Adaptation of Coccomyxa sp. to Extremely Low Light Conditions Causes Deep Chlorophyll and Oxygen Maxima in Acidic Pit Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. In Situ Measurements
2.3. Field Sampling
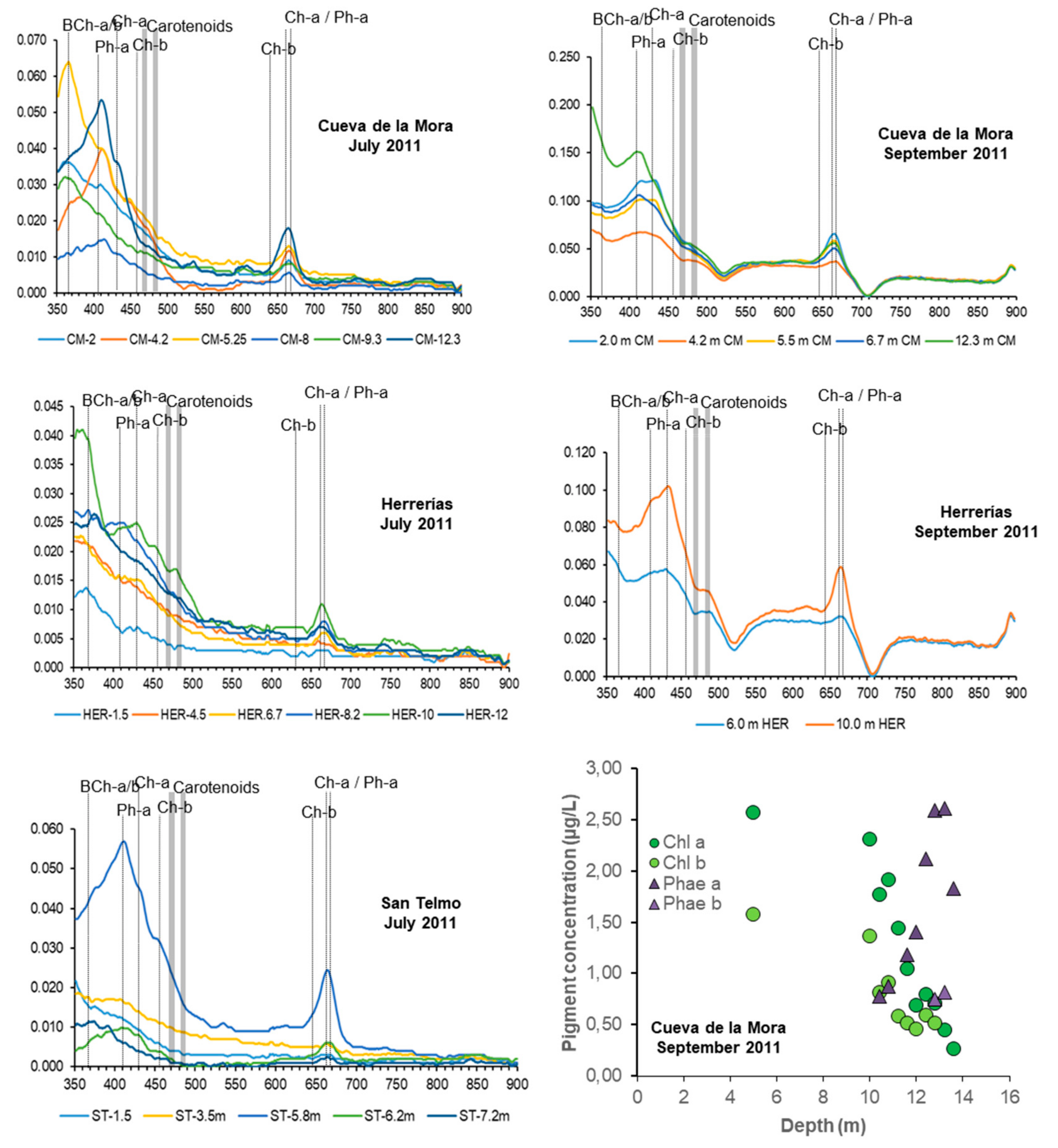
2.4. Nutrient and Iron Concentration, Pigment Analyses and Cell Counts
2.5. DNA and RNA Analyses
3. Results and Discussion
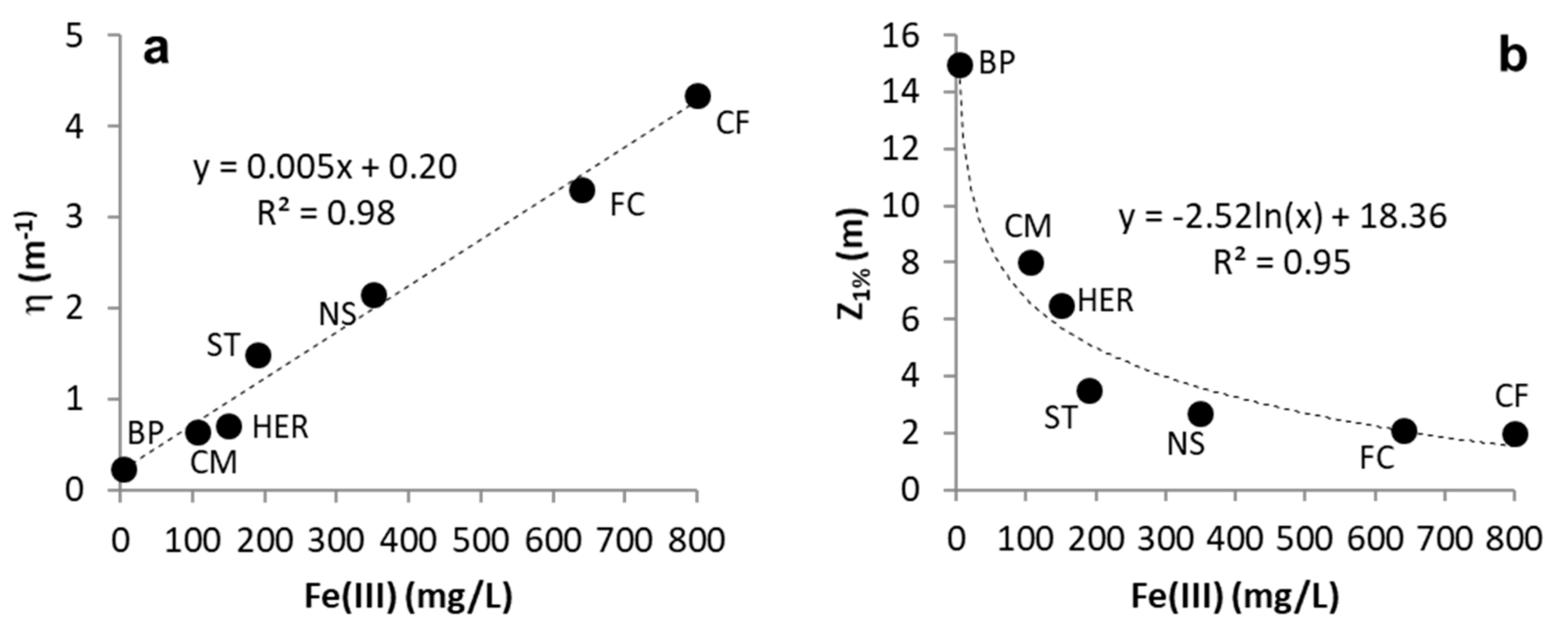
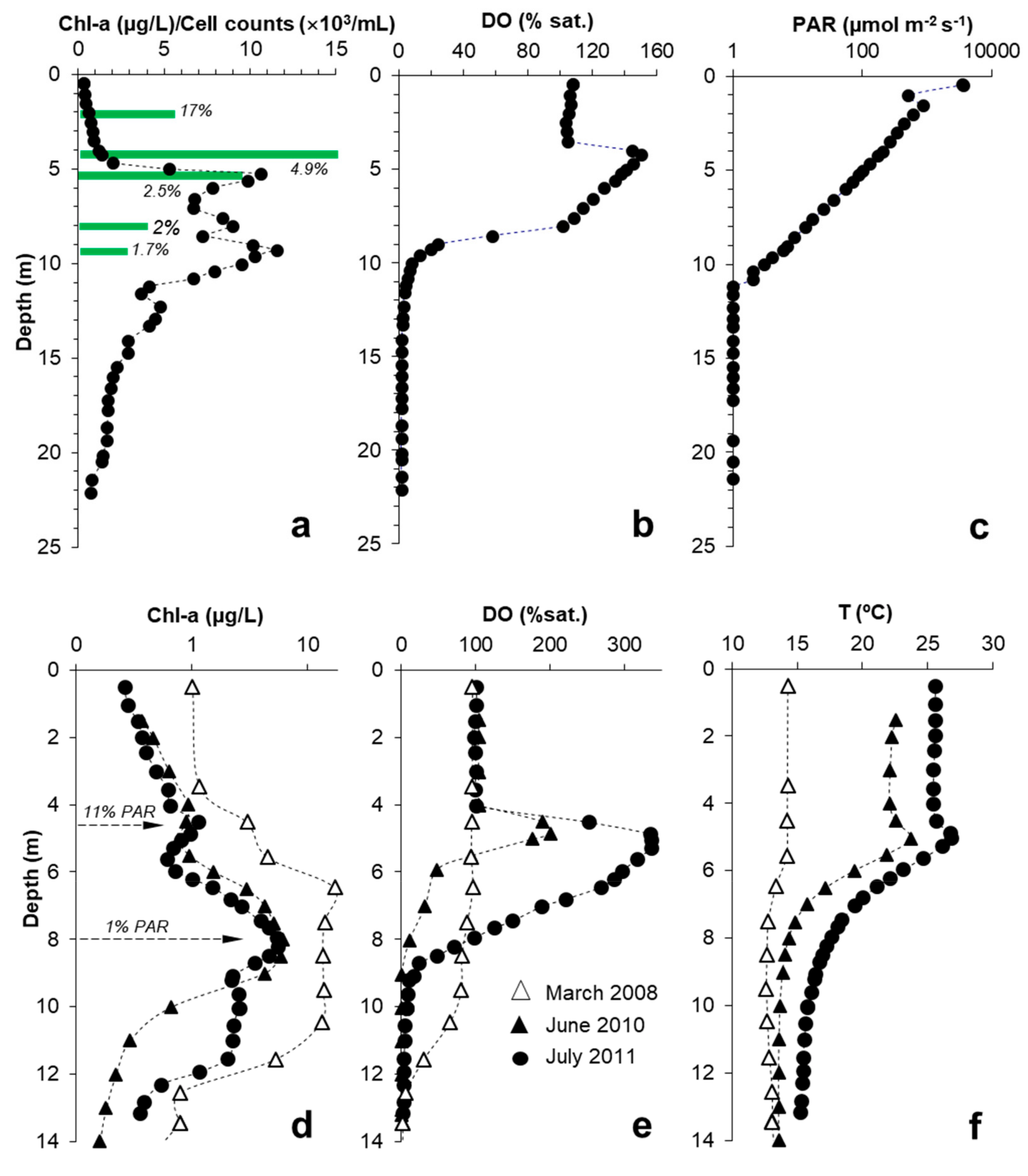
3.1. Light Attenuation by Dissolved Fe(III)
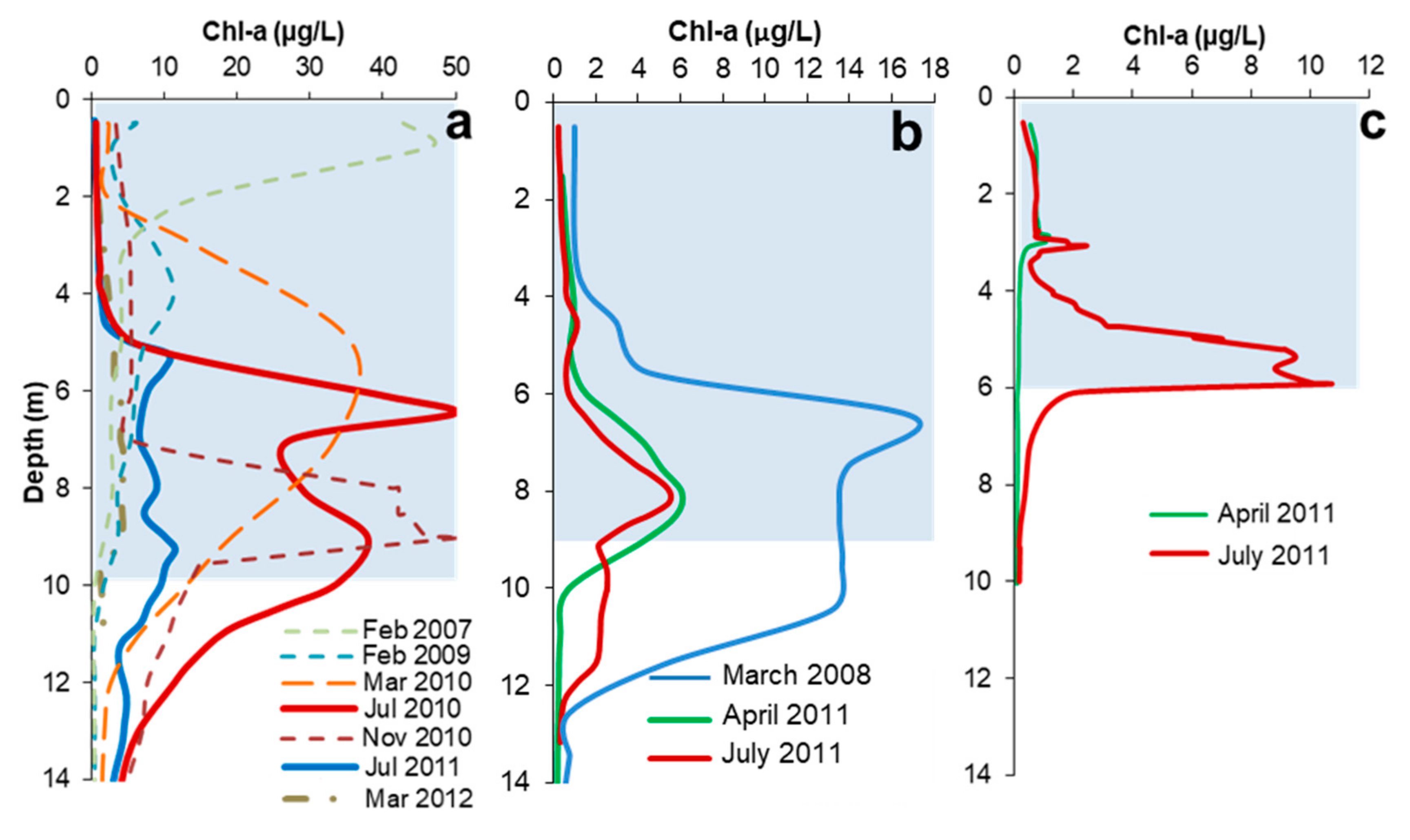
3.2. General Patterns of DCM Formation and Pigment Distribution
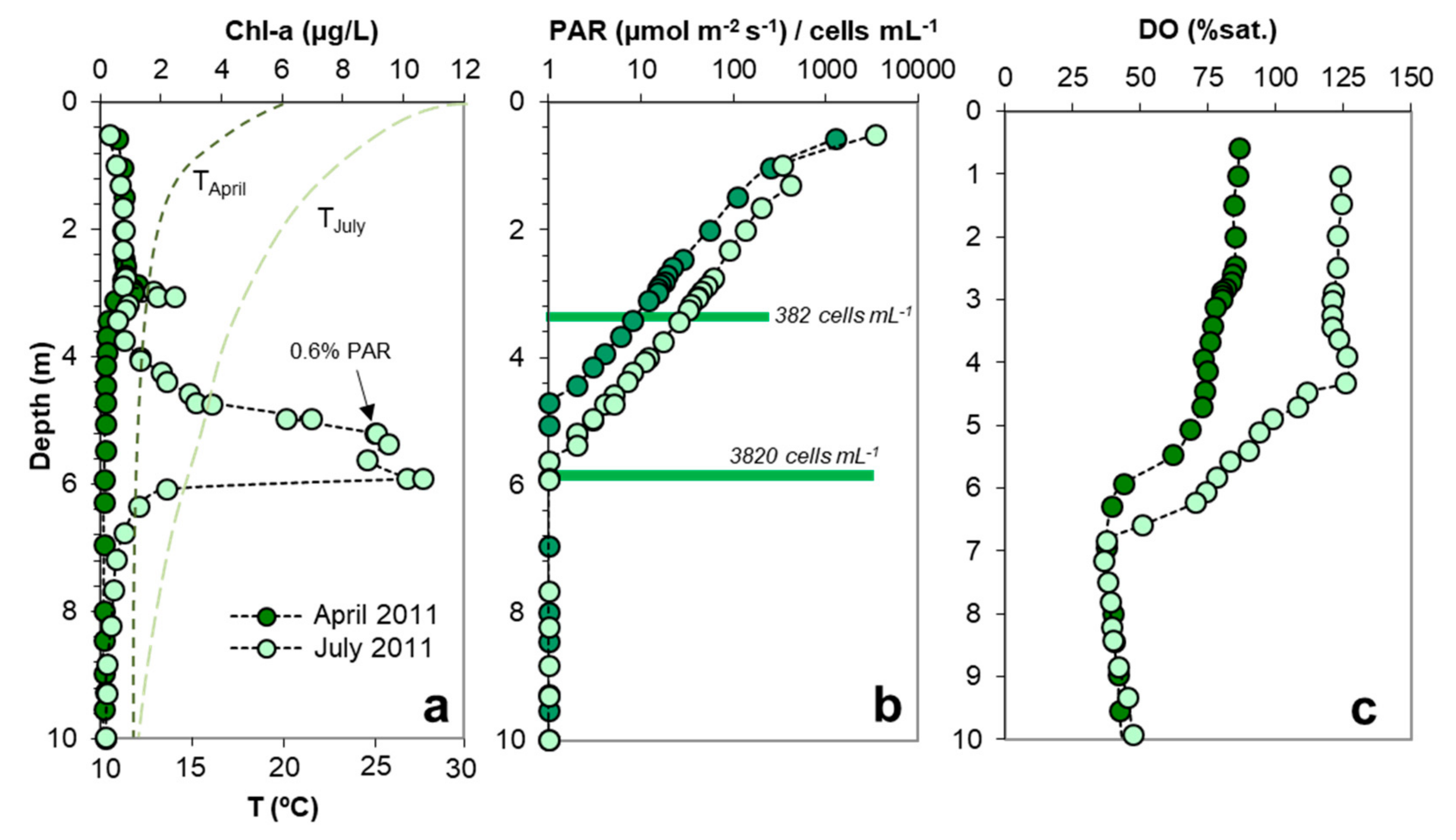
3.3. Relation of DCM with PAR
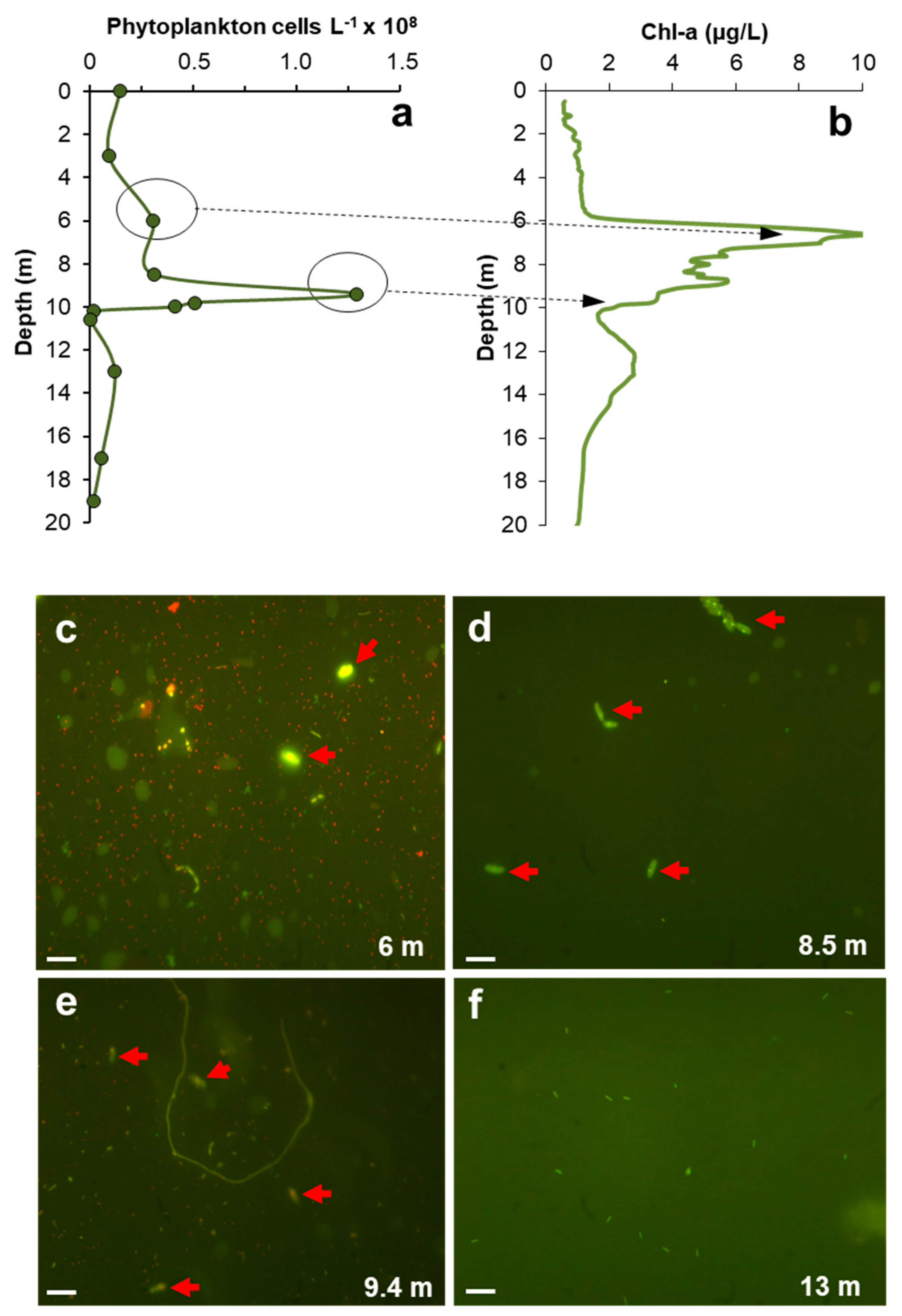
3.4. Relation between DCM, MOM and Microbial Biomass
3.5. Thermal Effects of MOM
3.6. Diurnal Phytoplankton and DO Dynamics
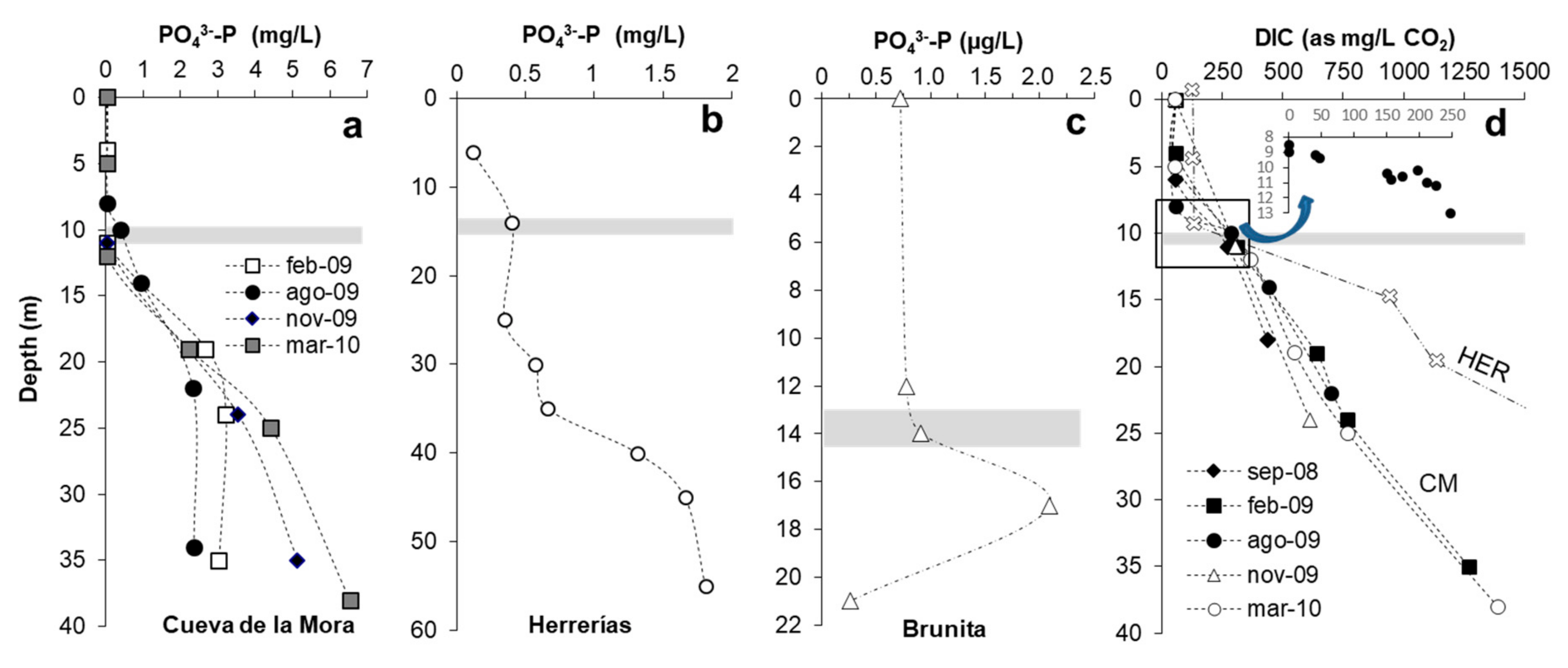
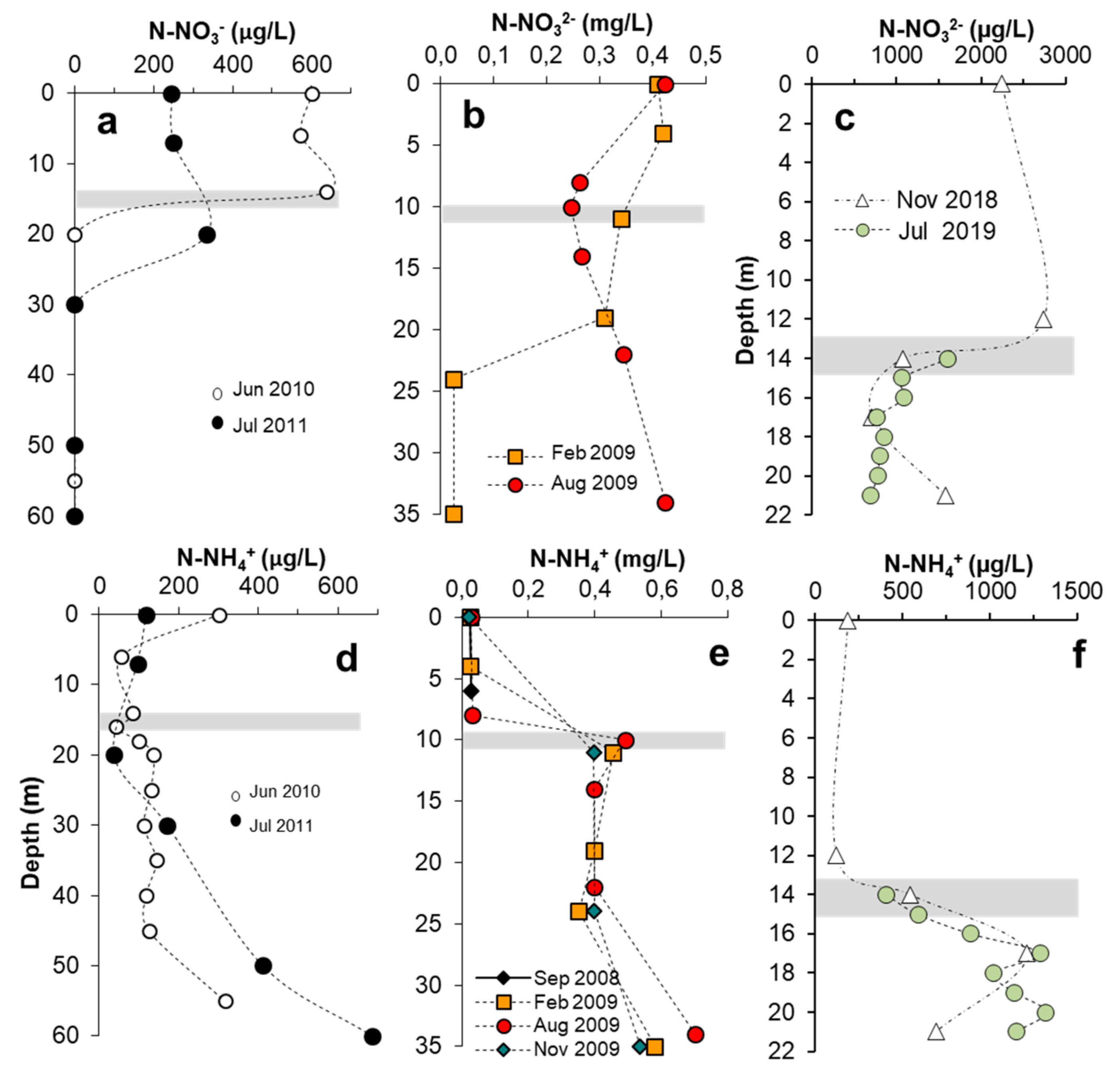
3.7. Nutrient Availability
3.7.1. Phosphorus
3.7.2. Dissolved Inorganic Carbon
3.7.3. Nitrogen
3.7.4. Silica
3.8. Phytoplankton Community Composition
3.9. General Considerations on the Phototrophic Communities and Their Role in DCM and MOM Development
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Elsevier-Academic Press: San Diego, CA, USA, 2001; p. 1001. [Google Scholar]
- Coon, T.G.; Lopez, M.M.; Richerson, P.J.; Powell, T.M.; Goldman, C.R. Summer dynamics of the deep chlorophyll maximum in Lake Tahoe. J. Plank. Res. 1987, 9, 327–344. [Google Scholar] [CrossRef]
- Leach, T.H.; Beisner, B.E.; Carey, C.C.; Pernica, P.; Rose, K.C.; Huot, Y.; Brentrup, J.A.; Domaizon, I.; Grossart, H.P.; Ibelings, B.W.; et al. Patterns and drivers of deep chlorophyll maxima structure in 100 lakes: The relative importance of light and thermal stratification. Limnol. Oceanogr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Cullen, J.J. Subsurface chlorophyll maximum layers: Enduring enigma or mystery solved? Ann. Rev. Mar. Sci. 2015, 7, 207–239. [Google Scholar] [CrossRef]
- Camacho, A. On the occurrence and ecological features of deep chlorophyll maxima (DCM) in Spanish stratified lakes. Limnetica 2006, 25, 453–478. [Google Scholar]
- Scofield, A.E.; Watkins, J.M.; Osantowski, E.; Rudstam, L.G. Deep chlorophyll maxima across a trophic state gradient: A case study in the Laurentian Great Lakes. Limnol. Oceanogr. 2020. [Google Scholar] [CrossRef]
- Wilkinson, G.M.; Cole, J.J.; Pace, M.L.; Johnson, R.A.; Kleinhans, M.J. Physical and biological contributions to metalimnetic oxygen maxima in lakes. Limnol. Oceanogr. 2015, 60, 242–251. [Google Scholar] [CrossRef]
- Miracle, M.R.; Vicente, E.; Pedrós-Alió, C. Biological studies of Spanish meromictic and stratified lakes. Limnética 1992, 8, 59–77. [Google Scholar]
- Cullen, J.J. The Deep Chlorophyll Maximum: Comparing Vertical Profiles of Chlorophyll a. Can. J. Fisher. Aquat. Sci. 1982, 39, 791–803. [Google Scholar] [CrossRef]
- Rogozin, D.; Zadereev, E.; Prokopkin, I.; Tolomeev, A.; Barkhatov, Y.; Khromechek, E.; Degermendzhi, D.; Drobotov, A.; Degermendzhi, A. Comparative study of the stability of stratification and the food web structure in the meromictic lakes Shira and Shunet (South Siberia, Russia). In Ecology of Meromictic Lakes. Ecological Studies (Analysis and Synthesis); Gulati, R., Zadereev, E., Degermendzhi, A., Eds.; Springer International Publishing: New York, NY, USA, 2017; Volume 228, pp. 89–124. [Google Scholar] [CrossRef]
- Modenutti, B.; Balseiro, E.; Callieri, C.; Queimalinos, C.; Bertoni, R. Increase in photosynthetic efficiency as a strategy of planktonic organisms exploiting deep lake layers. Freshwater Biol. 2004, 49, 160–169. [Google Scholar] [CrossRef]
- Berner, T.; Dubinsky, Z.; Whyman, K.; Falkowsky, P.G. Photoadaptation and the “package effect” in Dunaliella tertiolecta (Chlorophyceae). J. Phycol. 1989, 25, 70–78. [Google Scholar] [CrossRef]
- Rodríguez, F.; Chauton, M.; Johnsen, G.; Andresen, K.; Olsen, L.M.; Zapata, M. Photoacclimation in phytoplankton: Implications for biomass estimates, pigment functionality and chemotaxonomy. Mar. Biol. 2006, 148, 963–971. [Google Scholar] [CrossRef]
- Beamud, S.G.; Diaz, M.M.; Baccalá, N.B.; Pedrozo, F.L. Analysis of patterns of vertical and temporal distribution of phytoplankton using multifactorial analysis: Acidic Lake Caviahue, Patagonia, Argentina. Limnologica 2010, 40, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Friese, K.; Herzsprung, P.; Witter, B. Photochemical degradation of organic carbon in acidic mining lakes. Acta Hydrochim. Hydrobiol. 2002, 30, 141–148. [Google Scholar] [CrossRef]
- Nixdorf, B.; Mischke, U.; Leßmann, D. Chrysophytes and chlamydomonads: Pioneer colonists in extremely acidic mining lakes (pH <3) in Lusatia (Germany). Hydrobiologia 1998, 369, 315–327. [Google Scholar] [CrossRef]
- Gerloff-Elias, A.; Spijkerman, E.; Schubert, H. Light acclimation of Chlamydomonas acidophila accumulating in the hypolimnion of an acidic lake (pH 2.6). Freshwater Biol. 2005, 50, 1301–1314. [Google Scholar] [CrossRef]
- Kamjunke, N.; Gaedke, U.; Tittel, J.; Weithoff, G.; Bell, E.M. Strong vertical differences in the plankton composition of an extremely acidic lake. Arch. Hydrobiol. 2004, 161, 289–306. [Google Scholar] [CrossRef]
- Soria-Píriz, S.; Lara, M.; Jiménez-Arias, J.L.; Papaspyrou, S.; Úbeda, B.; Garcia-Robledo, E.; Bohórquez, J.; Gálvez, J.A.; Revsbech, N.P.; Corzo, A. What supports the deep chlorophyll maximum in acidic lakes? The role of the bacterial CO2 production in the hypolimnion. Limnol. Oceanogr. 2019. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-España, J.; López-Pamo, E.; Santofimia, E.; Diez-Ercilla, M. The acidic mine pit lakes of the Iberian Pyrite Belt: An approach to their physical limnology and hydrogeochemistry. Appl. Geochem. 2008, 23, 1260–1287. [Google Scholar] [CrossRef]
- López-Pamo, E.; Sánchez-España, J.; Diez, M.; Santofimia, E.; Reyes, J. Cortas mineras inundadas de la Faja Pirítica: Inventario e hidroquímica. Ser. Medio Ambient; IGME: Madrid, Spain, 2009. [Google Scholar]
- Sánchez-España, J.; López-Pamo, E.; Diez, M.; Santofimia, E. Physico-chemical gradients and meromictic stratification in Cueva de la Mora and other acidic pit lakes of the Iberian Pyrite Belt. Mine Water Environ. 2009, 28, 15–19. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Diez, M.; Santofimia, E. Mine pit lakes of the Iberian Pyrite Belt: Some basic limnological, hydrogeochemical and microbiological con-siderations. In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kleinmann, B., Wolkersdorfen, C., Eds.; Springer: Heidelberg, Germany, 2013; pp. 315–342. [Google Scholar]
- Diez-Ercilla, M.; Sánchez-España, J.; Yusta, I.; Wendt-Potthoff, K.; Koschorreck, M. Formation of biogenic sulfides in the water column of an acidic pit lake: Biogeochemical controls and effects on trace metal dynamics. Biogeochemistry 2014, 121, 519–536. [Google Scholar] [CrossRef]
- Falagán, C.; Sánchez-España, J.; Johnson, D.B. New insights into the biogeochemistry of extremely acidic environments revealed by a combined cultivation-based and culture-independent study of two stratified pit lakes. FEMS Microbiol. Ecol. 2014, 87, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ercilla, M.; López-Pamo, E.; Sánchez-España, J. The photoreduction of Fe(III) in the acidic mine pit lake of San Telmo (Huelva, Spain): Field and laboratory work. Aquat. Geochem. 2009, 15, 391–419. [Google Scholar] [CrossRef]
- Wendt-Potthoff, K.; Koschorreck, M.; Diez, M.; Sánchez-España, J. High microbial activity in a nutrient-rich, acidic pit lake. Limnologica 2012, 42, 175–188. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Ilin, A.; Van der Graaf, C.; Sánchez-Andrea, I. Microbial Geochemistry of the Acidic Saline Pit Lake of Brunita Mine (La Unión, SE Spain). Mine Water Environ. 2020. [Google Scholar] [CrossRef]
- Arar, E.J.; Collins, G.B. Method 445.0 In Vitro Determination of Chlorophyll A and Pheophytin A in Marine and Freshwater Algae by Fluorescence; Environmental Protection Agency: Washington, DC, USA, 1997. [Google Scholar]
- Rogozin, D.Y.; Degermendzhi, A.G. Hydraulically-operated thin-layer sampler for sampling heterogeneous water columns. J. Sib. Fed. Univ. Biol. 2008, 2, 111–117. [Google Scholar]
- Hach LCK Cuvette System. Available online: https://uk.hach.com/lck (accessed on 20 May 2020).
- Li, D.; Luo, R.; Liu, C.-M.; Leung, C.-M.; Ting, H.-F.; Sadakane, K.; Yamashita, H.; Lam, T.-W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.-L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef] [Green Version]
- Karin, L.E.; Mirdita, M.; Söding, J. MetaEuk—Sensitive, high-throughput gene discovery, and annotation for large-scale eukaryotic metagenomics. Microbiome 2020, 8, 48. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Huson, D.H.; Beier, S.; Flade, I.; Górska, A.; El-Hadidi, M.; Mitra, S.; Ruscheweyh, H.-J.; Tappu, R. MEGAN Community Edition—Interactive Exploration and Analysis of Large-Scale Microbiome Sequencing Data. PLoS Comput. Biol. 2016, 12, e1004957. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG ortholog assignment based on profile HMM and adaptive score threshold. BioRxiv 2019, 36, 2251–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopylova, E.; Noe, L.; Touzet, H. SortMeRNA: Fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Lorenzen, C.J. A note on the chlorophyll and phaeophytin content of the chlorophyll maximum. Limnol. Oceanogr. 1965, 10, 482–483. [Google Scholar] [CrossRef]
- Ross, O.N.; Sharples, J. Swimming for survival: A role of phytoplankton motility in a stratified turbulent environment. J. Mar. Syst. 2008, 70, 248–262. [Google Scholar] [CrossRef]
- Sosik, H.M.; Chisholm, S.W. Chlorophyll fluorescence from single cells: Interpretation of flow cytometric signals. Limnol. Oceanogr. 1989, 34, 1749–1761. [Google Scholar] [CrossRef]
- Vincent, A.; Mueller, D.R.; Vincent, W.F. Simulated heat storage in a perennially ice-covered high Arctic lake: Sensitivity to climate change. J. Geophys. Res. 2008, 113, C04036. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.R.; Cullen, J.J.; Platt, T. Phytoplankton and thermal structure in the upper ocean: Consequences of nonuniformity in chlorophyll profile. J. Geoph. Res. 1983, 88, 2565–2570. [Google Scholar] [CrossRef]
- Langman, O.C.; Hanson, P.C.; Carpenter, S.R.; Hu, Y.H. Control of dissolved oxygen in northern temperate lakes over scales ranging from minutes to days. Aquat. Biol. 2010, 9, 193–202. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Boehrer, B.; Yusta, I. Extreme carbon dioxide concentrations in acidic pit lakes provoked by water/rock interaction. Env. Sci. Technol. 2014, 48, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Salguero, D.M.; Fernández-Niño, M.; Serrano-Bermúdez, L.M.; Páez Melo, D.O.; Winck, F.V.; Caldana, C.; González Barrios, A.F. Development of a Chlamydomonas reinhardtii metabolic network dynamic model to describe distinct phenotypes occurring at different CO2 levels. PeerJ 2018, 6, e5528. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, C.; Falagán, C.; Knoeller, K.; Schultze, M.; Koschorreck, M. No nitrification in lakes below pH 3. Environ. Sci. Technol. 2013, 47, 14018–14023. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Giordano, M. The use of NH4+ rather than NO3− affects cell stoichiometry, C allocation, photosynthesis and growth in the cyanobacterium Synechococcus sp. UTEX LB 2380, only when energy is limiting. Plant. Cell Environ. 2017, 40, 227–236. [Google Scholar] [CrossRef]
- Falagán, C. Geomicrobiology of Meromictic, Metal-Mine Pit Lakes in the Iberian Pyrite Belt and Biotechnological Applications. Ph.D. Thesis, University of the Basque Country, Bilbao, Spain, 2015; p. 220. [Google Scholar]
- Fuentes, J.L.; Huss, V.A.R.; Montero, Z.; Torronteras, R.; Cuaresma, M.; Garbayo, I.; Vílchez, C. Phylogenetic characterization and morphological and physiological aspects of a novel acidotolerant and halotolerant microalga Coccomyxa onubensis sp. nov. (Chlorophyta, Trebouxiophyceae). J. Appl. Phycol. 2016, 28, 3269–3279. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Eggert, A.; Wolf, W.; Pröschold, T. Evaluating the Species Boundaries of Green Microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) Using Integrative Taxonomy and DNA Barcoding with Further Implications for the Species Identification in Environmental Samples. PLoS ONE 2015, 10, e0127838. [Google Scholar] [CrossRef]
- Malavasi, V.; Škaloud, P.; Rindi, F.; Tempesta, S.; Paoletti, M.; Pasqualetti, M. DNA-based taxonomy in ecologically versatile microalgae: A re-evaluation of the species concept within the coccoid green algal genus Coccomyxa (Trebouxiophyceae, Chlorophyta). PLoS ONE 2016, 11, e0151137. [Google Scholar] [CrossRef]
- Barcyté, D.; Nedbalová, L. Coccomyxa: A dominant planktic alga in two acid lakes of different origin. Extremophiles 2017, 21, 245–257. [Google Scholar] [CrossRef]
- Casal, C.; Cuaresma, M.; Vega, J.M.; Vilchez, C. Enhanced Productivity of a Lutein-Enriched Novel Acidophile Microalga Grown on Urea. Mar. Drugs 2010, 9, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Lehr, C.R.; Frank, S.D.; Norris, T.B.; D’Imperio, S.; Kalinin, A.V.; Toplin, J.A.; Castenholz, R.W.; McDermott, T.R. Cyanidia (cyanidiales) population diversity and dynamics in an acid-sulfate-chloride spring in Yellowstone National Park. J. Phycol. 2007, 43, 3–14. [Google Scholar] [CrossRef]
- Ciniglia, C.; Yoon, H.S.; Pollio, A.; Pinto, G.; Bhattacharya, D. Hidden biodiversity of the extremophilic Cyanidiales red algae. Mol. Ecol. 2004, 13, 1827–1838. [Google Scholar] [CrossRef] [PubMed]
- Donachie, S.P.; Christenson, B.W.; Kunkel, D.D.; Malahoff, A.; Alam, M. Microbial community in acidic hydrothermal waters of volcanically active White Island, New Zealand. Extremophiles 2002, 6, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, F.; Marasco, R.; Rolli, E.; Daffonchio, D.; Donachie, D.; Donachie, S.; Borin, S. Microbial life in volcanic lakes. In “Volcanic Lakes”, Advances in Volcanology; Rouwet, D., Christenson, B., Tassi, F., Vandemeulebrouck, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 507–522. [Google Scholar] [CrossRef]
- Dean, A.P.; Hartley, A.; McIntosh, O.A.; Smith, A.; Feord, H.K.; Holmberg, N.H.; King, T.; Yardley, E.; White, K.N.; Pittman, J.K. Metabolic adaptation of a Chlamydomonas acidophila strain isolated from acid mine drainage ponds with low eukaryotic diversity. Sci. Total. Environ. 2019, 647, 75–87. [Google Scholar] [CrossRef]
- Nishikawa, K.; Tominaga, N. Isolation, growth, ultrastructure, and metal tolerance of the green alga, Chlamydomonas acidophila (Chlorophyta). Biosci. Biotechnol. Biochem. 2001, 65, 2650–2656. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H. The Chlamydomonas Sourcebook: Introduction to Chlamydomonas and Its Laboratory Use, 2nd ed.; Academic Press: Cambridge, MA, USA, 2009; Volume 1, p. 480. [Google Scholar]
- Lessmann, D.; Fyson, A.; Nixdorf, B. Phytoplankton of the extremely acidic mining lakes of Lusatia (Germany) with pH ≤ 3. Hydrobiologia 2000, 433, 123–128. [Google Scholar] [CrossRef]
- Santofimia, E.; González-Toril, E.; López-Pamo, E.; Gomariz, M.; Amils, R.; Aguilera, A. Microbial diversity and its relationship to physicochemical characteristics of the water in two extreme acidic pit lakes from the Iberian Pyrite Belt (SW Spain). PLoS ONE 2013, 8, e66746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilera, A.; Manrubia, S.C.; Gómez, F.; Rodríguez, N.; Amils, R. Eukaryotic community distribution and its relationship to water physicochemical parameters in an extreme acidic environment, Río Tinto (Southwestern Spain). Appl. Environ. Microbiol. 2006, 72, 5325. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, A.; Zettler, E.; Gómez, F.; Amaral-Zettler, L.; Rodríguez, N.; Amils, R. Distribution and seasonal varibility in the benthic eukaryotic community of Río Tinto (SW, Spain), an acidic, high metal extreme environment. Syst. Appl. Microbiol. 2007, 30, 531–546. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-España, J.; Santofimia, E.; López-Pamo, E. Iron terraces in acid mine drainage systems: A discussion about the organic and inorganic factors involved in their formation through observations from the Tintillo acidic river (Riotinto mine, Huelva, Spain). Geosphere 2007, 3, 133–151. [Google Scholar] [CrossRef] [Green Version]
- González-Toril, E.; Aguilera, A.; Souza-Egipsy, V.; López Pamo, E.; Sánchez-España, J.; Amils, R. Geomicrobiology of La Zarza-Perrunal acid mine effluent (Iberian Pyritic Belt, Spain). Appl. Environ. Microbiol. 2011, 77, 2685–2694. [Google Scholar] [CrossRef] [Green Version]
- Rowe, O.F.; Sánchez-España, F.J.; Hallberg, K.D.; Johnson, D.B. Microbial communities and geochemical dynamics in an extremely acidic, metal-rich stream at an abandoned sulfide mine (Huelva, Spain) underpinned by two functional primary production systems. Environ. Microbiol. 2007, 9, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Huss, V.A.R.; Ciniglia, C.; Cennamo, P.; Cozzolino, S.; Pinto, G.; Pollio, A. Phylogenetic relationships and taxonomic position of Chlorella-like isolates from low pH environments (pH <3.0). BMC Evol. Biol. 2002, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Albertano, P.; Taddei, R. Chlorella protothecoides Krüger var. acidicola, a new variety from very low pH environments. Algol. Stud. Arch. für Hydrobiol. Suppl. Vol. 1984, 67, 401–408. [Google Scholar] [CrossRef]
- Toplin, J.A.; Norris, T.B.; Lehr, C.R.; McDermott, T.R.; Castenholz, R.W. Biogeographic and phylogenetic diversity of thermoacidophilic cyanidiales in Yellowstone National Park, Japan, and New Zealand. Appl. Environ. Microbiol. 2008, 74, 2822–2833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azúa-Bustos, A.; González-Silva, C.; Mancilla, R.A.; Salas, L.; Palma, R.E.; Wynne, J.J.; McKay, C.P.; Vicuña, R. Ancient Photosynthetic Eukaryote Biofilms in an Atacama Desert Coastal Cave. Microb. Ecol. 2009, 58, 485–496. [Google Scholar] [CrossRef] [PubMed]


| Mechanism | Description | Examples | Refs. |
|---|---|---|---|
| 1 | In situ growth of phototrophs promoted by higher nutrient availability in metalimnion or upper hypolimnion | DCM formed by cryptophytes or cyanobacteria | [8] |
| 2 | Differential predation pressure between near-surface and deep layers | Grazing of Cryptomonas by zooplankton; grazing of Clamydomonas by Ochromonas | [5] |
| 3 | Depth-differential sinking of eplimnetic algae (passive or active) | DCM formed by non-motile phytoplankton (e.g., diatoms) | [9,10] |
| 4 | Symbiotic association of algae with protozoa followed by blooming in metalimnion | Symbiosis between Chlorella and ciliated protozoa | [11] |
| 5 | Photo-acclimation of phytoplankton by increased chlorophyll content per cell | Photoadaptation of Dunaliella tertiolecta; lakes with clear waters | [12,13] |
| 6 | Photo-inhibition in near-surface waters due to high UV radiation exposure | Alpine lakes with very clear waters | [11] |
| Lake | Location | Area | Depth | Volume | pH | Fe(III) |
|---|---|---|---|---|---|---|
| m2 | m | m3 | mg/L | |||
| CM | IPB, Huelva | 17,900 | 40 | 3 × 105 | 2.3–3.2* | 108 |
| HER | IPB, Huelva | 19,000 | 70 | 3 × 105 | 2.0–3.1* | 140 |
| ST | IPB, Huelva | 145,500 | 130 | 7 × 106 | 2.2–3.0* | 202 |
| BRU | La Unión, Murcia | 45,000 | 21 | 9.5 × 105 | 2.0–2.4 | 450 |
| BP | Salamanca | 38,000 | 40 | 5 × 105 | 3.5 | 2.4 |
| NS | IPB, Huelva | 7000 | 32 | 1 × 105 | 2.5–2.8 | 330 |
| FC | IPB, Huelva | 38,000 | 50 | 6 × 105 | 2.0–2.4 | 650 |
| CF | IPB, Huelva | 24,800 | 80 | 1 × 105 | 2.3–2.5 | 812 |
| Lake | Depth | Date | Microorganisms | Technique | Source |
|---|---|---|---|---|---|
| (m) | |||||
| CM | 0 | July 2007 | Chlamydomonas, Zygnematales, Ochromonas, Diatoms | Confocal microscopy | [22] |
| CM | 3 | Sept 2011, March 2012, May 2018 | Coccomyxa sp., Chlorella, Micromonas, | T-RFLP, Confocal, Microscopy, SMA | [25,51] This study |
| CM | 5 | Sept 2017 | Cyanidium caldarium, Diatoms | Cultures, confocal microscopy | This study |
| CM | 6 | Sept 2012, Sept 2017 | Coccomyxa onubensis,Auxenochlorella, Cyanidium caldarium | T-RFLP, Cultures, confocal microscopy | [25,51] This study |
| CM | 7 | Sept 2017 | Cyanidium caldarium | Cultures and confocal microscopy | This study |
| CM | 8 | Sept 2011 | Coccomyxa onubensis,Auxenochlorella, Diatoms | T-RFLP, Confocal microscopy | [25,51] |
| CM | 11 | May 2018 | Coccomyxa sp., Chlorella, Micromonas | SMA | This study |
| HER | 5 | March 2012 | Coccomyxa onubensis,Chlamydomonas, Diatoms | T-RFLP, Confocal microscopy | [25,51] |
| HER | 7 | March 2012, Sept 2012 | Coccomyxa onubensis,Chlamydomonas, Diatoms | T-RFLP, Confocal microscopy | [25,51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-España, J.; Falagán, C.; Ayala, D.; Wendt-Potthoff, K. Adaptation of Coccomyxa sp. to Extremely Low Light Conditions Causes Deep Chlorophyll and Oxygen Maxima in Acidic Pit Lakes. Microorganisms 2020, 8, 1218. https://doi.org/10.3390/microorganisms8081218
Sánchez-España J, Falagán C, Ayala D, Wendt-Potthoff K. Adaptation of Coccomyxa sp. to Extremely Low Light Conditions Causes Deep Chlorophyll and Oxygen Maxima in Acidic Pit Lakes. Microorganisms. 2020; 8(8):1218. https://doi.org/10.3390/microorganisms8081218
Chicago/Turabian StyleSánchez-España, Javier, Carmen Falagán, Diana Ayala, and Katrin Wendt-Potthoff. 2020. "Adaptation of Coccomyxa sp. to Extremely Low Light Conditions Causes Deep Chlorophyll and Oxygen Maxima in Acidic Pit Lakes" Microorganisms 8, no. 8: 1218. https://doi.org/10.3390/microorganisms8081218
APA StyleSánchez-España, J., Falagán, C., Ayala, D., & Wendt-Potthoff, K. (2020). Adaptation of Coccomyxa sp. to Extremely Low Light Conditions Causes Deep Chlorophyll and Oxygen Maxima in Acidic Pit Lakes. Microorganisms, 8(8), 1218. https://doi.org/10.3390/microorganisms8081218






