Resilience of Aspergillus westerdijkiae Strains to Interacting Climate-Related Abiotic Factors: Effects on Growth and Ochratoxin A Production on Coffee-Based Medium and in Stored Coffee
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolate and Spores Preparation
2.2. Coffee-Based Agar Media Preparation
2.3. Inoculation for In Vitro Studies on Coffee-Based Media
2.4. Comparison of the In Vitro Resilience in Relation to aw × Temperature Stress on Growth of Strains of A. westerdijkiae on Green and Roasted Coffee-Based Agar Media
2.5. In Vitro Effect of Climate Change Related Interacting Abiotic Factors on Growth and Ochratoxin A Production by the A. westerdijkiae Strains
2.6. In Situ Effect of Climate Change Abiotic Factors on Ochratoxin A Contamination of Stored Coffee Beans Inoculated with A. westerdijkiae and A. ochraceus Strains
2.7. Ochratoxin A Extraction and Quantification
2.7.1. In Vitro Studies
- Mobile Phase: Acetonitrile (57%), water (41%), acetic acid (2%)
- Column: 120CC-C18 column (Poroshell 120, length 100 mm, diameter 4.6 mm, particle size 2.7 micron; 600 Bar)
- Temperature of column: 25 °C
- Excitation: 330 nm
- Emission: 460 nm
- Flow rate: 1 mL min−l
- Volume of sample injected: 20 µL
- Retention time: Approx. 2.49 min
- Run time: 17 min
- Limit of detection: 0.01 ng g−1
- Limit of Quantification: 0.039 ng g−1
2.7.2. In Situ Ochratoxin A Quantification
2.8. Statistical Analyses
3. Results
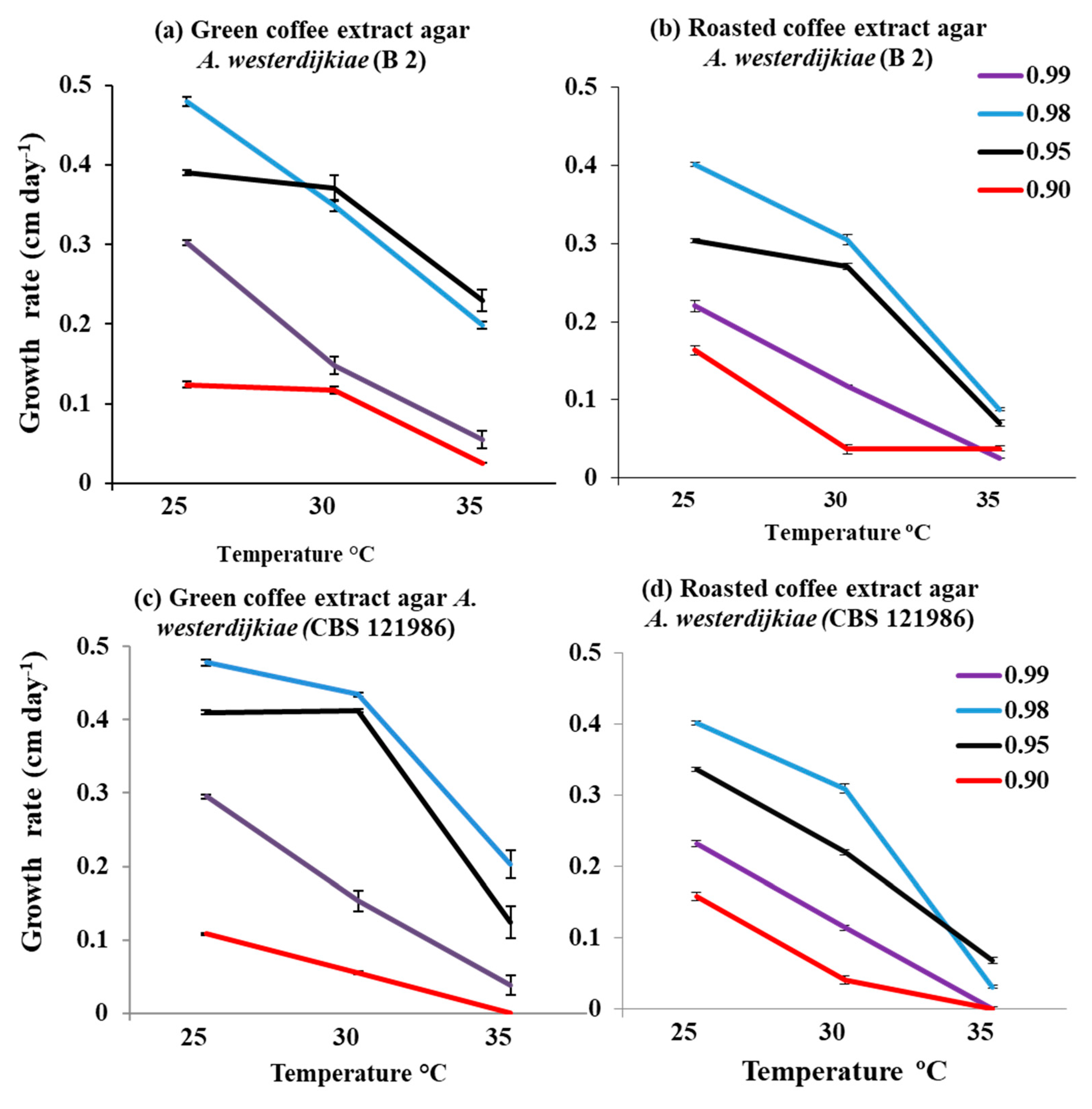
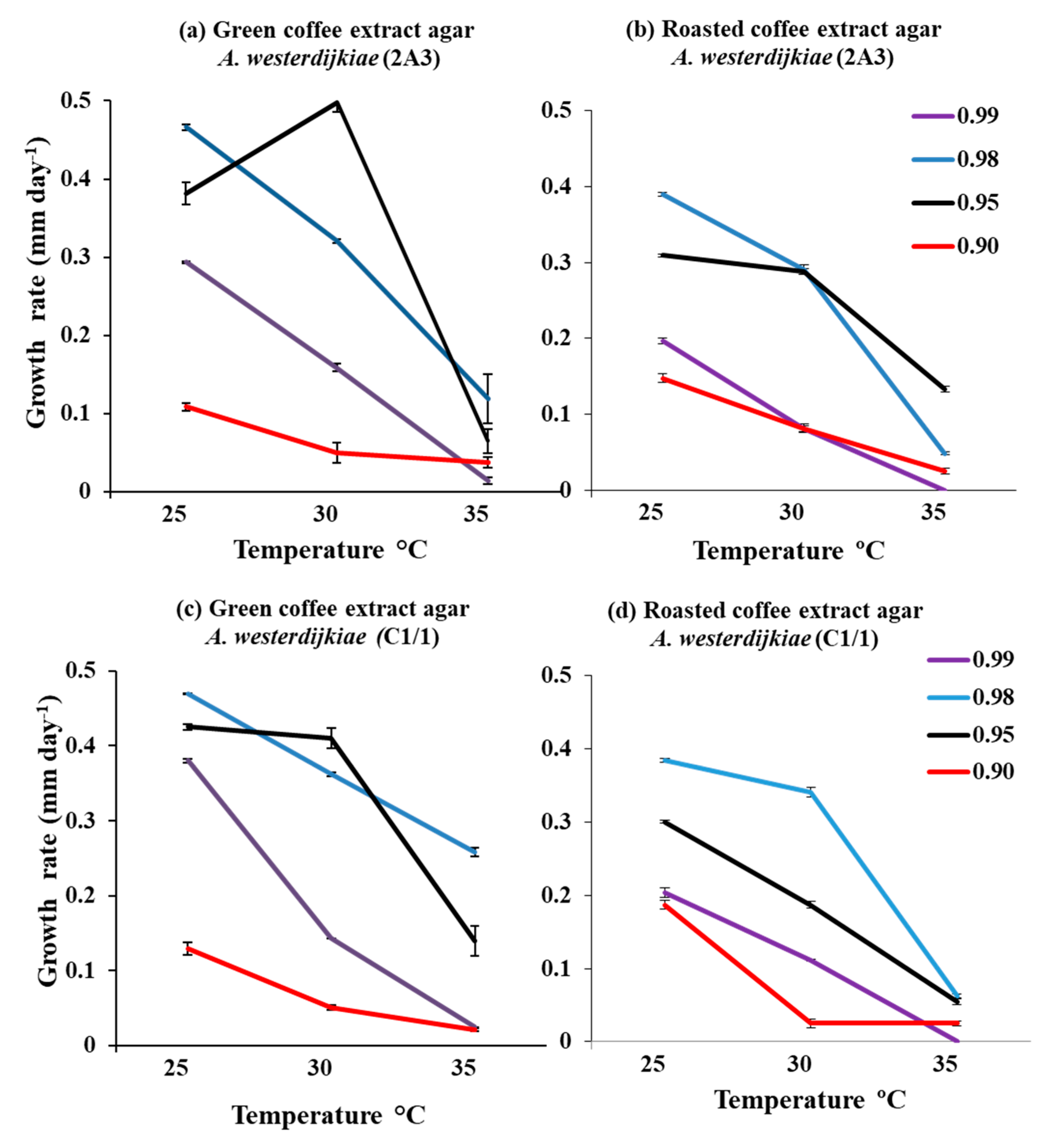
3.1. Comparison of Growth and Ochratoxin A Production by Strains of A. westerdijkiae on Green and Roasted Coffee-Based Media
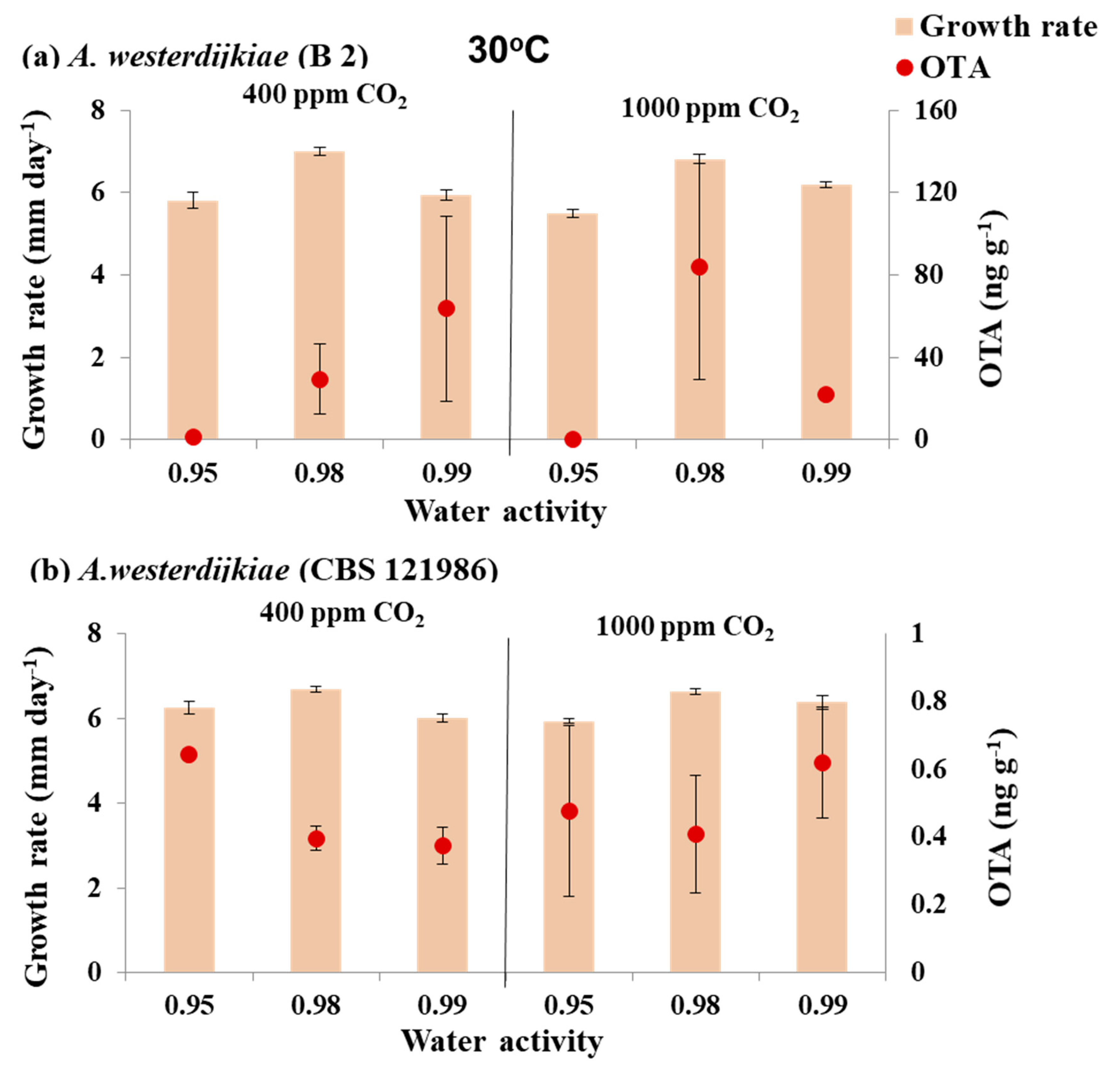
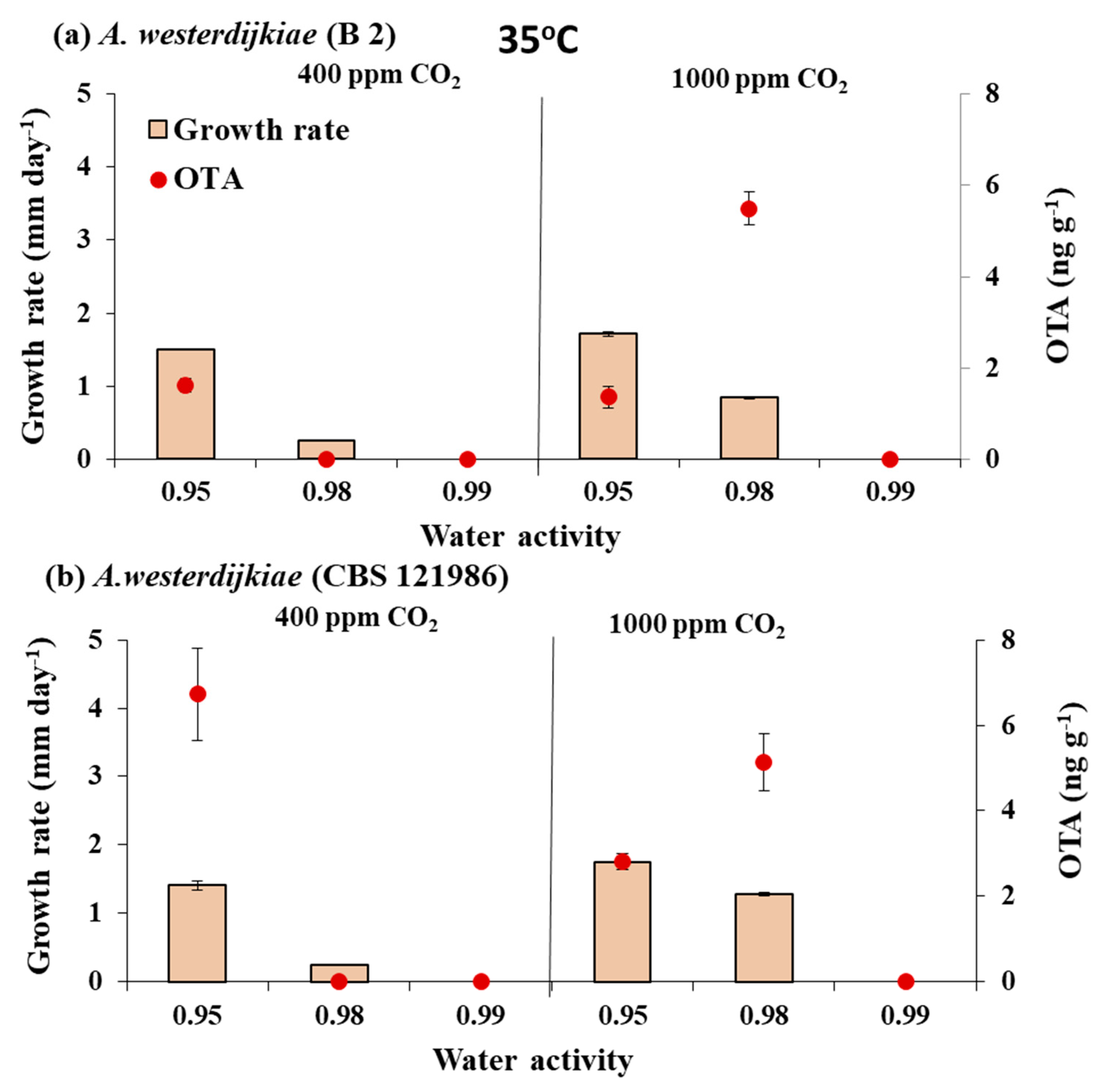
3.2. In Vitro Effect of Interacting Climate-Related Abiotic Factors of Water Activity × Elevated CO2 × Temperature on Growth and OTA Production by Strains of A. westerdijkiae and A. ochraceus
3.2.1. In Vitro Effects of Interacting Climate-Related Abiotic Factors on Growth
3.2.2. In Vitro Effects of Climate-Related Interaction of Abiotic Conditions on OTA Production
3.3. In Situ Effect of Water Activity × Elevated CO2 × Temperature on OTA Production at 30 and 35 °C in Stored Coffee Beans by A. westerdijkiae Strains
4. Discussion
4.1. In Vitro Effects of Climate-Related Abiotic Factors on Growth and OTA Production by Strains of A. westerdijkiae
4.2. In Situ Effect of Three-Way Interacting Climate-Related Abiotic Factors on OTA Production in Stored Green Coffee
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdel-Hadi, A.; Magan, N. Influence of environmental factors on growth, sporulation and ochratoxin A and B production of the new grouping of the A. ochraceus group. World Mycotoxin J. 2009, 2, 429–434. [Google Scholar] [CrossRef]
- European Commission Recommendation No 2006/576 of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9.
- Magan, N.; Medina, A. Integrating gene expression, ecology and mycotoxin production by Fusarium and Aspergillus species in relation to interacting environmental factors. World Mycotoxin J. 2016, 9, 863–874. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough. Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food. Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Wu, F.; Bhatnagar, D.; Bui-Klimke, T.; Carbone, I.; Hellmich, R.; Munkvold, G.; Paul, P.; Payne, G.; Takle, E. Climate change impacts on mycotoxin risks in US maize. World Mycotoxin J. 2011, 4, 79–93. [Google Scholar] [CrossRef]
- Akbar, A.; Medina, A.; Magan, N. Impact of climate change factors on growth and ochratoxin A production by Aspergillus sections Circumdati and Nigri species on coffee. World Mycotoxin J. 2016, 9, 863–874. [Google Scholar] [CrossRef]
- Magan, N.; Baxter, E.S. Effect of elevated CO2 and temperature on the phyllosphere mycoflora of winter wheat flag leaves during ripening. Ann. Appl. Biol. 1999, 129, 189–195. [Google Scholar] [CrossRef]
- Ramos, A.J.; Magan, N.; Sanchis, V. Osmotic and matric potential effects on growth, sclerotial production and partitioning of polyols and sugars in colonies and spores of Aspergillus ochraceus. Mycol. Res. 1999, 103, 141–147. [Google Scholar] [CrossRef]
- Abdelmohsen, S.; Verheecke-Vaessen, C.; Garcia-Cela, E.; Medina, A.; Magan, N. Solute and matric potential stress and Penicillium verrucosum: Impact on growth, gene expression and ochratoxin A production. World Mycotoxin J. 2020, 13, 345–351. [Google Scholar] [CrossRef]
- Gilbert, M.K.; Medina, A.; Mack, B.M.; Lebar, M.; Rodriguez, A.; Bhatnagar, D.; Magan, N.; Obrian, G.; Payne, G. Carbon dioxide mediates the response to temperature and water activity levels in Aspergillus flavus during infection of maize kernels. Toxins 2018, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.; Block, A.; Christensen, S.A.; Allen, L.H.; Schmelz, E.A. The effect of climate change associated abiotic stressors on phytochemical defences. Phytochem. Rev. 2017, 17, 37–49. [Google Scholar] [CrossRef]
- Vaughan, M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.; Sims, J.; Martins, V.F.; Swerbilow, J.A.Y.; Romero, M.; Alborn, H.T.; et al. Effects of elevated [CO2] on maize defense against mycotoxigenic Fusarium verticillioides. Plant. Cell Environ. 2014, 37, 2691–2706. [Google Scholar] [CrossRef] [PubMed]
- Verheecke-Vaessen, C.; Diez-Gutierrez, L.; Renaud, J.; Sumarah, M.; Medina, A.; Magan, N. Interacting climate change environmental factors effects on Fusarium langsethiae growth, expression of TRI genes and T-2/HT-2 mycotoxin production on oat-based media and in stored oats. Fungal Biol. 2019, 123, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Cabrera, H.; Taniwaki, M.H.; Menezes, H.C.; Iamanaka, B.T. The production of ochratoxin A by Aspergillus ochraceus in raw coffee at different equilibrium relative humidity and under alternating temperatures. Food Cont. 2004, 15, 531–535. [Google Scholar] [CrossRef]
- Sultan, Y.; Magan, N.; Medina, A. Comparison of five different C18 HPLC analytical columns for the analysis of ochratoxin A in different matrices. J. Chromat. B 2014, 971, 89–93. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Teixeira, A.A.; Teixeira, A.R.R.; Copetti, M.V.; Iamanaka, B.T. Ochratoxigenic fungi and ochratoxin A in defective coffee beans. Food Res. Int. 2014, 61, 161–166. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding Mycotoxin Contamination Across the Food Chain in Brazil: Challenges and Opportunities. Toxins 2019, 11, 411. [Google Scholar] [CrossRef]
- Pardo, E.; Ramos, A.J.; Sanchis, V.; Marin, S. Modelling of effects of water activity and temperature on germination and growth of ochratoxigenic isolates of Aspergillus ochraceus on a green coffee-based medium. Int. J. Food Microbiol. 2005, 98, 1–9. [Google Scholar] [CrossRef]
- Pardo, E.; Marin, S.; Ramos, A.J.; Sanchis, V. Effect of water activity and temperature on mycelial growth and Ochratoxin A production by isolates of Aspergillus ochraceus on irradiated green coffee beans. J. Food Prot. 2005, 68, 133–138. [Google Scholar] [CrossRef]
- Akbar, A.; Medina, A.; Magan, N. Efficacy of different caffeine concentrations on growth and ochratoxin A production by Aspergillus section Nigri and Circumdati species. Lett. Appl. Microbiol. 2016, 63, 25–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bucheli, P.; Taniwaki, M.H. Research on the origin, and on the impact of post-harvest handling and manufacturing on the presence of ochratoxin A in coffee. Food Addit. Contam. 2002, 19, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Cairns-Fuller, V.; Aldred, D.; Magan, N. Water, temperature and gas composition interactions affect growth and ochratoxin A production by isolates of Penicillium verrucosum on wheat grain. J. Appl. Microbiol. 2005, 99, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Paster, N.; Lisker, N.; Chet, I. Ochratoxin A pro-duction by Aspergillus ochraceus Wilhelm grown undercontrolled atmospheres. Appl. Environ. Microbiol. 1983, 45, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, M.; Dekanea, A.; Mitchell, D.; Lydakis, D.; Magan, N. Efficacy of sulphur dioxide, controlled atmospheres and water availability on in vitro germination, growth and ochratoxin A production by strains of Aspergillus carbonarius from grapes and vine fruits. Postharvest Biol. Technol. 2007, 44, 141–149. [Google Scholar] [CrossRef]
- Magan, N.; Lacey, J. The effect of gas composition and water activity on growth of field and storage fungi and their interactions. Trans. British. Mycol. Soc. 1984, 82, 305–314. [Google Scholar] [CrossRef]
- Valero, A.; Begum, M.; Hocking, A.D.; Marín, S.; Ramos, A.J.; Sanchis, V. Mycelial growth and ochratoxin A production by Aspergillus section Nigri on simulated grape medium in modified atmospheres. J. Appl. Microbiol. 2008, 105, 372–379. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Verheecke-Vaessen, C.; Gutierrez-Pozo, M.; Kiaitsi, E.; Gasperini, A.M.; Magan, N.; Medina, A. Unveiling the effect of interacting forecasted abiotic factors on growth and Aflatoxin B1 production kinetics by Aspergillus In Flavus. Fungal Biol. 2020. [Google Scholar] [CrossRef]
- Leitao, A.L. Occurrence of Ochratoxin A in Coffee: Threads and Solutions—A Mini-Review. Beverages 2019, 5, 36. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. Chemistry of coffee. In Comprehensive Natural Products II: Chemistry and Biology; Mander, L., Liu, H.W., Eds.; Elsevier: Amsterdam, Holland, 2010; Volume 3, pp. 1085–1117. [Google Scholar]
- Paterson, R.; Lima, N.; Taniwaki, M.H. Coffee, Mycotoxins and Climate Change. Food Res. Int. 2014, 61, 1–15. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Marin, S.; Sanchis, V.; Crespo-Sempere, A.; Ramos, A.J. Effect of ultraviolet radiation A and B on growth and mycotoxin production by Aspergillus carbonarius and Aspergillus parasiticus in grape and pistachio nuts. Fungal Biol. 2015, 119, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Cervini, C.; Verkeecke-Vaessen, C.; Ferrara, M.; García-Cela, E.; Magistàa, D.; Medina., A.; Gallo, A.; Magan, N.; Perron, G. Interacting climate change factors (CO2 and temperature cycles) on growth, secondary metabolite gene expression and phenotypic ochratoxin A production by Aspergillus carbonarius strains on a grape-based matrix. Fungal Biol. 2020. [Google Scholar] [CrossRef]
- Vary, Z.; Mullins, E.; Mcelwain, J.C.; Doohan, F. The severity of wheat diseases increases when plants and pathogens are acclimatised to elevated carbon dioxide. Glob. Chang. Biol. 2015, 21, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
| Temperature (°C) | 25 | 30 | 35 | ||||
|---|---|---|---|---|---|---|---|
| Strains/Medium/aw | GCEA | RCEA | GCEA | RCEA | GCEA | RCEA | |
| A. westerdijkiae (2A3) | 0.99 | ND | ND | ND | ND | ND | ND |
| 0.98 | 4.1 + 1.1. | <LOQ | 8.3 + 3.9 | <LOQ | 10.3 | ND | |
| 0.95 | 8.7 + 0.9 | 2.6 + 0.2 | <LOQ | <LOQ | 46.4 | ND | |
| 0.90 | ND | <LOQ | ND | ND | ND | ND | |
| A. westerdijkiae (C1/1) | 0.99 | <LOQ | ND | ND | ND | ND | ND |
| 0.98 | <LOQ | <LOQ | 48.3 + 13.8 | ND | 9.7 | ND | |
| 0.95 | 32.4 + 0.4 | <LOQ | ND | ND | ND | ND | |
| 0.90 | ND | <LOQ | ND | ND | ND | ND | |
| A. westerdijkiae (B 2) | 0.99 | ND | ND | 40.3 + 5.1 | ND | ND | ND |
| 0.98 | 9.4 + 5.2 | <LOQ | 1802.8 + 416 | ND | 5206.3 + 433 | ND | |
| 0.95 | 3.7 + 0.2 | <LOQ | 3.2 + 0.4 | <LOQ | 28.1 + 1.1 | <LOQ | |
| 0.90 | ND | ND | <LOQ | ND | <LOQ | ND | |
| A. westerdijkiae (CBS 121986) | 0.99 | <LOQ | ND | <LOQ | <LOQ | ND | ND |
| 0.98 | <LOQ | <LOQ | 280.4 + 17.2 | ND | 64.3 + 15.8 | ND | |
| 0.95 | 30.6 + 6.2 | <LOQ | 5.4 + 3.0 | ND | 52.81 | ND | |
| 0.90 | ND | <LOQ | <LOQ | ND | ND | ND | |
| Strain | Temperature | Media Type | Water Activity | Response |
|---|---|---|---|---|
| A. westerdijkiae (2A3) | S | NS | S | Growth |
| S | S | S | OTA (ng g−1) | |
| A. westerdijkiae (C1/1) | S | S | S | Growth |
| S | NS | S | OTA (ng g−1) | |
| A. westerdijkiae (B 2) | S | NS | S | Growth |
| S | S | S | OTA (ng g−1) | |
| A. westerdijkiae (CBS 121986) | S | NS | S | Growth |
| S | S | S | OTA (ng g−1) |
| Temperature 30 °C | ||||
| Strains | CO2 (1000 ppm) | Water Activity (aw) | CO2 × aw | Response |
| A. westerdijkiae (B 2) | NS a | S b | NS a | growth rate |
| A. westerdikiae (CBS 121986) | S a | NS a | - | growth rate |
| Temperature 35 °C | ||||
| A. westerdijkiae (B 2) | NS b | S b | NS a | growth rate |
| A. westerdijkiae (CBS 121986) | NS a | S a | - | growth rate |
| Temperatures 30 and 35 °C | ||||
| Strains | CO2 (1000 ppm) | aw | Temp (30 + 35 °C) | Response |
| A. westerdijkiae (B 2) | NS a | NS a | S a | growth rate |
| A. westerdijkiae (CBS 121986) | NS a | S a | S a | growth rate |
| Strains | CO2 (1000 ppm) | aw | CO2 × aw |
| A. westerdijkiae (B 2) | S a | S a | - |
| A. westerdijkiae (CBS 121986) | NS b | S b | NS b |
| Temperature 35 °C | |||
| A. westerdijkiae (B 2) | S a | S a | - |
| A. westerdijkiae (CBS 121986) | S a | S a | - |
| Temperature 30 and 35 °C | |||
| Strains | CO2 (1000 ppm) | aw | Temp 30 + 35 |
| A. westerdijkiae (B 2) | S a | S a | S a |
| A. westerdijkiae (CBS 121986) | S a | S a | S b |
| Temperature (°C) | 30 | 35 | |||
|---|---|---|---|---|---|
| CO2 Concentration (ppm) | 400 | 1000 | 400 | 1000 | |
| Strains | aw | ||||
| A. westerdijkiae (B 2) | 0.97 | 3976.9 ± 603.7 | 2760.4 ± 52.7 | 175.6 ± 0.2 | 14.4 ± 3.7 |
| 0.95 | 4243.3 ± 571.4 | 4767.1 ± 372.1 | 128.8 ± 31.6 | 63.1 ± 21.3 | |
| 0.90 | 1644.3 ± 545.3 | 4598.9 ± 426.4 | 8.2 ± 0.2 | 680.2 ± 187.2 | |
| A. westerdijkiae (CBS 121986) | 0.97 | 2681.3 ± 346.7 | 3395.5 ± 198.7 | 8.1 ± 0.4 | 12.7 ± 0.9 |
| 0.95 | 2842.3 ± 325.1 | 3087.9 ± 225.4 | 8.2 ± 0.6 | 51.3 ± 28.6 | |
| 0.90 | 2679.3 ± 391.3 | 3974.2 ± 101.6 | 7.1 ± 0.7 | 69.5 ± 21.2 | |
| Temperature (30 °C) | |||
| Strains | CO2 | aw | aw × CO2 |
| A. westerdijkiae (B 2) | S b | S b | S b |
| A. westerdijkiae (CBS 121986) | S b | NS | NS |
| Temperature (35 °C) | |||
| A. westerdijkiae (B 2) | S a | S a | N/A |
| A. westerdijkiae (CBS 121986) | S a | NS a | N/A |
| Strains | CO2 (1000 ppm) | aw | Temp: 30 + 35 °C |
| A. westerdijkiae (B 2) | NS a | NS a | S a |
| A. westerdijkiae (CBS 121986) | S a | NS a | S a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, A.; Medina, A.; Magan, N. Resilience of Aspergillus westerdijkiae Strains to Interacting Climate-Related Abiotic Factors: Effects on Growth and Ochratoxin A Production on Coffee-Based Medium and in Stored Coffee. Microorganisms 2020, 8, 1268. https://doi.org/10.3390/microorganisms8091268
Akbar A, Medina A, Magan N. Resilience of Aspergillus westerdijkiae Strains to Interacting Climate-Related Abiotic Factors: Effects on Growth and Ochratoxin A Production on Coffee-Based Medium and in Stored Coffee. Microorganisms. 2020; 8(9):1268. https://doi.org/10.3390/microorganisms8091268
Chicago/Turabian StyleAkbar, Asya, Angel Medina, and Naresh Magan. 2020. "Resilience of Aspergillus westerdijkiae Strains to Interacting Climate-Related Abiotic Factors: Effects on Growth and Ochratoxin A Production on Coffee-Based Medium and in Stored Coffee" Microorganisms 8, no. 9: 1268. https://doi.org/10.3390/microorganisms8091268
APA StyleAkbar, A., Medina, A., & Magan, N. (2020). Resilience of Aspergillus westerdijkiae Strains to Interacting Climate-Related Abiotic Factors: Effects on Growth and Ochratoxin A Production on Coffee-Based Medium and in Stored Coffee. Microorganisms, 8(9), 1268. https://doi.org/10.3390/microorganisms8091268





