Biosulfidogenesis Mediates Natural Attenuation in Acidic Mine Pit Lakes
Abstract
1. Introduction
2. Materials and Methods
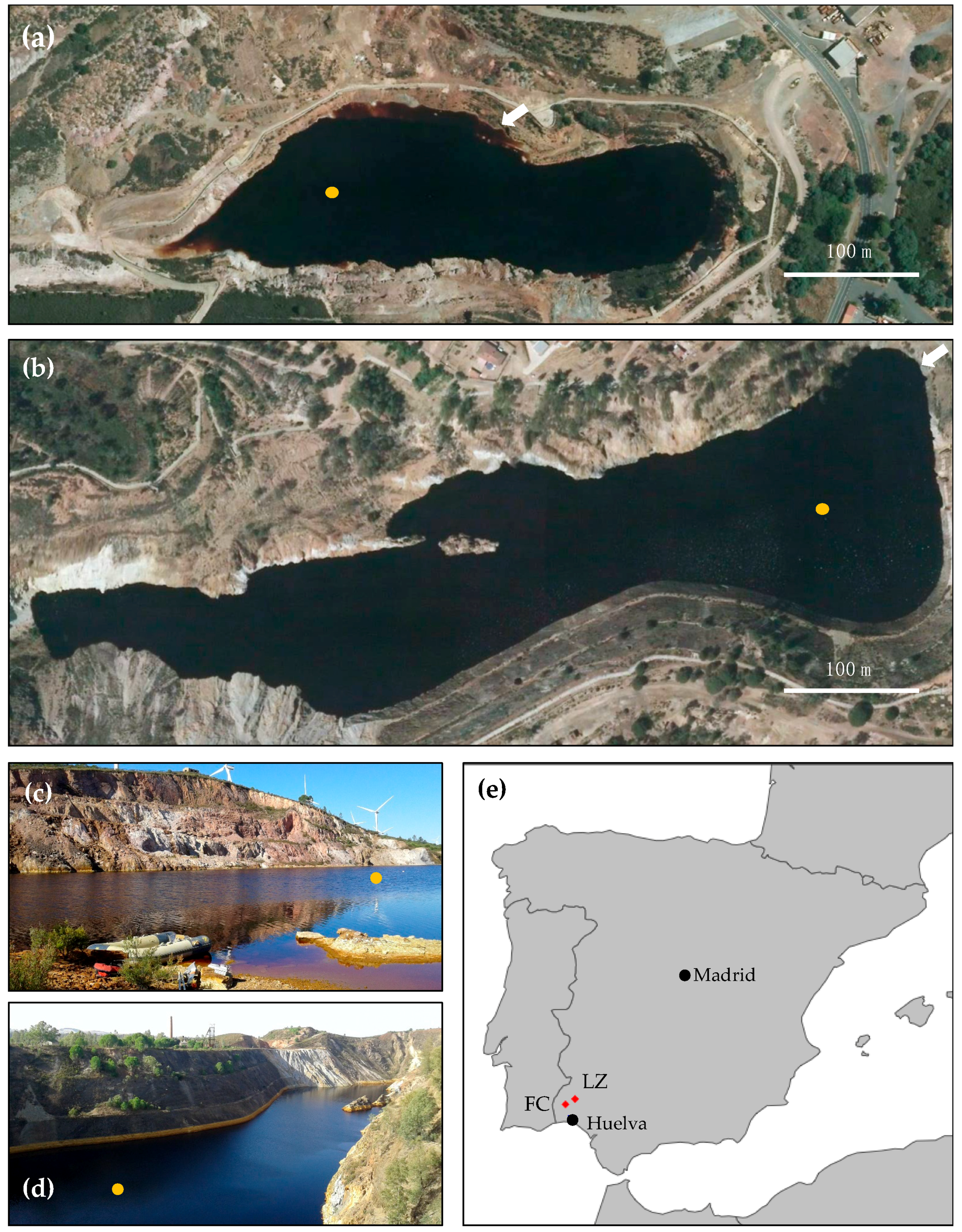
2.1. Study Sites
2.2. Field Site Exploration and Sample Collection
2.3. Chemical Analyses and Electron Microscopy
2.4. Microbial Community Analysis
2.5. Membrane Lipid Analysis
3. Results
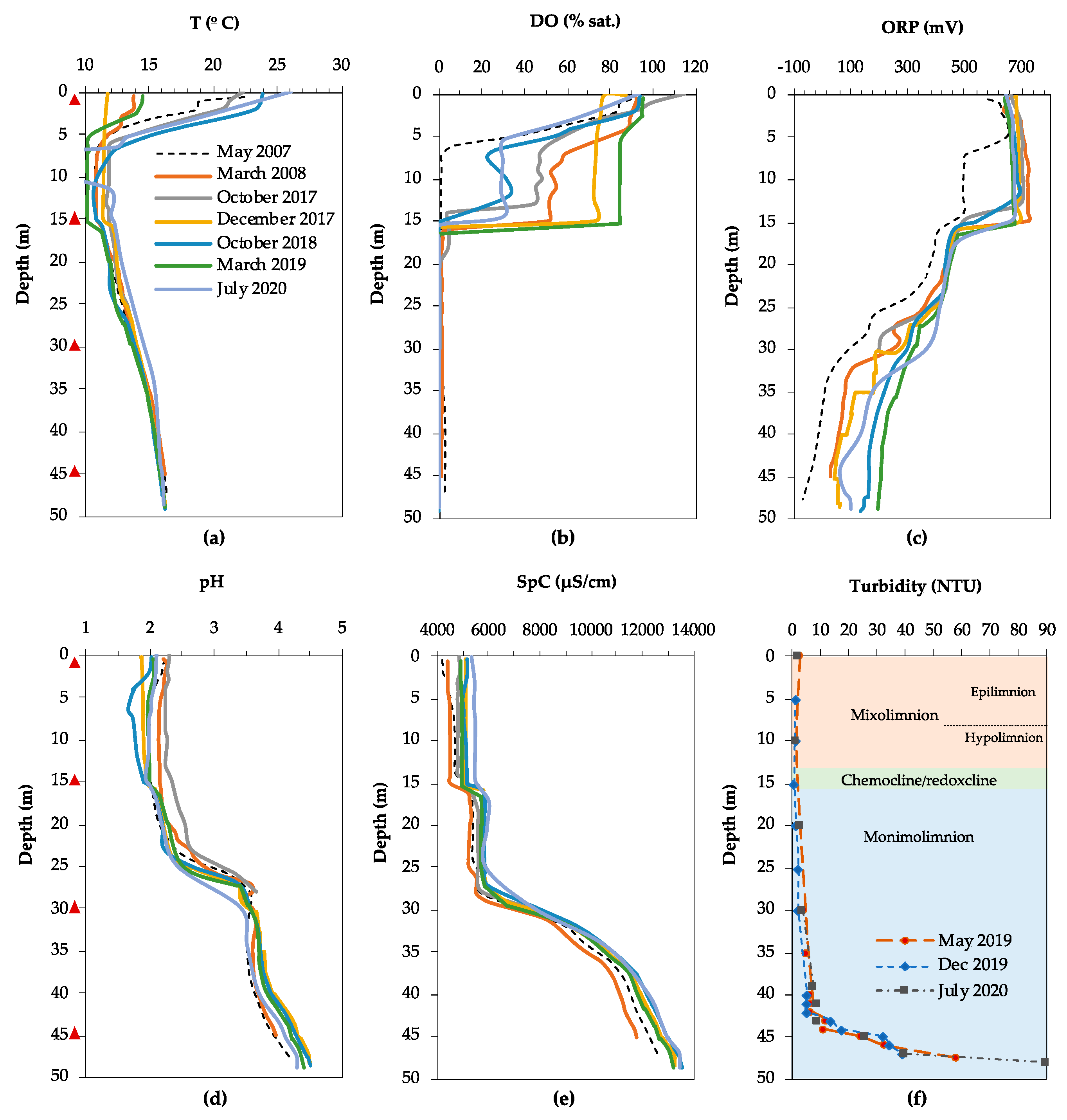
3.1. Physicochemical Characteristics and Water Chemistry
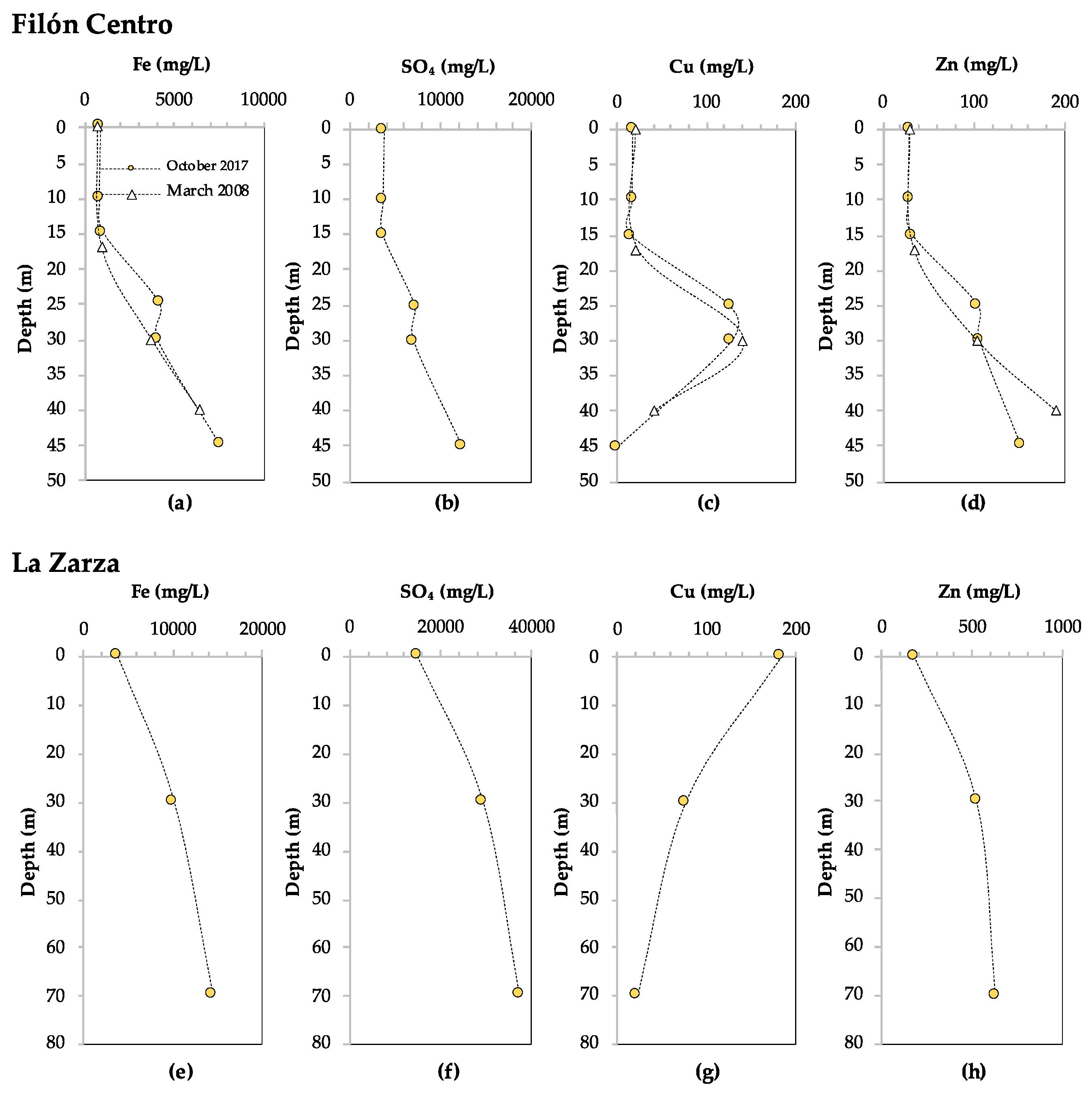
3.1.1. Filón Centro
3.1.2. La Zarza
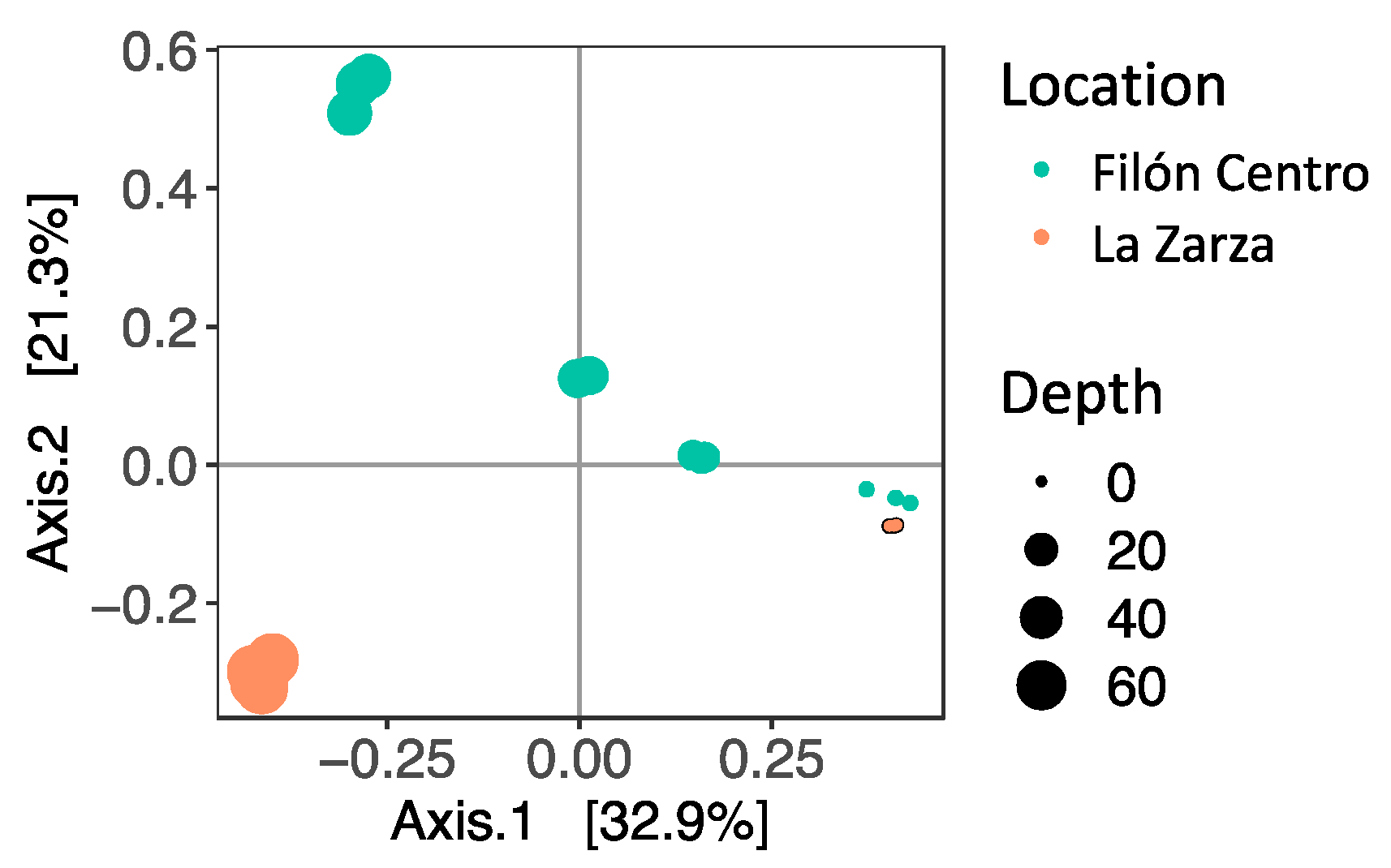
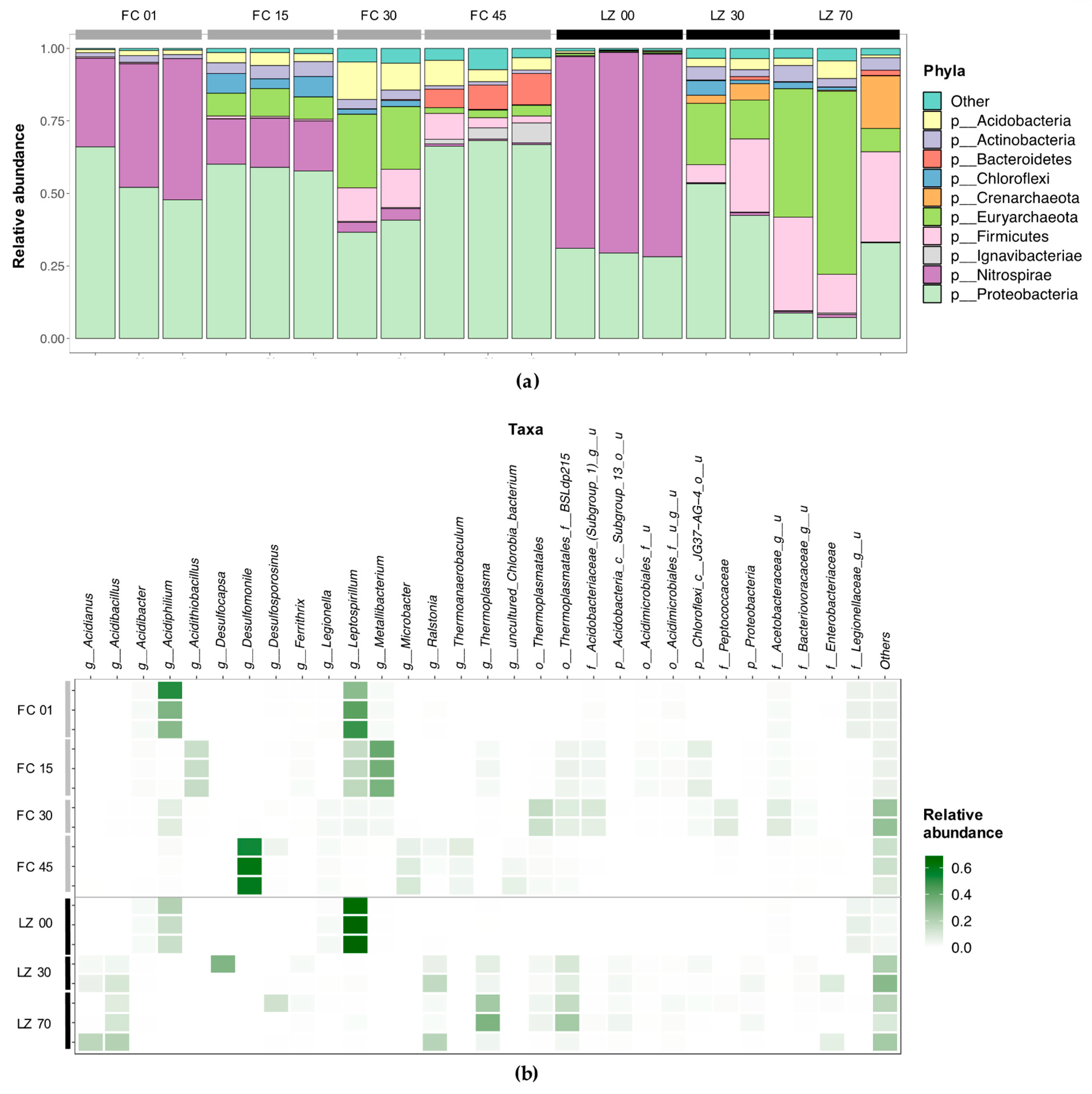
3.2. Microbial Diversity
3.2.1. Filón Centro
3.2.2. La Zarza
3.3. Microbial Membrane Lipids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leistel, J.M.; Marcoux, E.; Thiéblemont, D.; Quesada, C.; Sánchez, A.; Almodóvar, G.R.; Pascual, E.; Sáez, R. The volcanic-hosted massive sulphide deposits of the Iberian Pyrite Belt: Review and preface to the thematic Issue. Miner. Depos. 1997, 33, 2–30. [Google Scholar] [CrossRef]
- Tornos, F. La Geología y Metalogenia de la Faja Pirítica Ibérica. Macla 2008, 23, 10–13. [Google Scholar]
- Sánchez España, J.; Pamo, E.L.; Pastor, E.S.; Ercilla, M.D. The acidic mine pit lakes of the Iberian Pyrite Belt: An approach to their physical limnology and hydrogeochemistry. Appl. Geochemistry 2008, 23, 1260–1287. [Google Scholar] [CrossRef]
- Wendt-Potthoff, K.; Koschorreck, M.; Diez Ercilla, M.; Sánchez España, J. Microbial activity and biogeochemical cycling in a nutrient-rich meromictic acid pit lake. Limnologica 2012, 42, 175–188. [Google Scholar] [CrossRef]
- Falagán, C.; Sánchez-España, J.; Johnson, D.B. New insights into the biogeochemistry of extremely acidic environments revealed by a combined cultivation-based and culture-independent study of two stratified pit lakes. FEMS Microbiol. Ecol. 2014, 87, 231–243. [Google Scholar] [CrossRef]
- Santofimia, E.; González-Toril, E.; López-Pamo, E.; Gomariz, M.; Amils, R.; Aguilera, Á. Microbial diversity and Its relationship to physicochemical characteristics of the water in two extreme acidic pit lakes from the Iberian Pyrite Belt (SW Spain). PLoS ONE 2013, 8, 1–12. [Google Scholar] [CrossRef]
- Diez-Ercilla, M.; Sánchez-España, J.; Yusta, I.; Wendt-Potthoff, K.; Koschorreck, M. Formation of biogenic sulphides in the water column of an acidic pit lake: Biogeochemical controls and effects on trace metal dynamics. Biogeochemistry 2014, 121, 519–536. [Google Scholar] [CrossRef]
- González-Toril, E.; Águilera, Á.; Souza-Egipsy, V.; Pamo, E.L.; Sánchez-España, J.; Amils, R. Geomicrobiology of La Zarza-Perrunal acid mine effluent (Iberian Pyritic Belt, Spain). Appl. Environ. Microbiol. 2011, 77, 2685–2694. [Google Scholar] [CrossRef]
- Church, C.D.; Wilkin, R.T.; Alpers, C.N.; Rye, R.O.; Blaine, R.B. Microbial sulfate reduction and metal attenuation in pH 4 acid mine water. Geochem. Trans. 2007, 8, 1–14. [Google Scholar] [CrossRef]
- Rowe, O.F.; Sánchez-España, J.; Hallberg, K.B.; Johnson, D.B. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 2007, 9, 1761–1771. [Google Scholar] [CrossRef]
- García-Moyano, A.; González-Toril, E.; Aguilera, Á.; Amils, R. Comparative microbial ecology study of the sediments and the water column of the Río Tinto, an extreme acidic environment. FEMS Microbiol. Ecol. 2012, 81, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Toril, E.; Gómez, F.; Rodríguez, N.; Fernández-Remolar, D.; Zuluaga, J.; Marín, I.; Amils, R. Geomicrobiology of the Tinto River, a model of interest for biohydrometallurgy. Hydrometallurgy 2003, 71, 301–309. [Google Scholar] [CrossRef]
- Amils, R.; Fernández-Remolar, D.; Team, T. Río Tinto: A geochemical and mineralogical terrestrial analogue of Mars. Life 2014, 4, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Rodriguez, N.; Amils, R.; Sanz, J.L. Microbial diversity in anaerobic sediments at Rio Tinto, a naturally acidic environment with a high heavy metal content. Appl. Environ. Microbiol. 2011, 77, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-España, J.; Diez-Ercilla, M.; Santofimia, E. Mine pit lakes of the Iberian Pyrite Belt: Some basic limnological, hydrogeochemical and microbiologica considerations. In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kleinmann, B., Wolkersdorfer, C., Eds.; Springer-Verlag: Berlin, Germany, 2013; pp. 315–342. ISBN 9783642293832. [Google Scholar]
- Spiteri, C.; Van Cappellen, P.; Regnier, P. Surface complexation effects on phosphate adsorption to ferric iron oxyhydroxides along pH and salinity gradients in estuaries and coastal aquifers. Geochim. Cosmochim. Acta 2008, 72, 3431–3445. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. The microbiology of acidic mine waters. Res. Microbiol. 2003, 154, 466–473. [Google Scholar] [CrossRef]
- Johnson, D.B.; Aguilera, A. The Microbiology of Extremely Acidic Environments. In Manual of Environmental Microbiology; Yates, M., Nakatsu, C., Miller, R., Pillai, S., Eds.; ASM Press: Washington, DC, USA, 2015; pp. 4.3.1-1–4.3.1-24. [Google Scholar]
- González-Toril, E.; Llobet-Brossa, E.; Casamayor, E.O.; Amann, R.; Amils, R. Microbial Ecology of an Extreme Acidic Environment, the Tinto River. Appl. Environ. Microbiol. 2003, 69, 4853–4865. [Google Scholar] [CrossRef]
- Sand, W.; Gehrke, T.; Jozsa, P.-G.; Schippers, A. (Bio)chemistry of bacterial leaching—direct vs. indirect bioleaching. Hydrometallurgy 2001, 59, 159–175. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sánchez-Andrea, I. Dissimilatory reduction of sulfate and zero-valent sulfur at low pH and its significance for bioremediation and metal recovery. Adv. Microb. Physiol. 2019, 75, 205–231. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Nancucheo, I.; Bitencourt, J.A.P.; Sahoo, P.K.; Alves, J.O.; Siqueira, J.O.; Oliveira, G. Recent Developments for Remediating Acidic Mine Waters Using Sulfidogenic Bacteria. Biomed Res. Int. 2017, 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kim, H.; Kakegawa, T.; Hanada, S. A novel lineage of sulfate-reducing microorganisms: Thermodesulfobiaceae fam. nov., Thermodesulfobium narugense, gen. nov., sp. nov., a new thermophilic isolate from a hot spring. Extremophiles 2003, 7, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Frolov, E.N.; Kublanov, I.V.; Toshchakov, S.V.; Samarov, N.I.; Novikov, A.A.; Lebedinsky, A.V.; Bonch-Osmolovskaya, E.A.; Chernyh, N.A. Thermodesulfobium acidiphilum sp. nov., a thermoacidophilic, sulfate-reducing, chemoautotrophic bacterium from a thermal site. Int. J. Syst. Evol. Microbiol. 2017, 67, 1482–1485. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Stams, A.J.M.; Hedrich, S.; Ňancucheo, I.; Johnson, D.B. Desulfosporosinus acididurans sp. nov.: An acidophilic sulfate-reducing bacterium isolated from acidic sediments. Extremophiles 2015, 19, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Alazard, D.; Joseph, M.; Battaglia-Brunet, F.; Cayol, J.L.; Ollivier, B. Desulfosporosinus acidiphilus sp. nov.: A moderately acidophilic sulfate-reducing bacterium isolated from acid mining drainage sediments. Extremophiles 2010, 14, 305–312. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Stams, A.J.M.M.; Amils, R.; Sanz, J.L. Enrichment and isolation of acidophilic sulfate-reducing bacteria from Tinto River sediments. Environ. Microbiol. Rep. 2013, 5, 672–678. [Google Scholar] [CrossRef]
- Koschorreck, M. Microbial sulphate reduction at a low pH. FEMS Microbiol. Ecol. 2008, 64, 329–342. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Ilin, A.; van der Graaf, C.; Sánchez-Andrea, I. Microbial geochemistry of the acidic saline pit lake of Brunita mine (La Unión, SE Spain). Mine Water Environ. 2020, 1–21. [Google Scholar] [CrossRef]
- DeWeerd, K.A.; Mandelco, L.; Tanner, R.S.; Woese, C.R.; Suflita, J.M. Desulfomonile tiedjei gen. nov. and sp. nov., a novel anaerobic, dehalogenating, sulfate-reducing bacterium. Arch. Microbiol. 1990, 154, 23–30. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Cole, J.R.; Sun, B. Desulfomonile limimaris sp. nov., an anaerobic dehalogenating bacterium from marine sediments. Int. J. Syst. Evol. Microbiol. 2015, 51, 365–371. [Google Scholar] [CrossRef][Green Version]
- Tornos, F.; González Clavijo, E.; Spiro, B. The Filon Norte orebody (Tharsis, Iberian Pyrite Belt): A proximal low-temperature shale-hosted massive sulphide in a thin-skinned tectonic belt. Miner. Depos. 1997, 33, 150–169. [Google Scholar] [CrossRef]
- Pinedo Vara, I. Piritas De Huelva: Su Historia, Minería Y Aprovechamiento; Editorial Summa: Madrid, Spain, 1963. [Google Scholar]
- Checa Espinosa, M.; Carrasco Martiañez, I.; Muñoz López-Astilleros, A.; Gómez Delgado, B. Minería y actualidad en la Faja Pirítica Ibérica. Bocamina 2000, 5, 62–92. [Google Scholar]
- López-Pamo, E.; Sánchez-España, J.; Diez, M.; Santofimia, E.; Reyes, J. Cortas mineras inundadas de la Faja Pirítica: Inventario e hidroquímica. Inst. Geológico y Min. España, Ser. Medio Ambient. 2009. [Google Scholar]
- Klinger, M. More features, more tools, more CrysTBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Poncheewin, W.; Hermes, G.D.A.; van Dam, J.C.J.; Koehorst, J.J.; Smidt, H.; Schaap, P.J. NG-Tax 2.0: A semantic framework for high-throughput amplicon analysis. Front. Genet. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Quast, C.; Klindworth, A.; Pruesse, E.; Schweer, T.; Horn, M.; Glo, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Reseach 2013, 41, 1–11. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “all-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, 643–648. [Google Scholar] [CrossRef]
- Lahti, L.; Shetty, S. Tools for microbiome analysis in R 2017. Available online: https://bioconductor.org/packages/release/bioc/html/microbiome.html (accessed on 20 March 2020).
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Kassambara, A. ggpubr:“ggplot2” based publication ready plots. 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 20 March 2020).
- Bale, N.J.; Hopmans, E.C.; Schoon, P.L.; de Kluijver, A.; Downing, J.A.; Middelburg, J.J.; Sinninghe Damsté, J.S.; Schouten, S. Impact of trophic state on the distribution of intact polar lipids in surface waters of lakes. Limnol. Oceanogr. 2016, 61, 1065–1077. [Google Scholar] [CrossRef]
- Sturt, H.F.; Summons, R.E.; Smith, K.; Elvert, M.; Hinrichs, K.U. Intact polar membrane lipids in prokaryotes and sediments deciphered by high-performance liquid chromatography/electrospray ionization multistage mass spectrometry - New biomarkers for biogeochemistry and microbial ecology. Rapid Commun. Mass Spectrom. 2004, 18, 617–628. [Google Scholar] [CrossRef]
- Brandsma, J.; Hopmans, E.C.; Brussaard, C.P.D.; Witte, H.J.; Schouten, S.; Damsté, J.S.S. Spatial distribution of intact polar lipids in North Sea surface waters: Relationship with environmental conditions and microbial community composition. Limnol. Oceanogr. 2012, 57, 959–973. [Google Scholar] [CrossRef]
- Moore, E.K.; Hopmans, E.C.; Rijpstra, W.I.C.; Villanueva, L.; Sinninghe Damsté, J.S. Elucidation and identification of amino acid containing membrane lipids using liquid chromatography/high-resolution mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Sanz, J.L.; Stams, A.J.M. Microbacter margulisiae gen. nov., sp. nov., a novel propionigenic bacterium isolated from sediments of an acid rock drainage pond. Int. J. Syst. Evol. Microbiol. 2014, 64, 3936–3942. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. Environ. geochemistry Miner. Depos. 1999, 133–160. [Google Scholar]
- Sánchez España, J.; López Pamo, E.; Santofimia, E.; Aduvire, O.; Reyes, J.; Barettino, D. Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications. Appl. Geochem. 2005, 20, 1320–1356. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Advances in the hydrogeochemistry and microbiology of acid mine waters. Int. Geol. Rev. 2000, 42, 499–515. [Google Scholar] [CrossRef]
- McNaughton, S.; Wolf, L. Dominance and the Niche in Ecological Systems. J. Clin. Endocrinol. Metab. 1970, 167, 131–199. [Google Scholar]
- Sánchez-España, J.; López-Pamo, E.; Diez-Ercilla, M.; Santofimia, E. Physico-chemical gradients and meromictic stratification in Cueva de la Mora and other acidic pit lakes of the Iberian Pyrite Belt. Mine Water Environ. 2009, 28, 15–29. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Gray, J.; Burgos, W.D. Geochemistry of dissolved aluminum at low pH: Extent and significance of Al–Fe(III) coprecipitation below pH 4.0. Geochim. Cosmochim. Acta 2016, 175, 128–149. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Diez-Ercilla, M. Schwertmannite and hydrobasaluminite: A re-evaluation of their solubility and control on the iron and aluminium concentration in acidic pit lakes. Appl. Geochem. 2011, 26, 1752–1774. [Google Scholar] [CrossRef]
- Sánchez-España, J.; López Pamo, E.; Santofimia Pastor, E.; Reyes Andrés, J.; Martín Rubí, J.A. The impact of acid mine drainage on the water quality of the Odiel river (Huelva, Spain): Evolution of precipitate mineralogy and aqueous geochemistry along the Concepción-Tintillo segment. Water. Air. Soil Pollut. 2006, 173, 121–149. [Google Scholar] [CrossRef]
- Sand, W.; Gerke, T.; Hallmann, R.; Schippers, A. Sulfur chemistry, biofilm, and the (in)direct attack mechanism — a critical evaluation of bacterial leaching. Appl. Microbiol. Biotechnol. 1995, 43, 961–966. [Google Scholar] [CrossRef]
- Hippe, H. Leptospirillum gen. nov. (ex Markosyan 1972), nom. rev., including Leptospirillum ferrooxidans sp. nov. (ex Markosyan 1972), nom. rev. and Leptospirillum thermoferrooxidans sp. nov. (Golovacheva et al. 1992). Int. J. Syst. Evol. Microbiol. 2000, 50, 501–503. [Google Scholar] [CrossRef]
- Christel, S.; Herold, M.; Bellenberg, S.; El Hajjami, M.; Buetti-Dinh, A.; Pivkin, I.V.; Sand, W.; Wilmes, P.; Poetsch, A.; Dopson, M. Multi-omics Reveals the Lifestyle of the Acidophilic, Mineral-Oxidizing Model Species Leptospirillum ferriphilum T. Appl. Environ. Microbiol. 2017, 84, e02091-17. [Google Scholar] [CrossRef]
- González-Toril, E.; Aguilera, A.; Rodriguez, N.; Fernández-Remolar, D.; Gómez, F.; Diaz, E.; García-Moyano, A.; Sanz, J.L.; Amils, R. Microbial ecology of Río Tinto, a natural extreme acidic environment of biohydrometallurgical interest. Hydrometallurgy 2010, 104, 329–333. [Google Scholar] [CrossRef]
- Bond, P.L.; Smriga, S.P.; Banfield, J.F. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 2000, 66, 3842–3849. [Google Scholar] [CrossRef]
- Bond, P.L.; Druschel, G.K.; Banfield, J.F. Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems. Appl. Environ. Microbiol. 2000, 66, 4962–4971. [Google Scholar] [CrossRef]
- Rohwerder, T.; Janosch, C.; Sand, W. Elemental Sulfur Oxidation in Acidiphilium spp. Adv. Mater. Res. 2007, 20, 583. [Google Scholar] [CrossRef]
- Okamura, K.; Kawai, A.; Wakao, N.; Yamada, T.; Hiraishi, A. Acidiphilium iwatense sp. nov., isolated from an acid mine drainage treatment plant, and emendation of the genus Acidiphilium. Int. J. Syst. Evol. Microbiol. 2015, 65, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Nagashima, K.V.P.; Matsuura, K.; Shimada, K.; Takaichi, S.; Wakao, N.; Katayama, Y. Phylogeny and photosynthetic features of Thiobacillus acidophilus and related acidophilic bacteria: Its transfer to the genus Acidiphilium as Acidiphilium acidophilum comb. nov. Int. J. Syst. Bacteriol. 1998, 48, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.; Waidner, B.; Itoh, T.; Schumann, P.; Spring, S.; Gescher, J. Metallibacterium scheffleri gen. nov., sp. nov., an alkalinizing gammaproteobacterium isolated from an acidic biofilm. Int. J. Syst. Evol. Microbiol. 2013, 63, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Falagán, C.; Johnson, D.B. Acidibacter ferrireducens gen. nov., sp. nov.: An acidophilic ferric iron-reducing gammaproteobacterium. Extremophiles 2014, 18, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Hedrich, S.; Johnson, D.B. Acidocella aromatica sp. nov.: An acidophilic heterotrophic alphaproteobacterium with unusual phenotypic traits. Extremophiles 2013, 17, 841–850. [Google Scholar] [CrossRef]
- Auld, R.R.; Myre, M.; Mykytczuk, N.C.S.; Leduc, L.G.; Merritt, T.J.S. Characterization of the microbial acid mine drainage microbial community using culturing and direct sequencing techniques. J. Microbiol. Methods 2013, 93, 108–115. [Google Scholar] [CrossRef]
- Grettenberger, C.L.; Mccauley Rench, R.L.; Gruen, D.S.; Mills, D.B.; Carney, C.; Brainard, J.; Hamasaki, H.; Ramirez, R.; Watanabe, Y.; Amaral-Zettler, L.A.; et al. Microbial population structure in a stratified, acidic pit lake in the Iberian Pyrite Belt. Geomicrobiol. J. 2020, 0, 1–12. [Google Scholar] [CrossRef]
- Falagán, C.; Sánchez-España, F.J.; Johnson, D.B. Microbiological Communities in Two Acidic Mine Pit Lakes in the Iberian Pyrite Belt (IPB), Spain. Adv. Mater. Res. 2013, 825, 19–22. [Google Scholar] [CrossRef]
- Florentino, A.P.; Weijma, J.; Stams, A.J.M.; Sánchez-Andrea, I. Sulfur Reduction in Acid Rock Drainage Environments. Environ. Sci. Technol. 2015, 49, 11746–11755. [Google Scholar] [CrossRef]
- Janssen, P.H.; Schuhmann, A.; Bak, F.; Liesack, W. Disproportionation of inorganic sulfur compounds by the sulfate-reducing bacterium Desulfocapsa thiozymogenes gen, nov., sp. nov. Arch. Microbiol. 1996, 166, 184–192. [Google Scholar] [CrossRef]
- Finster, K.; Liesack, W.; Thamdrup, B. Elemental sulfur and thiosulfate disproportionation by Desulfocapsa sulfoexigens sp. nov., a new anaerobic bacterium isolated from marine surface sediment. Appl. Environ. Microbiol. 1998, 64, 119–125. [Google Scholar] [CrossRef]
- Zillig, W.; Yeats, S.; Holz, I.; Böck, A.; Rettenberger, M.; Gropp, F.; Simon, G. Desulfurolobus ambivalens, gen. nov., sp. nov., an autotrophic archaebacterium facultatively oxidizing or reducing sulfur. Syst. Appl. Microbiol. 1986, 8, 197–203. [Google Scholar] [CrossRef]
- Plumb, J.J.; Haddad, C.M.; Gibson, J.A.E.; Franzmann, P.D. Acidianus sulfidivorans sp. nov., an extremely acidophilic, thermophilic archaeon isolated from a solfatara on Lihir Island, Papua New Guinea, and emendation of the genus description. Int. J. Syst. Evol. Microbiol. 2007, 57, 1418–1423. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, R.; Yu, Y.; Jin, D.; Liang, C.; Yi, Y.; Zhu, W.; Xia, J. A novel acidophilic, thermophilic iron and sulfur-oxidizing archaeon isolated from a hot spring of Tengchong, Yunnan, China. Brazilian J. Microbiol. 2011, 42, 514–525. [Google Scholar] [CrossRef]
- Kletzin, A.; Urich, T.; Müller, F.; Bandeiras, T.M.; Gomes, C.M. Dissimilatory oxidation and reduction of elemental sulfur in thermophilic Archaea. J. Bioenerg. Biomembr. 2004, 36, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Kletzin, A. Oxidation of sulfur and inorganic sulfur compounds in Acidianus ambivalens. In Microbial Sulfur Metabolism; Springer: Berlin/Heidelberg, Germany, 2007; pp. 184–201. [Google Scholar]
- Amenabar, M.J.; Boyd, E.S. Mechanisms of mineral substrate acquisition in a thermoacidophile. Appl. Environ. Microbiol. 2018, 84, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Segerer, A.; Langworthy, T.A.; Stetter, K.O. Thermoplasma acidophilum and Thermoplasma volcanium sp. nov. from Solfatara Fields. Syst. Appl. Microbiol. 1988, 10, 161–171. [Google Scholar] [CrossRef]
- Druschel, G.K.; Baker, B.J.; Gihring, T.M.; Banfield, J.F. Acid mine drainage biogeochemistry at Iron Mountain, California. Geochem. Trans. 2004, 5, 13. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Rojas-Ojeda, P.; Amils, R.; Sanz, J.L. Screening of anaerobic activities in sediments of an acidic environment: Tinto River. Extremophiles 2012, 16, 829–839. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodríguez, N.; Díaz, E.E.; Amils, R. Methanogenesis in the sediments of Rio Tinto, an extreme acidic river. Environ. Microbiol. 2011, 13, 2336–2341. [Google Scholar] [CrossRef]
- Bräuer, S.L.; Cadillo-Quiroz, H.; Ward, R.J.; Yavitt, J.B.; Zinder, S.H. Methanoregula boonei gen. nov., sp. nov., an acidiphilic methanogen isolated from an acidic peat bog. Int. J. Syst. Evol. Microbiol. 2011, 61, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Sprott, G.D.; Fein, J.E. Isolation and characterization of Methanobacterium espanolae sp. nov., a mesophilic, moderately acidiphilic methanogen. Int. J. Syst. Bacteriol. 1990, 40, 12–18. [Google Scholar] [CrossRef]
- Savant, D.V.; Shouche, Y.S.; Prakash, S.; Ranade, D.R. Methanobrevibacter acididurans sp. nov., a novel methanogen from a sour anaerobic digester. Int. J. Syst. Evol. Microbiol. 2002, 52, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Rahim, R.A.; Abdullah, N.; Wright, A.D.G.; Shirai, Y.; Sakai, K.; Sulaiman, A.; Hassan, M.A. Importance of the methanogenic archaea populations in anaerobic wastewater treatments. Process Biochem. 2010, 45, 1214–1225. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Ertefai, T.F.; Fisher, M.C.; Fredricks, H.F.; Lipp, J.S.; Pearson, A.; Birgel, D.; Udert, K.M.; Cavanaugh, C.M.; Gschwend, P.M.; Hinrichs, K.U. Vertical distribution of microbial lipids and functional genes in chemically distinct layers of a highly polluted meromictic lake. Org. Geochem. 2008, 39, 1572–1588. [Google Scholar] [CrossRef]
- Panevska, A.; Skočaj, M.; Križaj, I.; Maček, P.; Sepčić, K. Ceramide phosphoethanolamine, an enigmatic cellular membrane sphingolipid. Biochim. Biophys. Acta - Biomembr. 2019, 1861, 1284–1292. [Google Scholar] [CrossRef]
- Gaidos, E.; Marteinsson, V.; Thorsteinsson, T.; Jóhannesson, T.; Rúnarsson, Á.R.; Stefansson, A.; Glazer, B.; Lanoil, B.; Skidmore, M.; Han, S.; et al. An oligarchic microbial assemblage in the anoxic bottom waters of a volcanic subglacial lake. ISME J. 2009, 3, 486–497. [Google Scholar] [CrossRef]
- Dann, A.L.; Cooper, R.S.; Bowman, J.P. Investigation and optimization of a passively operated compost-based system for remediation of acidic, highly iron-and sulfate-rich industrial waste water. Water Res. 2009, 43, 2302–2316. [Google Scholar] [CrossRef]
- Lindsay, M.B.J.; Wakeman, K.D.; Rowe, O.F.; Grail, B.M.; Ptacek, C.J.; Blowes, D.W.; Johnson, D.B. Microbiology and geochemistry of mine tailings amended with organic carbon for passive treatment of pore water. Geomicrobiol. J. 2011, 28, 229–241. [Google Scholar] [CrossRef]
- Olsen, I.; Jantzen, E. Sphingolipids in bacteria and fungi. Anaerobe 2001, 7, 103–112. [Google Scholar] [CrossRef]
- Grossi, V.; Mollex, D.; Vinçon-Laugier, A.; Hakil, F.; Pacton, M.; Cravo-Laureau, C. Mono- and dialkyl glycerol ether lipids in anaerobic bacteria: Biosynthetic insights from the mesophilic sulfate reducer Desulfatibacillum alkenivorans PF2803 T. Appl. Environ. Microbiol. 2015, 81, 3157–3168. [Google Scholar] [CrossRef] [PubMed]
- Rütters, H.; Sass, H.; Cypionka, H.; Rullkötter, J. Monoalkylether phospholipids in the sulfate-reducing bacteria Desulfosarcina variabilis and Desulforhabdus amnigenus. Arch. Microbiol. 2001, 176, 435–442. [Google Scholar] [CrossRef] [PubMed]


| Polar Headgroup | PC | PG | PE | MMPE | DMPE | MGDG (6) | DGDG (4) | SQDG (3) | BL (18) | OL (8) | GDGT (14) | AR (4) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Core Structure | DAG (6) | AEG (42) | DEG (23) | DAG/AEG (3) | DEG (1) | DAG (10) | AEG (8) | DEG (6) | CR (5) | DAG/AEG (6) | CR (1) | DAG/AEG (5) | |||||||
| Location | |||||||||||||||||||
| FC 1 | 1.1 | 19.6 | 7.9 | 5 | 0.2 | 6.5 | 1.8 | nd | nd | nd | nd | 7 | 0.1 | 1.7 | 4.7 | 36.1 | 0.6 | nd | 7.5 |
| FC 15 | 1.3 | 14.4 | 3 | 1.7 | 0.4 | 16.4 | 5.8 | nd | nd | 11.7 | nd | 11.1 | 0.2 | 0.8 | 1.6 | 14 | 6.2 | 7.3 | 4.1 |
| FC 30 | 2.4 | 21.5 | 2.4 | 1.4 | 1 | 10 | 5 | nd | nd | 6.3 | nd | 10.4 | 0.3 | 0.8 | 1.5 | 12.1 | 3 | 19.3 | 2.6 |
| FC 45 | 0.9 | 44.6 | 1.4 | 0.7 | 1.2 | 3.9 | 1.9 | 3 | 4.3 | 3 | 0.7 | 4.6 | 0.4 | 0.9 | 2.5 | 12.9 | 1.9 | 9.3 | 1.8 |
| LZ 30 | 16.7 | 23.2 | 0.2 | 0.3 | 0.6 | 5.9 | 6.8 | 2.7 | nd | nd | nd | 2.9 | 1.9 | 9.1 | 0 | 6.8 | 1 | 15.2 | 6.8 |
| LZ 70 | 5.1 | 17.1 | nd | nd | 0.3 | 6.9 | 5.8 | 4.2 | nd | nd | nd | 3.7 | 2 | 12.8 | 0 | 9 | nd | 26.3 | 6.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Graaf, C.M.; Sánchez-España, J.; Yusta, I.; Ilin, A.; Shetty, S.A.; Bale, N.J.; Villanueva, L.; Stams, A.J.M.; Sánchez-Andrea, I. Biosulfidogenesis Mediates Natural Attenuation in Acidic Mine Pit Lakes. Microorganisms 2020, 8, 1275. https://doi.org/10.3390/microorganisms8091275
van der Graaf CM, Sánchez-España J, Yusta I, Ilin A, Shetty SA, Bale NJ, Villanueva L, Stams AJM, Sánchez-Andrea I. Biosulfidogenesis Mediates Natural Attenuation in Acidic Mine Pit Lakes. Microorganisms. 2020; 8(9):1275. https://doi.org/10.3390/microorganisms8091275
Chicago/Turabian Stylevan der Graaf, Charlotte M., Javier Sánchez-España, Iñaki Yusta, Andrey Ilin, Sudarshan A. Shetty, Nicole J. Bale, Laura Villanueva, Alfons J. M. Stams, and Irene Sánchez-Andrea. 2020. "Biosulfidogenesis Mediates Natural Attenuation in Acidic Mine Pit Lakes" Microorganisms 8, no. 9: 1275. https://doi.org/10.3390/microorganisms8091275
APA Stylevan der Graaf, C. M., Sánchez-España, J., Yusta, I., Ilin, A., Shetty, S. A., Bale, N. J., Villanueva, L., Stams, A. J. M., & Sánchez-Andrea, I. (2020). Biosulfidogenesis Mediates Natural Attenuation in Acidic Mine Pit Lakes. Microorganisms, 8(9), 1275. https://doi.org/10.3390/microorganisms8091275






