrids during early stage development in the sand fly vector. PLoS ONE 2011, 6, e19851. [Google Scholar] [CrossRef]
- Sadlova, J.; Seblova, V.; Votypka, J.; Warburg, A.; Volf, P. Xenodiagnosis of Leishmania donovani in BALB/c mice using Phlebotomus orientalis: A new laboratory model. Parasite. Vector. 2015, 8, 158. [Google Scholar] [CrossRef]
- Alcolea, P.J.; Alonso, A.; Sánchez-Gorostiaga, A.; Moreno-Paz, M.; Gómez, M.; Ramos, I.; Parro, V.; Larraga, V. Genome-wide analysis reveals increased levels of transcripts related with infectivity in peanut lectin non-agglutinated promastigotes of Leishmania infantum. Genomics 2009, 93, 551–564. [Google Scholar] [CrossRef]
- Sadlova, J.; Price, H.P.B.; Smith, A.; Votypka, J.; Volf, P.; Smith, D.F. The stage-regulated HASPB and SHERP proteins are essential for differentiation of the protozoan parasite Leishmania major in its sand fly vector, Phlebotomus papatasi. Cell. Microbiol. 2010, 12, 1765–1779. [Google Scholar] [CrossRef]
- Dostálová, A.; Volf, P. Leishmania development in sand flies: Parasite-vector interactions overview. Parasite. Vector. 2012, 5, 276. [Google Scholar] [CrossRef] [PubMed]
- Seblova, V.; Volfova, V.; Dvorak, V.; Pruzinova, K.; Votypka, J.; Kassahun, A.; Gebre-Michael, T.; Hailu, A.; Warburg, A.; Volf, P. Phlebotomus orientalis sand flies from two geographically distant Ethiopian localities: Biology, genetic analyses and susceptibility to Leishmania donovani. PLoS Negl. Trop. Dis. 2013, 7, e2187. [Google Scholar] [CrossRef] [PubMed]
- Jaouadi, K.; Haouas, N.; Chaara, D.; Gorcii, M.; Chargui, N.; Augot, D.; Pratlong, F.; Dedet, J.-P.; Ettlijani, S.; Mezhoud, H.; et al. First detection of Leishmania killicki (Kinetoplastida, Trypanosomatidae) in Ctenodactylus gundi (Rodentia, Ctenodactylidae), a possible reservoir of human cutaneous leishmaniasis in Tunisia. Parasite Vectors 2011, 4, 159. [Google Scholar] [CrossRef]
- Ghawar, W.; Bettaieba, J.; Salema, S.; Snoussia, M.-A.; Jaouadia, K.; Yazidia, R.; Ben-Salah, A. Natural infection of Ctenodactylus gundi by Leishmania major in Tunisia. Acta Trop. 2018, 177, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Davami, M.H.; Motazedian, M.H.; Kalantari, M.; Asgari, Q.; Mohammadpour, I.; Sotoodeh-Jahromi, A.; Solhjoo, K.; Pourahmad, M. Molecular survey on detection of leishmania infection in rodent reservoirs in Jahrom District, Southern Iran. J. Arthropod. Borne. Dis. 2014, 8, 139–146. [Google Scholar]
- Parhizkari, M.; Motazedian, M.H.; Asqari, Q.; Mehrabani, D. The PCR-based detection of Leishmania major in Mus musculus and other rodents caught in southern Iran: A guide to sample selection. Ann. Trop. Med. Parasitol. 2011, 105, 319–323. [Google Scholar] [CrossRef]
- Mohebali, M.; Kanari, M.N.; Kanani, A.; Edrissian, H.; Anvari, S.; Nadim, A. Cricetulus migratorius (gray hamster), another possible animal reservoir of Kala-Azar in Meshkin-Shahr, Iran. Iran. J. Public Health 1995, 24, 27–30. [Google Scholar]
- Mohebali, M.; Moradi-Asl, E.; Rassi, Y. Geographic distribution and spatial analysis of Leishmania infantum infection in domestic and wild animal reservoir hosts of zoonotic visceral leishmaniasis in Iran: A systematic review. J. Vector Borne Dis 2018, 55, 173–183. [Google Scholar]
- Bradley, R.D. Family Cricetidae. In Handbook of the Mammals of the World. Rodents II; Wilson, D.E., Lacher, T.E., Mittermeier, R.A., Eds.; Lynx Edicions: Barcelona, Spain, 2017; Volume 7, pp. 181–313. [Google Scholar]
- Ashford, R.W. Leishmaniasis reservoirs and their significance in control. Clin. Dermatol. 1996, 14, 523–532. [Google Scholar] [CrossRef]
- McCall, L.I.; Zhang, W.W.; Matlashewski, G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathog. 2013, 9, e1003053. [Google Scholar] [CrossRef]
- Loeuillet, C.; Bañuls, A.; Hide, M. Study of Leishmania pathogenesis in mice: Experimental considerations. Parasit. Vector. 2016, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Aslan, H.; Dey, R.; Meneses, C.; Castrovinci, P.; Bezerra Jeronimo, S.M.; Oliva, G.; Fischer, L.; Duncan, R.C.; Nakhasi, H.L.; Valenzuela, J.G.; et al. A new model of progressive visceral leishmaniasis in hamsters by natural transmission via bites of vector sand flies. JID 2013, 207, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.E.; Ilg, T.; Nikolaev, A.V.; Ferguson, M.A.J.; Bates, P.A. Transmission of cutaneous leishmaniasis by sand flies is enhanced by regurgitation of fPPG. Nature 2004, 430, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R.; Dieckmann, B.S.; Childs, G.E. Leishmania braziliensis and Leishmania mexicana: Experimental cutaneous infections in golden hamsters. Exp. Parasitol. 1979, 47, 270–283. [Google Scholar] [CrossRef]
- Martín-Martín, I.; Jiménez, M.; González, E.; Eguiluz, C.; Molina, R. Natural transmission of Leishmania infantum through experimentally infected Phlebotomus perniciosus highlights the virulence of Leishmania parasites circulating in the human visceral leishmaniasis outbreak in Madrid, Spain. Vet. Res. 2015, 46, 138. [Google Scholar] [CrossRef] [PubMed]
- Lestinova, T.; Rohousova, I.; Sima, M.; de Oliveira, C.I.; Volf, P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017, 11, 1–26. [Google Scholar] [CrossRef]
- Silverman, J.M.; Clos, J.; Horakova, E.; Wang, A.Y.; Wiesgigl, M.; Kelly, I.; Lynn, M.A.; McMaster, W.R.; Foster, L.J.; Levings, M.K.; et al. Leishmania exosomes modulate innate and adaptive immune responses through effects on monocytes and dendritic cells. J. Immunol. 2010, 185, 5011–5022. [Google Scholar] [CrossRef]
- Kimblin, N.; Peters, N.; Debrabant, A.; Secundino, N.; Egen, J.; Lawyer, P.; Fay, M.P.; Kamhawi, S.; Sacks, D. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc. Natl. Acad. Sci. USA 2008, 105, 10125–10130. [Google Scholar] [CrossRef]
- Maia, C.; Seblova, V.; Sadlova, J.; Votypka, J.; Volf, P. Experimental transmission of Leishmania infantum by two major vectors: A comparison between a viscerotropic and a dermotropic strain. PLoS Negl. Trop. Dis. 2011, 5, e1181. [Google Scholar] [CrossRef]
- Secundino, N.F.C.; De Freitas, V.C.; Monteiro, C.C.; Pires, A.-C.A.M.; David, B.A.; Pimenta, P.F.P. The transmission of Leishmania infantum chagasi by the bite of the Lutzomyia longipalpis to two different vertebrates. Parasite. Vector. 2012, 5, 20. [Google Scholar] [CrossRef]
- Doehl, J.S.P.; Sadlova, J.; Aslan, H.; Pruzinova, K.; Metangmo, S.; Votypka, J.; Kamhawi, S.; Volf, P.; Smith, D.F. Leishmania HASP and SHERP genes are required for in vivo differentiation, parasite transmission and virulence attenuation in the host. PLoS Pathog. 2017, 13, e1006130. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Kamhawi, S.; Modi, G.; Valenzuela, J.; Noben-Trauth, N.; Rowton, E.; Ribeiro, J.; Sack, D.L. Development of a natural model of cutaneous leishmaniasis: Powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp.Med. 1998, 188, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Mendez, S.; Lira, R.; Kadambi, N.; Milon, G.; Sacks, D. A natural model of Leishmania major infection reveals a prolonged phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 2000, 165, 969–977. [Google Scholar] [CrossRef]
- Ong, H.B.; Clare, S.; Roberts, A.J.; Wilson, M.E.; Wright, G.J. Establishment, optimisation and quantitation of a bioluminescent murine infection model of visceral leishmaniasis for systematic vaccine screening. Sci Rep. 2020, 10, 4689. [Google Scholar] [CrossRef] [PubMed]
- Carrión, J.; Nieto, A.; Iborra, S.; Iniesta, V.; Soto, M.; Folgueira, C.; Abanades, D.R.; Requena, J.M.; Alonso, C. Immunohistological features of visceral leishmaniasis in BALB/c mice. Parasite Immunol. 2006, 28, 173–183. [Google Scholar] [CrossRef]
- Conter, C.C.; Camila Alves Mota, C.A.; dos Santos, B.A.; de Souza Braga, L.; de Souza Terrona, M.; Navasconia, T.R.; Bekner Silva Fernandes, A.C.; Galhardo Demarchi, I.; Reinhold de Castroa, K.R.; Alessi Aristides, S.M.; et al. Experimental Parasitology PCR primers designed for new world Leishmania: A systematic review. Exp. Parasitol. 2019, 207, 107773. [Google Scholar] [CrossRef]
- Conter, C.C.; Neitzke-Abreu, H.C.; Bocchi Pedroso, R.; Campana Lonardoni, K.V.; Verzignassi Silveira, T.G.; Alessi Aristides, S.M. Detection of Leishmania (Viannia) DNA in leucocytes from the blood of patients with cutaneous leishmaniasis. Rev. Soc. Bras. Med. Trop. 2015, 48, 626–628. [Google Scholar] [CrossRef]
- Fagundes, A.; Schubach, A.; de Paula, C.C.; Bogio, A.; de Fátima Antonio, L.; Botelho Schiavoni, P.; de Souza Monteiro, V.; de Fátima Madeira, M.; Pereira Quintella, L.; Valete-Rosalino, C.M.; et al. Evaluation of polymerase chain reaction in the routine diagnosis for tegumentary leishmaniasis in a referral centre. Mem. Inst. Oswaldo Cruz 2010, 105, 109–112. [Google Scholar] [CrossRef][Green Version]
- Sadlova, J.; Vojtkova, B.; Hrncirova, K.; Lestinova, T.; Spitzova, T.; Becvar, T.; Votypka, J.; Bates, P.; Volf, P. Host competence of African rodents Arvicanthis neumanni, A. niloticus and Mastomys natalensis for Leishmania major. Int. J. Parasitol. Parasites Wildl. 2019, 8, 118–126. [Google Scholar] [CrossRef]
- Neumann, N.F.; Gyurek, L.L.; Gammie, L.; Finch, G.R.; Belosevic, M. Comparison of animal infectivity and nucleic acid staining for assessment of Cryptosporidium parvum viability in water. Appl. Environ. Microbiol. 2000, 66, 406–412. [Google Scholar] [CrossRef]
- Staalsoe, T.; Giha, H.A.; Dodoo, D.; Theander, T.G.; Hviid, L. Detection of antibodies to variant antigens on Plasmodium falciparum -infected erythrocytes by flow cytometry. Cytology 1999, 336, 329–336. [Google Scholar]
- Di Giorgio, C.D.I.; Ridoux, O.; Delmas, F.; Azas, N.; Gasquet, M.; Diseases, T. Flow cytometric detection of Leishmania parasites in human monocyte-derived macrophages: Application to antileishmanial-drug testing. Antimicrob. Agents Chemother. 2000, 44, 3074–3078. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.L.; Giorgio, S.; Merjan, A.J.C.; Figueiredo, E.N. Glycosphingolipid antigens of Leishmania (Leishmania) amazonensis amastigotes identified by use of a monoclonal antibody. Infect. Immun. 1993, 61, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Schmid, I.; Uittenbogaart, C.; Jamieson, B.D. Live-cell assay for detection of apoptosis by dual-laser flow cytometry using Hoechst 33342 and 7-amino-actinomycin D. Nat. Protoc. 2007, 2, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Thalhofer, C.J.; Graff, J.W.; Love-Homan, L.; Hickerson, S.M.; Noah Craft, N.; Beverley, S.M.; Wilson, M.E. In vivo imaging of transgenic Leishmania parasites in a live host. J. Vis. Exp. 2010, 41, 3–9. [Google Scholar]
- Melo, G.D.; Goyard, S.; Lecoeur, H.; Rouault, E.; Pescher, P.; Fiette, L.; Boissonnas, A.; Minoprio, P.; Lang, T. New insights into experimental visceral leishmaniasis: Real-time in vivo imaging of Leishmania donovani virulence. PLoS Negl. Trop. Dis. 2017, 11, 1–18. [Google Scholar] [CrossRef]
- Melby, P.C.; Chandrasekar, B.; Zhao, W.; Coe, J.E. The hamster as a model of human visceral leishmaniasis: Progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J. Immunol. 2001, 166, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Domínguez-Bernal, G.; Orden, J.A.; La Fuente, R.D.; Madrid-Elena, N.; Carrión, J. Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus syrian hamster model. Vet. Res. 2011, 42, 39. [Google Scholar] [CrossRef]
- Wilson, M.E.; Innes, D.J.; de Sousa, A.; Pearson, R.D. Early histopathology of experimental infection with Leishmania donovani in hamsters. J. Parasitol 1987, 73, 55–63. [Google Scholar] [CrossRef]
- das Dores Moreira, N.; Vitoriano-Souya, J.; Rooatt, B.M.; de Abreu Vieira, P.M.; Coura-Vital, W.; de Oliveira Cardoso, J.M.; Rezende, M.T.; Ker, H.G.; Giunchetti, R.C.; Carneiro, C.M.; et al. Clinical, hematological and biochemical alterations in hamster (Mesocricetus auratus) experimentally infected with Leishmania infantum through different routes of inoculation. Parasit. Vector. 2016, 9, 181. [Google Scholar] [CrossRef]
- Gomes, R.; Teixeira, C.; Teixeira, M.J.; Oliveira, F.; Menezes, M.J.; Silva, C.; de Oliveira, C.I.; Miranda, J.C.; Elnaiem, D.E.; Kamhawi, S.; et al. Immunity to a salivary protein of a sand fly vector protects against the fatal outcome of visceral leishmaniasis in a hamster model. Proc. Natl. Acad. Sci. USA 2008, 105, 7845–7850. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Noben-Trauth, N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2002, 2, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.W.; Masur, H.; Keithly, J.S. Cell-mediated immune response in experimental visceral leishmaniasis. I. Correlation between resistance to Leishmania donovani and lymphokine-generating capacity. J. Immunol. 1982, 129, 344–350. [Google Scholar] [PubMed]
- Rocha, F.J.S.; Schleicher, U.; Mattner, J.; Alber, G.; Bogdan, C. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect. Immun. 2007, 75, 3823–3832. [Google Scholar] [CrossRef]
- De Moura, T.R.; Novais, F.O.; Oliveira, F.; Clarencio, J.; Noronha, A.; Barral, A.; Brodskyn, C.; de Oliveira, C.I. Toward a novel experimental model of infection to study american cutaneous leishmaniasis caused by Leishmania braziliensis. Infect. Immun. 2005, 73, 5827–5834. [Google Scholar] [CrossRef]
- Wilson, M.E.; Jeronimo, S.M.B.; Pearson, R.D. Immunopathogenesis of infection with the visceralizing Leishmania species. Microb. Pathog. 2005, 38, 147–160. [Google Scholar] [CrossRef]
- The Working Group on Research Priorities for Development of Leishmaniasis Vaccines; Nery Costa, C.H.; Peters, N.C.; Maruyama, S.R.; de Brito, E.C., Jr.; Santos, I.K. Vaccines for the leishmaniases: Proposal for a research agenda. PLoS Negl. Trop. Dis. 2011, 5, e943. [Google Scholar] [CrossRef]
| Rodent Species | No. of Samples | Median (in Thousands) | Minimum (in Thousands) | Maximum (in Thousands) | p Values 1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FC | PCR | FC | PCR | FC | PCR | FC | PCR | FC | PCR | |
| BALB/c mice | 12 | 12 | 430 | 438 | 2 | 0.3 | 2756 | 14,270 | - | - |
| P. sungorus | 5 | 11 | 732 | 293 | 28 | 46 | 820 | 2240 | 0.93 | 0.19 |
| C. griseus | 13 | 16 | 744 | 152 | 6 | 0 | 1947 | 2240 | 0.89 | 0.03 |
| L. lagurus | 16 | 16 | 7 | 3 | 0.04 | 0.1 | 6982 | 974 | 0.09 | 0.0003 |
| Rodent Species | Rodent Numbe 1 | No. of Sand Fly Females | No. and (%) of Positive Females | Rodent Species | Rodent Number | No. of Sand Fly Females | No. and (%) of Positive Females |
|---|---|---|---|---|---|---|---|
| BALB/c mice p = 0.58 | C1 | 30 | 0 | P. sungorus p = 0.80 | C1 | 33 | 5 (15) |
| C2 | 32 | 7 (21) | C2 | 25 | 5 (20) | ||
| C3 | 24 | 3 (12) | C3 | 22 | 6 (27) | ||
| C4 | 30 | 4 (13) | C4 | 24 | 11 (46) | ||
| C5 | 29 | 8 (27) | C5 | 22 | 8 (36) | ||
| C6 | 30 | 5 (17) | ∑ | 126 | 35 (28) | ||
| ∑ | 175 | 27 (15) | S1 | 32 | 6 (19) | ||
| S1 | 32 | 6 (18) | S2 | 25 | 9 (36) | ||
| S2 | 30 | 5 (17) | S3 | 33 | 9 (27) | ||
| S3 | 31 | 8 (26) | S4 | 22 | 10 (45) | ||
| S4 | 34 | 0 | S5 | 23 | 6 (26) | ||
| S5 | 39 | 5 (13) | ∑ | 135 | 40 (30) | ||
| S6 | 33 | 0 | Total | 261 | 75 (29) | ||
| ∑ | 199 | 24 (12) | L. lagurus p = 0.36 | C1 | 4 | 0 | |
| Total | 374 | 51 (14) | C2 | 2 | 0 | ||
| C. griseus p = 0.84 | C1 | 23 | 6 (26) | C3 | 2 | 0 | |
| C2 | 24 | 3 (13) | C4 | 20 | 2 (10) | ||
| C3 | 27 | 2 (7) | C5 | 25 | 0 | ||
| C4 | 23 | 1 (4) | C6 | 23 | 1 (4) | ||
| C5 | 23 | 0 | C7 | 24 | 2 (8) | ||
| C6 | 24 | 5 (21) | ∑ | 100 | 5 (5) | ||
| C7 | 20 | 0 | S1 | 3 | 0 | ||
| ∑ | 164 | 17 (10) | S2 | 2 | 2 (100) | ||
| S1 | 28 | 0 | S3 | 2 | 0 | ||
| S2 | 15 | 5 (33) | S4 | 22 | 2 (9) | ||
| S3 | 26 | 0 | S5 | 18 | 1 (6) | ||
| S4 | 23 | 8 (35) | S6 | 13 | 3 (23) | ||
| S5 | 15 | 2 (13) | S7 | 26 | 3 (12) | ||
| S6 | 19 | 0 | S8 | 22 | 0 | ||
| ∑ | 126 | 15 (12) | ∑ | 108 | 11 (10) | ||
| Total | 290 | 32 (11) | Total | 208 | 16 (8) |
| Rodent Species | Week p.i. | No. of Animals Exposed | No. of Sand Fly Females | No. and % of Positive Females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 105 CDP | 107 CDP | SDP | 105 CDP | 107 CDP | SDP | 105 CDP | 107 CDP | SDP | ||
| BALB/c mice | 10 | - | 4 | 4 | - | 21 | 32 | - | 0 | 0 |
| 15 | - | 7 | 7 | - | 73 | 79 | - | 0 | 0 | |
| 20 | - | 4 | 4 | - | 55 | 76 | - | 0 | 0 | |
| 25 | - | 3 | 4 | - | 45 | 49 | - | 0 | 0 | |
| 30 | - | 2 | 4 | - | 25 | 59 | - | 0 | 0 | |
| ∑ | 219 | 295 | 0 | 0 | ||||||
| C. griseus | 10 | - | 4 | 4 | - | 11 | 7 | - | 0 | 0 |
| 15 | - | 7 | 7 | - | 22 | 33 | - | 0 | 0 | |
| 20 | - | 4 | 4 | - | 11 | 28 | - | 0 | 0 | |
| 25 | - | 4 | 4 | - | 24 | 23 | - | 1 (4.2 | 1 (4.4) | |
| 30 | - | 4 | 4 | - | 25 | 22 | - | 2 (8.0) | 1 (4.6) | |
| ∑ | 93 | 113 | 3 (3.2) | 2 (1.8) | ||||||
| M. auratus | 15 | 3 | 3 | 3 | 3 | 7 | 25 | 0 | 0 | 0 |
| 20 | 3 | 3 | 3 | 26 | 20 | NA 1 | 0 | 0 | NA | |
| 25 | 3 | 3 | 3 | 6 | 4 | 29 | 0 | 0 | 0 | |
| 30 | 3 | 3 | 6 | 13 | 14 | 60 | 0 | 0 | 0 | |
| ∑ | 48 | 45 | 114 | 0 | 0 | 0 | ||||
| L. lagurus | 15 | 3 | 3 | 3 | 9 | 17 | 4 | 0 | 0 | 0 |
| 20 | 3 | 3 | 3 | 14 | 3 | NA | 0 | 0 | NA | |
| 25 | 3 | 3 | 3 | 14 | 12 | 16 | 0 | 3 (25.0) | 4 (25.0) | |
| 30 | 3 | 3 | 6 | 14 | 14 | 27 | 0 | 8 (57.1) | 5 (18.5) | |
| ∑ | 51 | 46 | 47 | 0 | 11 (23.9) | 9 (19.1) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojtkova, B.; Spitzova, T.; Votypka, J.; Lestinova, T.; Kominkova, I.; Hajkova, M.; Santos-Mateus, D.; Miles, M.A.; Volf, P.; Sadlova, J. Central Asian Rodents as Model Animals for Leishmania major and Leishmania donovani Research. Microorganisms 2020, 8, 1440. https://doi.org/10.3390/microorganisms8091440
Vojtkova B, Spitzova T, Votypka J, Lestinova T, Kominkova I, Hajkova M, Santos-Mateus D, Miles MA, Volf P, Sadlova J. Central Asian Rodents as Model Animals for Leishmania major and Leishmania donovani Research. Microorganisms. 2020; 8(9):1440. https://doi.org/10.3390/microorganisms8091440
Chicago/Turabian StyleVojtkova, Barbora, Tatiana Spitzova, Jan Votypka, Tereza Lestinova, Iveta Kominkova, Michaela Hajkova, David Santos-Mateus, Michael A. Miles, Petr Volf, and Jovana Sadlova. 2020. "Central Asian Rodents as Model Animals for Leishmania major and Leishmania donovani Research" Microorganisms 8, no. 9: 1440. https://doi.org/10.3390/microorganisms8091440
APA StyleVojtkova, B., Spitzova, T., Votypka, J., Lestinova, T., Kominkova, I., Hajkova, M., Santos-Mateus, D., Miles, M. A., Volf, P., & Sadlova, J. (2020). Central Asian Rodents as Model Animals for Leishmania major and Leishmania donovani Research. Microorganisms, 8(9), 1440. https://doi.org/10.3390/microorganisms8091440






