Abstract
Thermokarst lakes are one of the most abundant types of microbial ecosystems in the circumpolar North. These shallow basins are formed by the thawing and collapse of ice-rich permafrost, with subsequent filling by snow and ice melt. Until now, permafrost thaw lakes have received little attention for isolation of microorganisms by culture-based analysis. The discovery of novel psychrophiles and their biomolecules makes these extreme environments suitable sources for the isolation of new strains, including for potential biotechnological applications. In this study, samples of bottom sediments were collected from three permafrost thaw lakes in subarctic Québec, Canada. Their diverse microbial communities were characterized by 16S rRNA gene amplicon analysis, and subsamples were cultured for the isolation of bacterial strains. Phenotypic and genetic characterization of the isolates revealed affinities to the genera Pseudomonas, Paenibacillus, Acinetobacter, Staphylococcus and Sphingomonas. The isolates were then evaluated for their production of extracellular enzymes and exopolymers. Enzymes of potential biotechnological interest included α and β-glucosidase, α and β-maltosidase, β-xylosidase and cellobiohydrolase. One isolate, Pseudomonas extremaustralis strain 2ASCA, also showed the capability to produce, in the loosely bound cell fraction, a levan-type polysaccharide with a yield of 613 mg/L of culture, suggesting its suitability as a candidate for eco-sustainable alternatives to commercial polymers.
1. Introduction
High latitude environments are characterized by persistent cold temperatures, freeze-thaw cycles and extreme seasonality, and these conditions impose severe constraints on biological processes. However, the Polar Regions provide diverse habitats for microbial colonization and growth, including ice-covered seas, perennial snowbanks, glaciers and ice shelves, tundra, polar desert soils, volcanoes and a variety of ice-influenced lakes, rivers and wetlands [1,2]. These extreme environments are increasingly looked to as a source of novel microbes and biomolecules that have potential biotechnological and biomedical applications [3,4]. For example, cold-active enzymes have been identified in microbial cultures from north polar marine environments [5], antibiotic-producing bacteria have been isolated from High Arctic permafrost soils [6], polar glaciers are sites of novel psychrophilic fungi and microalgae [7,8,9], and a variety of exopolysaccharides (EPS) are produced by bacterial isolates from Arctic and Antarctic environments [10,11,12,13]. Many of these EPS compounds have interesting properties, such as metal adsorption, cryoprotection, and emulsifying activities, which suggests that they have potential applications in cosmetic, environmental, and food biotechnological fields as alternatives to the commercial polymers that are currently used [11].
Permafrost landscapes are a typical feature of high latitude environments, and are characterized by the presence of various types of liquid water habitats where microbial communities may grow to high population densities—for example in cold-water springs [14], water tracks beneath the soil surface [15], and ice-covered lakes [16]. One of the most abundant types of waterbody in the Arctic is generated as a consequence of the thawing and collapse of ice-rich permafrost, followed by the filling of these shallow basins by snow and ice melt to produce thermokarst lakes and ponds (‘permafrost thaw lakes’). These waterbodies collectively cover more than 100,000 km2 of the northern lands [17], and are capped by ice and snow throughout much of the year. They are known to be transient, microbiological hotspots in the landscape [18], but to date, they have received little attention by microbiologists for culture-based analysis of bacterial isolates.
To our knowledge, this study reports the first attempt to isolate bacterial taxa from the sediments of permafrost thaw lakes, and to characterize their biomolecules, with special attention to exopolymers. These compounds are known to play a major role in low-temperature tolerance and freeze survival of bacteria, and high latitude environments are therefore an excellent set of locations to look for their presence and diversity [13]. We undertook this study at the southern margin of northern permafrost, in subarctic Québec, Canada—where the thawing and collapse of ice-rich permafrost mounds (‘palsas’) produces abundant thaw lakes. The lakes lie in permafrost peatlands, and their waters contain high concentrations of organic matter and diverse planktonic communities of bacteria, archaea and microbial eukaryotes [19,20]. The organic lake sediments are covered by liquid water throughout the year, providing a carbon-rich habitat for continuous growth despite the prolonged winter freeze-up of the surrounding landscape; nevertheless, little is known about their microbial communities. We sampled the bottom sediments from three permafrost thaw lakes during the open water period in summer, and first characterized their sediment bacterial diversity by 16S rRNA gene amplicon analysis to assess the microbial richness of this habitat. With subsamples from the same sediment cores, we then cultured and isolated bacterial strains, and characterized extracellular enzymes and EPS produced by cultures of each isolate.
2. Materials and Methods
2.1. Sampling
The study sites were located in the Sasapimakwananisikw (SAS) River Valley, in the sporadic permafrost zone of subarctic Quebec, Canada, around 10 km southwest of the village Whapmagoostui-Kuujjuarapik. The valley contains dozens of lakes associated with peatlands and collapsing palsas (a photograph of the valley is given as Figure 7 in reference [21]), and was accessed by helicopter in September 2017. Sediments were sampled from three permafrost thaw ponds (thermokarst lakes) in the valley, identified as SAS2A, SAS2B, and SAS2C. Site physicochemical conditions have been previously described [20]; these black-water lakes are slightly acidic with high concentrations of dissolved organic carbon and anoxic bottom waters. The lakes have high emission rates of methane, and low sulfate concentrations. Sampling was from a Zodiac boat and performed with a MiniGlew sediment coring sampler from the middle of each lake. The sediment material was maintained in the dark at 4 °C prior to subsampling for DNA and culture analyses.
2.2. Culture-Independent Approach: Genomic Analysis
To characterize the full bacterial diversity of the benthic microbiome in these peatland thaw lakes, we extracted environmental DNA from the upper cm of the three SAS sediment cores, and analyzed the bacterial 16S rRNA genes by high throughput MiSeq Illumina sequencing. Nucleic acids were extracted from 1g of sediments using the AllPrep DNA/RNA Mini Kit (Qiagen Sciences, Germantown, MD, USA). The V4 region of the 16S rRNA gene was amplified by PCR using the S-D-Bact-0516-a-S-18/S-D-Bact-0907-a-A-20 primer set fused with MiSeq adaptors. PCR reactions were carried out in duplicate in a final volume of 25 μL using Brilliant III Ultra-Fast Q-PCR Master Mix (Agilent, Santa Clara, CA, USA), 0.5 nM of primers and 1 ng of DNA template. The PCR program included 30 cycles of denaturation at 95 °C for 30 s then annealing at 58 °C for 30 s and extension at 72 °C for 1 min. Illumina MiSeq adaptors and barecodes were added during a second PCR step. Each reaction contained 5 μL of GreenGoTaq Reaction Buffer (Promega Corporation, Madison, WI, USA), 0.5 μL of dNTP mix (40 mmol L–1 total), 1 μL of each primer (10 μM), 0.125 μL of GoTaq DNA polymerase (Promega), and 1 μL of corresponding PCR1 product. The final volume was adjusted to 25 μL with sterile water. PCR conditions were 95 °C for 2 min; 10 cycles of 95 °C for 40 s, 55 °C for 45 s, and 72 °C for 60 s; and a final extension at 72 °C for 10 min. PCR amplifications were then pooled and purified on agarose gel using the QIAquick Gel Extraction Kit (Qiagen). Libraries were quantified using a QuBit DNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA) and a QuBit 3.0 Fluorometer (Life Tech-nologies) following the manufacturer’s instructions and then paired-end sequenced on an Illumina MiSeq sequencer using a V3 MiSeq sequencing kit (2 × 300 bp) at the Institut de Biologie Intégrative et des Systèmes (IBIS) sequencing platform (Université Laval, Quebec, Canada). Sequences were quality filtered as previously detailed [22] then clustered into operational taxonomic units (OTUs) at 97% of sequence similarity using VSEARCH [23]. The taxonomic affiliations of the OTUs were performed using the Mothur version of the Bayesian classifier with the SILVA 128 database.
2.3. Culture-Dependent Approach: Isolation of Psychrophilic Strains
Surface sediment samples (about 1.0 g) were used to inoculate, separately, 30 mL of Tryptone Soya Broth (TSB), 30 mL of medium R2A and 30 mL of medium YN. The temperature of incubation was 10 °C for one week, under slow agitation (40 rpm). The TSB medium contained (g/L): pancreatic digest of casein 17.0; enzymatic digest of soya bean 3.0; NaCl 5.0; K2HPO4 2.5; glucose 2.5, pH 7.2. The R2A medium contained (g/L): yeast extract 0.50; proteose peptone 0.50; casamino acids 0.50; glucose 0.50; Na-pyruvate 0.30; K2HPO4 0.30; MgSO4 7H2O 0.05, pH 7.0. Medium YN contained (g/L): yeast extract 6.0; NaCl 6.0, pH 5.6.
Solid media were obtained by adding 1.8% agar to the compositions described above. After one week of incubation, microbial growth occurred in all tested liquid media, and subsamples were then spread on the corresponding solid media in plates. The colonies were purified by using the serial dilution-plating method at 10 °C, followed by repeated re-streaking on the same solid medium until uniform colonies were obtained.
Genomic DNA was isolated from the cultures using DNAzol (Molecular Research Centre, Inc. Cincinnati, OH, USA) according to the manufacturer’s instruction. PCR amplification and sequencing of the almost full-length 16S rRNA genes of twenty-one strains were obtained from the genomic-DNAs amplification by using universal primers 8F and 1517R of broad specificity in a polymerase chain reaction (PCR). The nucleotide sequence of 16S rRNA genes was analyzed by EzTaxon-e server (https://www.ezbiocloud.net), and the values for pairwise 16S rRNA gene sequence similarity among the closest species were determined using the EzTaxon-e server. A phylogenetic tree was constructed using the software package MEGA X [24] after multiple alignments of the data by CLUSTAL_X [25]. Distances (distance options according to Kimura 2-parameter method) [26] and clustering were based on neighbor-joining [27]. Tree topologies were tested by the bootstrap method of resampling [28] using 1000 replications.
2.4. Phenotypic Characterization of Isolates
Cell and colony morphologies were determined by phase contrast microscopy (Nikon Eclipse E400) and by stereomicroscopy (M8, Leica), respectively. Gram reactions were performed by the KOH lysis method, according to Halebian et al. [29], and by testing aminopeptidase activity through commercial strips (Bactident® Merck Millipore, Darmstadt, Germany). Oxidase activity was determined by assessing the oxidation of tetramethyl-p-phenylenediamine, and catalase activity by assessing bubble production in a 3% (v/v) hydrogen peroxide solution. Starch hydrolysis was tested by flooding cultures with Lugol’s iodine solution on solid medium containing 0.2% (w/v) starch. Xylan and cellulose hydrolysis were tested by flooding cultures with 0.1% Congo Red dye followed by rinsing with 1.0 M NaCl solution on solid medium containing 0.2% (w/v) xylan and carboxymethyl-cellulose (CMC), respectively.
2.5. Exopolymer Production and Recovery
Isolated strains showing mucoid accumulation on agar plates were investigated for exopolymer (EP) production. The bacterial isolates were cultivated in 250 mL of modified buffered broth R2A with reduced complex carbon sources, and enriched by a simple carbon source (1%, w/v) when their capability was tested, to utilize the selected carbon source and release an exopolymer (EP). Modified buffered medium R2A contained (g/L): yeast extract 0.2, peptone 0.2, casamino acid 0.2, Na-pyruvate 0.3, MgSO4 7H2O 0.05 with 0.1 M phosphate buffer pH 7.0 (Na2HPO4 7H2O 16.35 g/L and NaH2PO4 5.4 g/L) plus carbon source 1.0, sterilized by filtration (0.22µm size-pore) and added to the medium R2A after autoclaving. The tested carbon sources were: galactose (Applichem, Darmstadt, Germany), glucose (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), mannose (Applichem) and sucrose (Sigma-Aldrich).
Bacterial growth was monitored at 10°C under agitation (130 rpm) by measuring the optical density at 540 nm and pH values until the stationary phase was reached (four days of incubation). The cultures were then centrifuged for 30 min at 10,000 rpm, 4 °C. Polymers contained in the extracellular fraction (S) were precipitated by adding 1 volume of cold absolute ethanol, kept at −20 °C overnight and then centrifuged (10,000 rpm, 30 min, 4 °C). The pellet was dissolved in warm distilled water, dialyzed in dialysis tubes (Spectra/Por MWCO; molecular weight cut-off 12–14 kDa) against tap water for 3 days, lyophilized (Heto drywinner) and then weighed.
Cells showed a gelatinous aspect after centrifugation, suggesting that exopolymers could be bound to the cell surface. Procedures to recover the tightly bound (TB) and loosely bound (LB) exopolymer fractions were therefore applied. For recovery of the LB fraction, cells were washed three times with 0.15 M phosphate buffered saline (PBS) pH 7.2 (containing g/L: Na2HPO4 1.44, KH2PO4 0.24, NaCl 8.0, KCl 0.20), and then centrifuged at 4000× g for 40 min at 4 °C. The supernatants were collected and filtered (0.22 µm size-pore) to remove residual cells, dialyzed in tubes (Spectra/Por MWCO; molecular weight cut-off 6–8 kDa) and lyophilized. To recovery the TB fraction, the cells were suspended with 0.1 M NaOH for 4 h at room temperature under agitation, then centrifuged at 20,000× g for 20 min at 4 °C to separate the supernatant containing the EP from the residual cell membranes. The supernatant was then dialyzed and lyophilized [11]. EPS, TB and LB fractions were assayed for total carbohydrates according to the phenol-sulfuric acid method [30], for proteins according to Bradford method [31] and uronic acid content according to Blumenkrantz and Asboe-Hansen [32]. All experiments were carried out in duplicate.
2.6. Chemical Characterization of Exopolymers
The exopolymer composition was determined after acidic hydrolysis of lyophilized samples (3–4 mg) with 1 mL of 0.5 M trifluoroacetic acid (TFA) at a temperature of 120 °C for 2 h. Monomers were identified by thin layer chromatography (TLC) on silica plates (Silica GelF60, Merck). TLC plates were developed with the following mobile phase: n-butanol/acetic acid/water (60/20/20; v/v/v)). The sugar and amino acid fractions were identified by spraying plates with α-naphthol and ninhydrin reagents, respectively [33]. The sugar composition was also identified by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD) using a CarboPAC PA1 column and standards for identification and calibration curves [34].
2.7. NMR Spectroscopy
1H and 13C nuclear magnetic resonance (NMR) and heteronuclear single quantum coherence (HSQC) spectra of the exopolysaccharides were performed on a Bruker 600 MHz Bruker spectrometer at 50 °C. For 1H analysis, the sample Lev_2ASCA was exchanged twice with D2O with an intermediate lyophilizing step and then dissolved in 700 µL D2O. Chemical shifts were reported in parts per million, relative to sodium 2,2,3,3-d 4-(trimethylsilyl) propanoate for 1H, and CDCl3 for 13C-NMR spectra [35].
2.8. Chromogenic Substrate Hydrolysis: Screening of Glycoside Hydrolase (GH) Activities
Isolates were investigated for the presence of enzymatic activities in the extracellular compartment. For this purpose, 100 mL of cultures in the standard media were collected at stationary phase and centrifuged (20 min at 10,000 rpm, 4 °C). The cell-free supernatants were partially purified with 80% (w/v) (NH4)2SO4 precipitation and then dialyzed against 50 mM phosphate buffer pH 7.0. The extracellular fractions were assayed for protein content according to Bradford method [29], and tested for glycoside hydrolase activities. The compounds ortho-nitrophenyl β-D-galactopyranoside, o-nitrophenyl β-D-glucopyranoside, p-nitrophenyl β-D-cellobioside, p-nitrophenyl β-D-glucopyranoside, o-nitrophenyl β-D-cellobioside, p-nitrophenyl β-D-lactopyranoside, p-nitrophenyl β-D-maltoside, p-nitrophenyl β-D-galactopyranoside, p-nitrophenyl β-D-xylopyranoside, p-nitrophenyl α-D-galactopyranoside, p-nitrophenyl α-D-glucopyranoside, p-nitrophenyl α-D-maltoside, p-nitrophenyl α-L-arabinofuranoside, p-nitrophenyl α-L-arabinopyranoside and o-nitrophenyl α-D-galactopyranoside were used as colorimetric substrates for β-galactosidase, β-glucosidase, exoglucanase (cellobiohydrolase), β-lactase, β-maltosidase, β-xylosidase, α-galactosidase, α-glucosidase, α-maltosidase, α-arabinofuranosidase and α-arabinopiranosidase enzymatic activities. One hundred μL of 100 mM substrate solution were mixed with 15 μg of proteins in the extracellular fraction and suspended in 50 mM phosphate buffer (1 mL final volume). The reactions were incubated at 37 °C for 1 h. The release of the chromophore p-nitrophenol (pNP) from the chromogenic substrates was measured by absorbance at 420 nm. One unit of activity was defined as the activity releasing 1 μmol of p-nitrophenol in 1 h under the above assay conditions [36].
3. Results
3.1. Microbial Diversity of the Thaw Lake Sediments
Sediment samples from the permafrost thaw lakes SAS2A, SAS2B and SAS2C were examined by combining two complementary approaches: Genomic analysis of amplicons and culture-dependent analysis, to assess the microbial diversity of these extreme high-latitude environments, and to identify biomolecules such as enzymes and exopolymers of potential biotechnological interest.
3.1.1. Genomic Analysis
The bacterial community composition of SAS2A, SAS2B and SAS2C sediments was analyzed in detail by 16S rRNA gene sequencing, with an average of 96,800 sequences per sample. The bacterial communities in all three lake sediments had a diverse composition, with up to 1208 different OTUs (at the 97% similarity level). The communities of SAS2A and SAS2B surface sediments were similar and were dominated by Chlorobium and Bacteroidetes OTUs, whereas SAS2C sediments showed a reduced proportion of the Chlorobium OTUs (Figure 1).
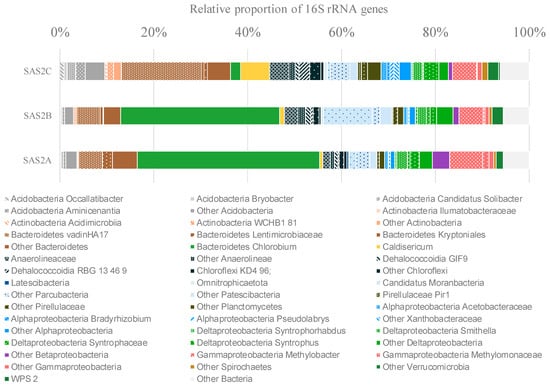
Figure 1.
Bacterial community composition determined by Illumina MiSeq 16S rRNA gene sequencing. Only operational taxonomy units (OTUs) with a relative proportion of >1% are shown.
Both aerobic and anaerobic taxa were identified in the sediments. Putative aerobic taxa were represented by members of the Acidobacteria (5.34% of the sequences), Actinobacteria (1.52%), Alpha- (3.66%), Beta- (1.92%) and Gamma-proteobacteria (7.33%), including Methylomonaceae and Pseudomonadaceae families. Anaerobic lineages were mainly represented by Chlorobium (24.73%), Deltaproteobacteria (7.94%) and Chloroflexi (8.10% of the sequences). In addition, candidate division bacteria, including Candidatus Moranbacteria, represented a substantial proportion of the sediment community (9.68%) (Figure 1).
Once the cultured isolates were obtained (see below), we re-examined the 16S rRNA gene sequences from the amplicon analysis to determine whether the same taxonomic groups could be detected in the environmental DNA. For the full data set, the genus Pseudomonas and the order Sphingomonadales were represented, each contributing up to 0.11% of the reads. Paenibacillus accounted for up to 0.007% of the reads, while Acinetobacter was not detected. Staphylococcus was removed from this analysis, given its presence in a DNA extraction negative control.
3.1.2. Culture-Dependent Analysis
The sediment samples were enriched by using TSB, R2A and YN media for the isolation of psychrophilic bacteria. Several colonies were purified, and twenty-one isolates were obtained. Morphological and biochemical features of isolates are summarized in Table 1. All isolates were rod-shaped except for the strain 2A that showed a cocci shape. Colonies of some isolates, such as strains 2AP, 2CS2, 2CSA, 2CSB, and 2CSC showed fluorescent staining when grown on YN medium; the colonies of strain 2BG were yellow. They were all Gram-positive except for strain 2B that was Gram-negative. They were oxidase positive and most of them showed amylase activity. No strains showed cellulase and xylanase enzymatic activities on agar-plates when grown at a temperature of 10 °C.

Table 1.
Main properties of strains isolated from SAS2A, SAS2B, and SAS2C sampled sites.
Microbial isolates were identified using the EzTaxon-e server (www.ezbiocloud.net/eztaxon), based on the 16S rRNA gene sequence data. Most belonged to the genus Pseudomonas; in particular, from SAS2A site five isolates showed the highest similarity to Pseudomonas extremaustralis (the similarity ranging from 99.51% to 99.71%) and two strains to Pseudomonas frederiksbergensis; other two isolates from SAS2A were most closely related to Pseudomonas fluorescens; likewise, from SAS2C site five isolates exhibited highest similarity to Pseudomonas fluorescens (similarity ranging from 99.71 to 99.93%). From this latter site, five strains of Pseudomonas yamanorum, whose similarity ranged from 99.18 to 99.85%, were also isolated. The isolates also included single strains of the genera Acinetobacter, Staphylococcus, Pseudomonas, Sphingomonas and Paenibacillus.
The 16S rRNA gene sequences were deposited in GenBank/EMBL/DDBJ under accession numbers as reported in Table 2 and were used for the construction of the neighbor-joining phylogenetic tree (Figure 2). Newly identified isolates were compared to appropriate type species within each genus.

Table 2.
Identification of isolates by 16S rRNA gene sequencing.
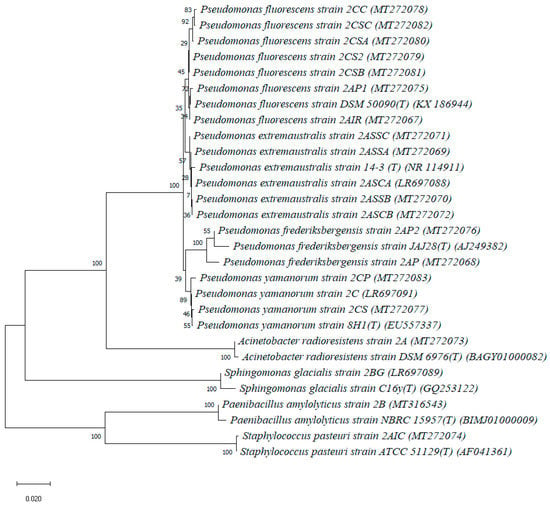
Figure 2.
Neighbor-joining tree showing the phylogenetic position of bacterial strains isolated in the present study and the closest relatives based on partial 16S rRNA gene sequences, reconstructed using the Kimura 2-parameter method with 1000 bootstrap replications. The scale represents a genetic distance of 0.02 nucleotide substitutions per site.
3.2. Exopolymer Production and Partial Purification
The isolates with mucoid aspect on agar plates, namely, Pseudomonas extremaustralis strain 2ASCA, Sphingomonas glacialis strain 2BG, Paenibacillus amylolyticus strain 2B and Pseudomonas yamanorum strain 2C, were grown on simple carbon sources, such as glucose, galactose, mannose and sucrose in minimal growth media, with the aim to evaluate the exopolymer production capability. Sphingomonas glacialis strain 2BG was able to utilize only galactose and glucose for growth, reaching an optical density at 540 nm of 1.054 and 1.018, respectively, after four days of incubation at a temperature of 10 °C; only a small amount of exoproduct (about 55 mg/L) was recovered into the supernatants from each sugar tested, and no carbohydrate polymers were detected. Similarly, Pseudomonas yamanorum strain 2C grew on galactose, glucose, and mannose as main carbon sources, reaching an optical density at 540 nm of 1.150, 1.181, and 1.079, respectively, after four days of incubation at a temperature of 10 °C; a small amount of exoproduct (15–26 mg/L) was recovered from the supernatants when the strain was grown in the presence of the tested sugars tested, and no carbohydrate polymers were detected. Paenibacillus amylolyticus strain 2B and Pseudomonas extremaustralis strain 2ASCA showed good growth on all four sugar sources tested with an O.D. at 540 nm > 1.000 (Table 3).

Table 3.
Growth and exopolymer yields of Paenibacillus amylolyticus strain 2B and Pseudomonas extremaustralis strain 2ASCA in the presence of single carbon sources.
Analysis of the three purified fractions, extracellular (S), tightly bound (TB) and loosely bound (LB) fractions, revealed that Paenibacillus amylolyticus strain 2B and Pseudomonas extremaustralis strain 2ASCA were able to synthesize exopolymers. Strain 2B produced an exoproduct showing both a proteic and saccharidic nature, in different ratios, when it was grown in mR2A medium plus galactose or plus sucrose as main carbon sources, at 10 °C under agitation; these products, named “P_2BG” and “P_2BS” were both located in the tightly bound cell fraction and showed a yield of 6.76 and 23 mg/L, respectively (Table 3). Thin layer chromatography analyses of these products, carried out after acidic hydrolysis reactions, revealed the presence of two monomers giving a positive ninhydrine reaction and showing the retention factor of glutamic acid and glutamine for P_2BG. The hydrolyzed P_2BS exhibited on TLC four bands positive for ninhydrine color reaction with the same retention factor of phenylalanine, glutamic acid, galactosamine and glutamine (data not showed).
Strain 2ASCA produced an exopolysaccharide when it was grown in mR2A medium plus sucrose as sole carbon source, at temperature of 10°C under agitation; this exopolymer, named “Lev_2ASCA” was located in the loosely bound cell fraction and it was produced with a yield of 613.2 mg/L, of which almost all was polysaccharide (Table 3). Preliminary studies of the Lev_2ASCA polymer were carried out by analyzing the monomer composition by TLC after acidic hydrolysis, and Lev_2ASCA polymer was found to be composed essentially of fructose units. This composition was confirmed by HPLC-Dionex analysis. The chromatogram showed a main peak with a retention time typical of the fructose standard (15.68 min), indicating that Lev_2ASCA has a sugar composition consisting of fructose residues (data not shown).
3.3. NMR Spectroscopy
Structural analysis of the Lev_2ASCA polymer was performed by means of NMR. The comparison between 1D (Figure 3) and 2D (Figure 4) spectra confirmed that the isolated polymer is a levan type polysaccharide. In the 1H spectrum (upper trace in Figure 3), the main signals were found in the bulk region were the ring protons resonate; i.e., at 4.21 ppm, 4.12 ppm, 3.97 ppm, 3.92 ppm, 3.78 ppm, 3.72 ppm and 3.61 ppm, which is typical of the furanose ring of a levan polymer (Poli et al., 2009). The 13C spectrum (lower trace in Figure 3) also showed the typical signals of a levan, with resonances at 105.5 ppm, 81.15 ppm, 77.51ppm, 76.23 ppm, 64.24 ppm and 61.19 ppm. The analysis of the HSQC spectrum (Figure 4) showed main cross peaks at 81.15 ppm/3.97 ppm (C5/H5), 77.51 ppm/4.21 ppm (C3/H3), 76.23 ppm/4.12 ppm (C4/H4), 64.24 ppm/3.92 and 3.61 ppm (C6/H6a,b), 61.19 ppm/3.78 and 3.72 ppm (C1/H1a,b).
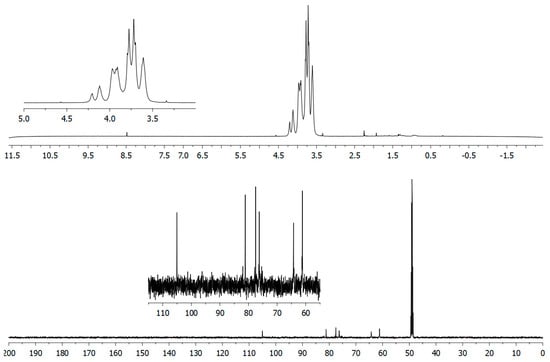
Figure 3.
1D-NMR analysis of the Lev_2ASCA polymer: 1H (upper) and 13C (lower) traces were recorded with a 600 MHz Bruker spectrometer. The x-axis for each spectrum were in chemical shifts reported as parts per million (ppm) with reference to D2O and to CD3OD for 1H and 13C spectrum, respectively.
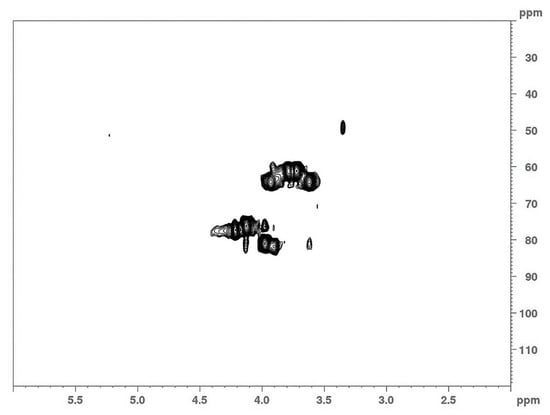
Figure 4.
2D-NMR analysis of the Lev_2ASCA polymer: The HSQC spectrum was recorded with a 600 MHz Bruker spectrometer.
3.4. Chromogenic Substrate Hydrolyses: Screening of Glycoside Hydrolase (GH) Activities
Some isolates, namely, Pseudomonas extremaustralis strains 2ASSA and 2ASCA, Acinetobacter radioresistens strain 2A, Paenibacillus amylolyticus strain 2B, Sphingomonas glacialis strain 2BG, Pseudomonas fluorescens strains 2CSB and 2CS2 and Pseudomonas yamanorum strain 2C were investigated for the presence of glycoside hydrolase activities in the cell free supernatant, given that species belonging to the same genera are described in the literature as being capable of producing glycoside hydrolase activities (Table 4).

Table 4.
Glycoside hydrolase (GH) activities detected through nitrophenyl (NP) glycosides in the extracellular compartment of selected isolates.
For this purpose, nitrophenyl glycosides were utilized as substrates for enzymatic assays at 37 °C. After 1 h, all tested microorganisms exhibited β-galactosidase and glucosidase activities when a pyranose ring form of substrate was used, while analogous substrates in which the saccharidic moieties were in furanose forms were not hydrolyzed. In addition, Pseudomonas extremaustralis strains 2ASSA and 2ASCA also released an α-glucosidase enzyme, with activities almost nine-times higher than the beta form. Pseudomonas fluorescens strain 2CSB and Pseudomonas yamanorum strain 2C expressed a β-glucosidase hydrolysis function that exclusively attacked bonds in the ortho position. Paenibacillus amylolyticus strain 2A displayed a versatile maltosidase for α and β linkages, while Pseudomonas extremaustralis strain 2ASCA had only a week α-maltosidase, unlike Pseudomonas yamanorum strain 2C and Pseudomonas fluorescens strain 2CS2 that exhibited strong β-maltosidase activity. All tested isolates showed β-xylosidase activity and did not produce β-lactase; in addition, they were able to hydrolyze β bonds linking cellobiose units in the para, but not ortho, position.
4. Discussion
Permafrost thaw lakes are abundant habitats for microbial life, yet their sediment microbial diversity has been little explored. In this study, we examined the microbial communities of sediments collected in three sites in the rapidly thawing southern margin of permafrost, in subarctic Québec, Canada. The diverse microbial communities inhabiting the thaw lake sediments were analyzed by means of next-generation sequencing of 16S rRNA gene amplicons from environmental DNA, and Sanger sequencing of the 16S rRNA genes in cultured psychrophilic isolates. Based on the literature data and their observed phenotypic features, bacterial isolates were selected to investigate their potential as producers of extracellular biomolecules.
The Sanger sequencing of cultures allowed us to identify 21 diverse isolates, mostly Gammaproteobacteria (86%), but also Bacilli (9.5%) and Alphaproteobacteria (4.5%). In particular, 18 isolates were in the genus Pseudomonas, consistent with the detection of Pseudomonas in the environmental DNA analysis. Other taxa detected by the amplicon analysis were likely precluded by the culture conditions chosen here, such as the designated temperature, pH, sodium chloride content, organic matter, oxygen availability and absence of light. These conditions selected for specific chemoorganoheterotrophs and would have excluded anaerobes such as Chlorobium, Deltaproteobacteria and Chloroflexi that were well represented in the amplicon sequences. The isolates accounted for only a small portion of the total microbial diversity of the thaw lake sediments, as expected, and were rare taxa that proliferated in culture.
Cold-dwelling microbes isolated from the low-temperature habitats are known to release biomolecules of broad interest in several biotechnological sectors [12,37,38,39,40,41,42]. In this study, the cultured isolates were investigated for the production of exopolymers and extracellular enzymes by means of glycoside hydrolase activities. Pseudomonas extremaustralis strain 2ASCA, isolated from the sample SAS2A, produced the levan-type exopolymer Lev_2ASCA. This compound was recovered from the loosely bound cell fraction when the bacterium was grown at 10 °C in the presence of sucrose as main carbon source. Levan is the homopolymer of fructose and is known to be synthesized as an exopolysaccharide by many bacterial genera, including Acetobacter, Azotobacter, Bacillus, Corynebacterium, Gluconobacter, Halomonas, Mycobacterium, Pseudomonas, Streptococcus, and Zymomonas [43]. Studies have drawn attention to the wide potential of levan for its anti-cancer, anti-bacterial and anti-viral activities, in addition to its capabilities to enhance calcium absorption, to decrease plasma glucose content in diabetic rats, to inhibit metal corrosion, to aid drug delivery and to act as an adhesive [44].
Levan production by strain 2ASCA had a novel feature in that the polymer was cell-associated and not dispersed in the supernatant. Therefore, polymer recovery was simplified and faster relative to other exopolysaccharide producers, since by means of only a single centrifuge step, all polymers were precipitated by forming a gel layer above the cellular pellet. An important aspect of this biochemical isolation procedure is that it did not require the use of organic solvents. This raises the value of levan from Pseudomonas extremaustralis strain 2ASCA, since it would lend itself to eco-friendly production systems, without the need to use organic chemicals that create waste disposal problems. The isolate Paenibacillus amylolyticus strain 2B from the SAS2B sediments, produced a biofilm with both protein and saccharide constituents when grown in the presence of galactose or sucrose as main carbon sources at 10 °C. These products were also located in the tightly bound cell fraction, which may similarly facilitate biochemical extraction of exopolymers from this isolate. Further studies are needed to more completely understand the chemical composition of these exoproducts, and their role in permafrost microbial ecosystems.
Although the optimization of enzyme expression was not the focus of this study, our preliminary results show levels of activity that are consistent with those reported in the literature [45,46,47,48,49]. Under the tested conditions, the most promising activities were of α-glucosidase from Pseudomonas extremaustralis strains 2ASSA and 2ASCA, and of β-galactosidase, β-glucosidase β-xylosidase and cellobiohydrolase from several isolates. These results encourage future studies to purify these glycoside hydrolase enzymes, to identify their exact cellular localization and to assess the culture and substrate conditions for their maximum expression.
The results of this study are consistent with the view that little-explored areas of our planet such as extreme Arctic environments contain high microbial biodiversity, with a level of genomic and metabolomic richness that has been largely unrecognized to date [50]. As shown here for permafrost thaw lake sediments, these habitats contain microbiota that can produce a wide array of interesting biomolecules, including enzymes and extracellular polysaccharides. These bacterial biomolecules offer potential for eco-sustainable biotechnology applications.
This study delivered insights into the microbial diversity of communities present in the sediments collected in three permafrost thaw lakes in subarctic Québec, Canada, using both culture-dependent and -independent approaches. Next Generation Sequencing provides technologies complementary to conventional isolation, amplification and Sanger sequencing of 16S rRNA genes for bacterial identification, especially in case of complex samples, because they provide rapid information, avoid any preliminary culturing step, and permit classification to family and genus levels with accuracy. At the species level, however, there are limitations due to the restricted discriminatory capacity of the 16S rRNA gene for rare taxa, thereby underestimating the full bacterial diversity. Parallel use of both procedures is desirable for obtaining complementary information about the taxonomic composition, genetic richness, and potential metabolic pathways of microbial communities [51]. On the one hand, the cultivable microbial populations represent only a minute fraction of the whole community, and are strictly limited to the growing parameters set up by the microbiologist. However, on the other hand, culture-based methods remain indispensable for the recovery and characterization of biomolecules synthesized by microorganisms, and for studying microbial adaptation mechanisms and ecological roles. Only through a culture-dependent approach is it possible to access and fully characterize the wide range of microbial metabolic products that are of interest in biotechnology.
Author Contributions
Conceptualization: I.F., A.V., A.P., B.N. and W.F.V.; methodology and analysis: I.F., A.V., L.L., A.S.M., P.D.D. and A.P.; writing—initial drafts: I.F., A.V., A.P. and W.F.V., writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the program Sentinel North financed by the Canada First Research Excellence Fund (CFREF); the Natural Sciences and Engineering Research Council of Canada (NSERC); the CNR (Italy)—Université Laval UMI-MicroMeNu program (JOINT INTERNATIONAL RESEARCH UNIT FOR CHEMICAL AND BIOMOLECULAR RESEARCH ON THE MICROBIOME AND ITS IMPACT ON METABOLIC HEALTH AND NUTRITION); the Fonds de Recherche du Québec-Nature et Technologies (FQRNT); and the Network of Centres of Excellence ArcticNet.
Acknowledgments
The authors thank the manager and staff of the Centre d’études nordiques (CEN) research station at Whapmagoostui-Kuujjuarapik for their support and the NMR Service of Institute of Biomolecular Chemistry of CNR (Pozzuoli, Italy).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vincent, W.F. Microbial Ecosystems of Antarctica; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Miller, R.V.; Whyte, L. Polar Microbiology: Life in a Deep Freeze; ASM Press: Washington, DC, USA, 2012. [Google Scholar]
- Di Donato, P.; Poli, A.; Taurisano, V.; Abbamondi, G.R.; Nicolaus, B.; Tommonaro, G. Recent advances in the study of marine microbial biofilm: From the involvement of quorum sensing in its production up to biotechnological application of the polysaccharide fractions. J. Mar. Sci. Eng. 2016, 4, 34. [Google Scholar] [CrossRef]
- Di Donato, P.; Romano, I.; Mastascusa, V.; Poli, A.; Orlando, P.; Pugliese, M.; Nicolaus, B. Survival and adaptation of the thermophilic species Geobacillus thermantarcticus in simulated spatial conditions. Orig. Life Evol. Biosph. 2018, 48, 141–158. [Google Scholar] [CrossRef] [PubMed]
- De Santi, C.; Altermark, B.; de Pascale, D.; Willassen, N.-P. Bioprospecting around Arctic islands: Marine bacteria as rich source of biocatalysts. J. Basic Microbiol. 2016, 56, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Marcolefas, E.; Leung, T.; Okshevsky, M.; McKay, G.; Hignett, E.; Hamel, J.; Aguirre, G.; Blenner-Hassett, O.; Boyle, B.; Lévesque, R.C.; et al. Culture-dependent bioprospecting of bacterial isolates from the Canadian High Arctic displaying antibacterial activity. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- De Menezes, G.C.A.; Porto, B.A.; Amorim, S.S.; Zani, C.L.; de Almeida Alves, T.M.; Junior, P.A.S.; Murta, S.M.F.; Simões, J.C.; Cota, B.B.; Rosa, C.A.; et al. Fungi in glacial ice of Antarctica: Diversity, distribution and bioprospecting of bioactive compounds. Extremophiles 2020, 24, 367–376. [Google Scholar] [CrossRef]
- Perini, L.; Gostinčar, C.; Anesio, A.M.; Williamson, C.; Tranter, M.; Gunde-Cimerman, N. Darkening of the Greenland Ice Sheet: Fungal abundance and diversity are associated with algal bloom. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Vishniacozyma ellesmerensis sp. nov., a psychrophilic yeast isolated from a retreating glacier in the Canadian High Arctic. Int. J. Syst. Evol. Microbiol. 2019, 69, 696–700. [Google Scholar] [CrossRef]
- Finore, I.; Lama, L.; Di Donato, P.; Romano, I.; Tramice, A.; Leone, L.; Nicolaus, B.; Poli, A. Parageobacillus thermantarcticus, an Antarctic cell factory: From crop residue valorization by green chemistry to astrobiology studies. Diversity 2019, 11, 128. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Poli, A.; Finore, I.; Rizzo, C. Peculiarities of extracellular polymeric substances produced by Antarctic bacteria and their possible applications. Appl. Microbiol. Biotechnol. 2020, 104, 2923–2934. [Google Scholar] [CrossRef]
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms 2017, 5, 25. [Google Scholar] [CrossRef]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, E.; Mykytczuk, N.C.S.; Pellerin, A.; Goordial, J.; Twine, S.M.; Wing, B.; Foote, S.J.; Fulton, K.; Whyte, L.G. Thiomicrorhabdus streamers and sulfur cycling in perennial hypersaline cold springs in the Canadian high Arctic. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Steven, B.; Lionard, M.; Kuske, C.R.; Vincent, W.F. High bacterial diversity of biological soil crusts in water tracks over permafrost in the High Arctic polar desert. PLoS ONE 2013, 8, e071489. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.F.; Laybourn-Parry, J. Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Grosse, G.; Jones, B.; Arp, C. Thermokarst lakes, drainage, and drained basins. In Treatise on Geomorphology; Shroder, J., Giardino, R., Harbor, J., Eds.; Academic Press: San Diego, CA, USA, 2013; Volume 8, pp. 325–353. [Google Scholar]
- Vigneron, A.; Cruaud, P.; Bhiry, N.; Lovejoy, C.; Vincent, W.F. Microbial community structure and methane cycling potential along a thermokarst pond-peatland continuum. Microorganisms 2019, 7, 486. [Google Scholar] [CrossRef]
- Przytulska, A.; Comte, J.; Crevecoeur, S.; Lovejoy, C.; Laurion, I.; Vincent, W.F. Phototrophic pigment diversity and picophytoplankton in permafrost thaw lakes. Biogeosciences 2016, 13, 13–26. [Google Scholar] [CrossRef]
- Vigneron, A.; Lovejoy, C.; Cruaud, P.; Kalenitchenko, D.; Culley, A.; Vincent, W.F. Contrasting winter versus summer microbial communities and metabolic functions in a permafrost thaw lake. Front. Microbiol. 2019, 10, 1656. [Google Scholar] [CrossRef]
- Vincent, W.F.; Lemay, M.; Allard, M. Arctic permafrost landscapes in transition: Towards an integrated Earth system approach. Arct. Sci. 2017, 3, 39–64. [Google Scholar] [CrossRef]
- Cruaud, P.; Vigneron, A.; Fradette, M.-S.; Dorea, C.C.; Culley, A.I.; Rodriguez, M.J.; Charette, S.J. Annual bacterial community cycle in a seasonally ice-covered river reflects environmental and climatic conditions. Limnol. Oceanogr. 2020, 65, S21–S37. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acid. Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Halebian, S.; Harris, B.; Finegold, S.M.; Rolfe, R.D. Rapid method that aids in distinguishing Gram-positive from Gram-negative anaerobic bacteria. J. Clin. Microbiol. 1981, 13, 444–448. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Finore, I.; Orlando, P.; Di Donato, P.; Leone, L.; Nicolaus, B.; Poli, A. Nesterenkonia aurantiaca sp nov., an alkaliphilic actinobacterium isolated from Antarctica. Int. J. Syst. Evol. Micr. 2016, 66, 1554–1560. [Google Scholar] [CrossRef]
- Poli, A.; Kazak, H.; Gürleyendağ, B.; Tommonaro, G.; Pieretti, G.; Öner, E.T.; Nicolaus, B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr. Polym. 2009, 78, 651–657. [Google Scholar] [CrossRef]
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Finore, I.; Nicolaus, B.; Di Marco, G.; Michaud, L.; Lo Giudice, A. Production and biotechnological potential of extracellular polymeric substances from sponge-associated Antarctic bacteria. App. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Finore, I.; Poli, A.; Di Donato, P.; Lama, L.; Trincone, A.; Fagnano, M.; Mori, M.; Nicolaus, B.; Tramice, A. The hemicellulose extract from Cynara cardunculus: A source of value-added biomolecules produced by xylanolytic thermozymes. Green Chem. 2016, 18, 2460–2472. [Google Scholar] [CrossRef]
- Mao, X.; Hong, Y.; Shao, Z.; Zhao, Y.; Liu, Z. A novel cold-active and alkali-stable beta-glucosidase gene isolated from the marine bacterium Martelella mediterranea. Appl. Biochem. Biotechnol. 2010, 162, 2136–2148. [Google Scholar] [CrossRef] [PubMed]
- Casillo, A.; Ståhle, J.; Parrilli, E.; Sannino, F.; Mitchell, D.E.; Pieretti, G.; Gibson, M.I.; Marino, G.; Lanzetta, R.; Parrilli, M.; et al. Structural characterization of an all-aminosugar-containing capsular polysaccharide from Colwellia psychrerythraea 34H. Antonie Van Leeuwenhoek 2017, 110, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Srimathi, S.; Jayaraman, G.; Feller, G.; Danielsson, B.; Narayanan, P.R. Intrinsic halotolerance of the psychrophilic alpha-amylase from Pseudoalteromonas haloplanktis. Extremophiles 2007, 11, 505–515. [Google Scholar] [CrossRef]
- Qin, Y.; Huang, Z.; Liu, Z. A novel cold-active and salt-tolerant alpha-amylase from marine bacterium Zunongwangia profunda: Molecular cloning, heterologous expression and biochemical characterization. Extremophiles 2014, 18, 271–281. [Google Scholar] [CrossRef]
- Carillo, S.; Pieretti, G.; Lindner, B.; Parrilli, E.; Sannino, F.; Tutino, M.L.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Structural characterization of the core oligosaccharide isolated from the lipopolysaccharide of the psychrophilic bacterium Colwellia psychrerythraea strain 34H. Eur. J. Org. Chem. 2013, 18, 3771–3779. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, X.; Wang, P.; Wang, F. Screening and characterization of psychrotrophic lipolytic bacteria from deep-sea sediments. J. Microbiol. Biotechnol. 2004, 14, 952–958. [Google Scholar]
- Kazak, H.S.; Ates, O.; Ozdemir, G.; Arga, K.Y.; Toksoy, E.O. Effective stimulating factors for microbial levan production by Halomonas smyrnensis AAD6T. J. Biosci. Bioeng. 2015, 119, 455–463. [Google Scholar] [CrossRef]
- Toksoy, E.Ö.; Hernández, L.; Combie, J. Review of levan polysaccharide: From a century of past experiences to future prospects. Biotechnol. Adv. 2016, 36, 827–844. [Google Scholar] [CrossRef]
- Hansen, W.; Yourassowsky, E. Detection of glycosidases in Pseudomonas of the fluorescent group: Relation between serotype and glycosidase activities in P. aeruginosa. Ann. Microbiol. 1983, 134B, 411–419. [Google Scholar]
- Isorna, P.; Polaina, J.; Latorre-García, L.; Cañada, F.J.; González, B.; Sanz-Aparicio, J. Crystal structures of Paenibacillus polymyxa beta-glucosidase B complexes reveal the molecular basis of substrate specificity and give new insights into the catalytic machinery of family I glycosidases. J. Mol. Biol. 2007, 371, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yin, Z.; Wu, L.; Yin, C. Diversity of cultivable β-glycosidase-producing micro-organisms isolated from the soil of a ginseng field and their ginsenosides-hydrolysing activity. Lett. Appl. Microbiol. 2014, 58, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, S.S. Acinetobacter kyonggiensis sp. nov., a β-glucosidase-producing bacterium, isolated from sewage treatment plant. J. Microbiol. 2010, 48, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Chesneau, O.; Morvan, A.; Grimont, F.; Labischinski, H.; El Solh, N. Staphylococcus pasteuri sp. nov., isolated from human, animal, and food specimens. Int. J. Syst. Evol. Micr. 1993, 43, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Cameron, K.A.; Cook, J.M.; Debbonaire, A.R.; Furness, E.; Hay, M.C.; Rassner, S.M. Microbial genomics amidst the Arctic crisis. Microb. Gen. 2020, 6, e000375. [Google Scholar] [CrossRef]
- Li, A.-Z.; Han, X.-B.; Zhang, M.-X.; Zhou, Y.; Chen, M.; Yao, Q.; Zhu, H.-H. Culture-dependent and -independent analyses reveal the diversity, structure, and assembly mechanism of benthic bacterial community in the Ross Sea, Antarctica. Front. Microbiol. 2019, 10, 2523. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).