Organic Carbon and Nitrogen Isoscapes of Reef Corals and Algal Symbionts: Relative Influences of Environmental Gradients and Heterotrophy
Abstract
1. Introduction
2. Materials and Methods
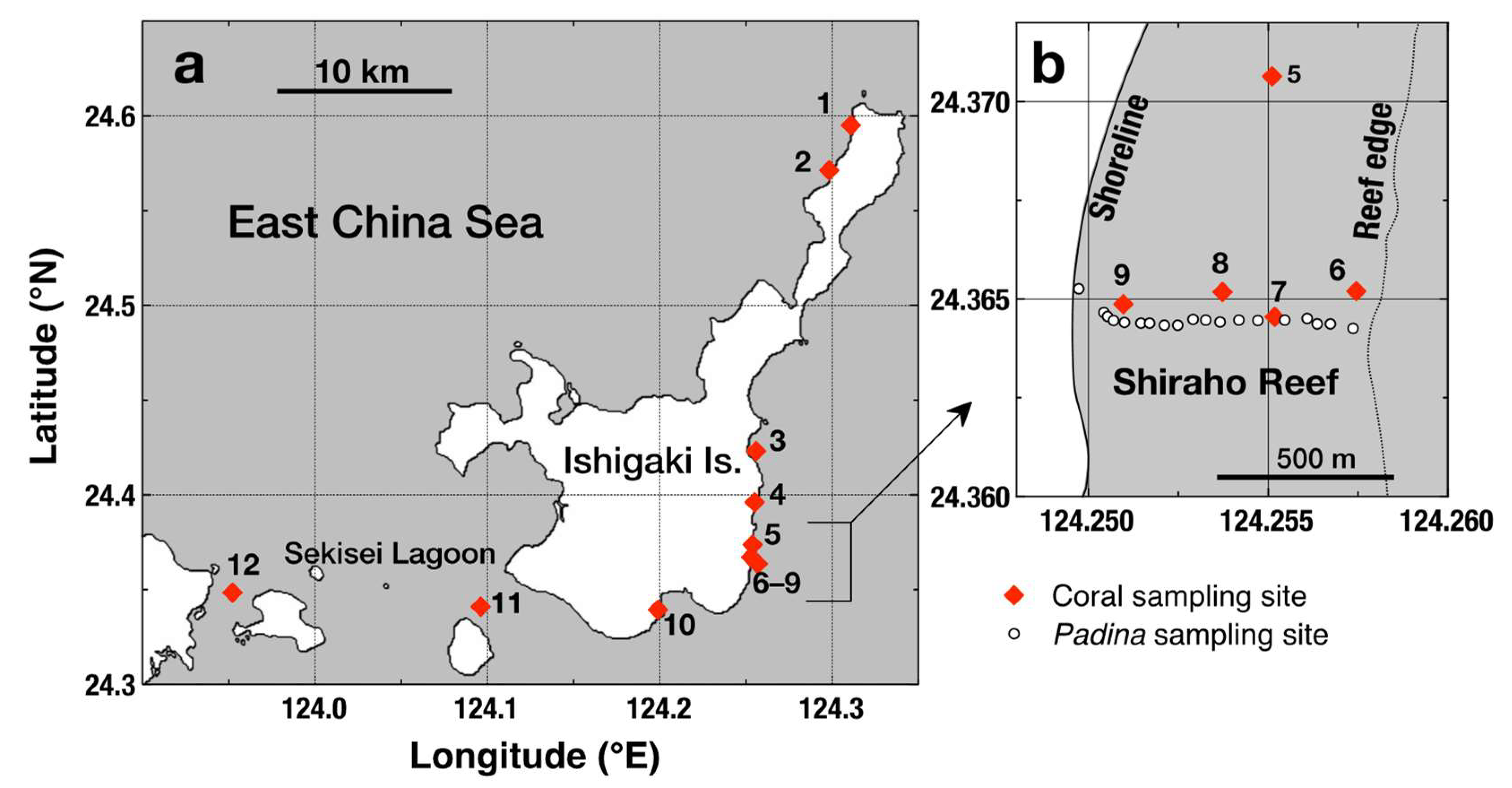
2.1. Field Sampling
2.2. Bulk Stable Isotope Analysis
2.3. Amino Acid Composition
2.4. Compound-Specific δ15N Analysis of Amino Acids
2.5. Data Treatment and Statistical Analysis
3. Results
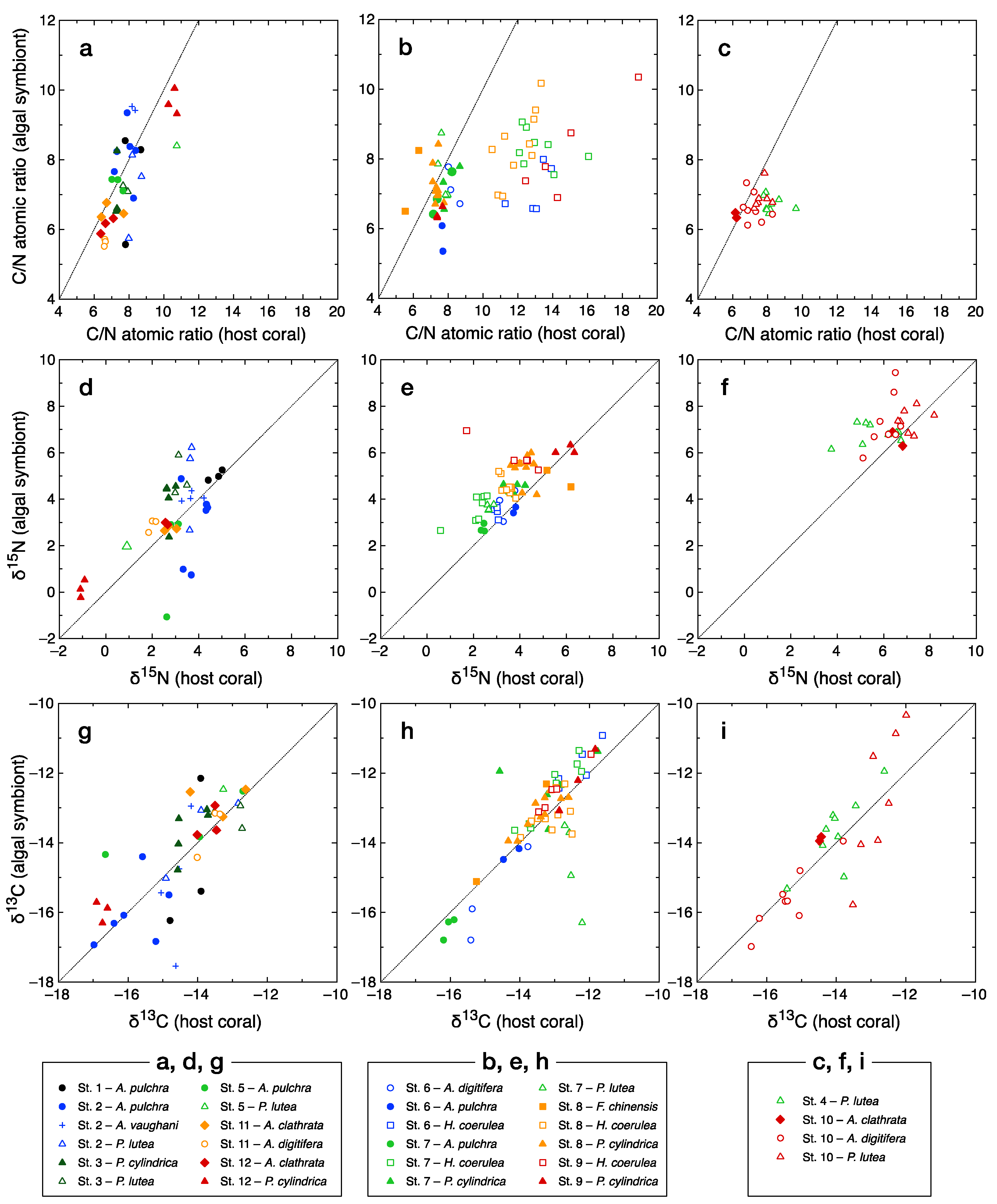
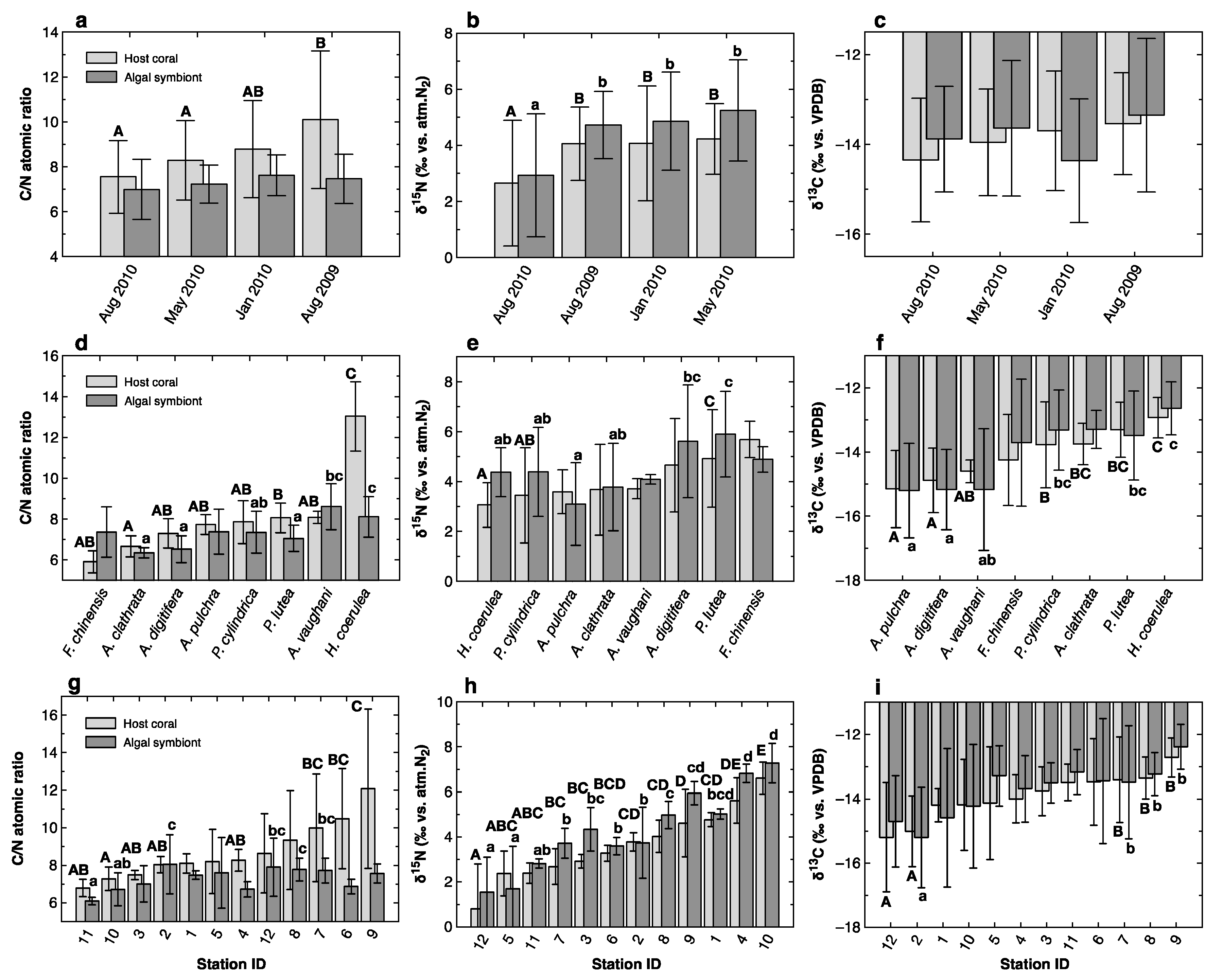
3.1. C/N Ratio and Bulk Carbon and Nitrogen Isotope Ratios
3.2. Amino Acid Composition and Compound-Specific δ15N Values of Amino Acids
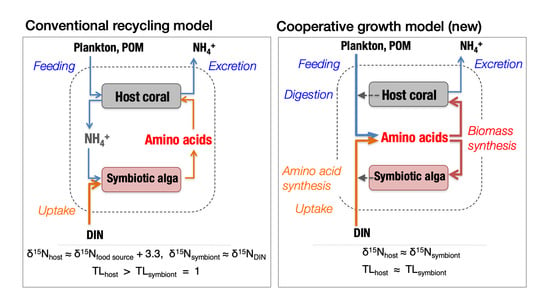
4. Discussion
4.1. Seasonal Variations
4.2. Species-Specific Differences
4.3. Spatial Variations
4.4. Contribution of Heterotrophic Nutrition
5. Conclusions
- C/N ratios were species-specific and were not very sensitive to changes in environmental conditions. However, excess organic C production under nutrient-limited conditions may be reflected in higher C/N ratios of algal endosymbionts.
- δ13C values appeared to be driven by overall isotope fractionation during DIC uptake and fixation related to the hydrodynamic conditions of the microhabitat and coral morphology, which constrain the thickness of the diffusion boundary layer. Seasonal changes in water temperature and insolation also influenced δ13C.
- δ15N values primarily reflected the δ15N values of DIN and varied along reef-scale pollution gradients. The relative influence of pollution-derived N on coral nutrition can be evaluated using the δ15N signature of host coral tissues or their symbiotic algae.
- Heterotrophy by coral holobionts was shown to cause significant shifts in both δ15N and δ13C values. To use δ15N and δ13C values to evaluate nutrient sources and coral health, the dependence of coral holobionts on heterotrophy should be assessed and, if necessary, the effect of heterotrophy on δ15N and δ13C values should be adequately corrected, e.g., based on the compound-specific δ15N values of amino acids.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Elia, C.F. The uptake and release of dissolved phosphorus by reef corals. Limnol. Oceanogr. 1977, 22, 301–315. [Google Scholar] [CrossRef]
- Muscatine, L.; D’Elia, C.F. The uptake, retention, and release of ammonium by reef corals. Limnol. Oceanogr. 1978, 23, 725–734. [Google Scholar] [CrossRef]
- Burris, R.H. Uptake and assimilation of 15NH4+ by a variety of corals. Mar. Biol. 1983, 75, 151–155. [Google Scholar] [CrossRef]
- Wyatt, A.S.J.; Falter, J.L.; Lowe, R.J.; Humphries, S.; Waite, A.M. Oceanographic forcing of nutrient uptake and release over a fringing coral reef. Limnol. Oceanogr. 2012, 57, 401–419. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Tambutté, E.; Richard, C.; Ferrier-Pagès, C. Importance of a micro-diet for scleractinian corals. Mar. Ecol. Prog. Ser. 2004, 282, 151–160. [Google Scholar] [CrossRef]
- Houlbrèque, F.; Ferrier-Pagès, C. Heterotrophy in tropical scleractinian corals. Biol. Rev. 2009, 84, 1–17. [Google Scholar] [CrossRef]
- Patten, N.L.; Wyatt, A.S.J.; Lowe, R.J.; Waite, A.M. Uptake of picophytoplankton, bacterioplankton and virioplankton by a fringing coral reef community (Ningaloo Reef, Australia). Coral Reefs 2011, 30, 555–567. [Google Scholar] [CrossRef]
- Rosenfeld, M.; Bresler, V.; Abelson, A. Sediment as a possible source of food for corals. Ecol. Lett. 1999, 2, 345–348. [Google Scholar] [CrossRef]
- Mills, M.M.; Sebens, K.P. Ingestion and assimilation of nitrogen from benthic sediments by three species of coral. Mar. Biol. 2004, 145, 1097–1106. [Google Scholar] [CrossRef]
- Lesser, M.P.; Falcón, L.I.; Rodríguez-Román, A.; Enríquez, S.; Hoegh-Guldberg, O.; Iglesias-Prieto, R. Nitrogen fixation by symbiotic cyanobacteria provides a source of nitrogen for the scleractinian coral Montastraea cavernosa. Mar. Ecol. Prog. Ser. 2007, 346, 143–152. [Google Scholar] [CrossRef]
- Suzuki, Y.; Casareto, B.E. The role of dissolved organic nitrogen (DON) in coral biology and reef ecology. In Coral Reefs: An Ecosystem in Transition; Dubinsky, Z., Stambler, N., Eds.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2011; pp. 207–229. [Google Scholar] [CrossRef]
- Miyajima, T.; Hata, H.; Umezawa, Y.; Kayanne, H.; Koike, I. Distribution and partitioning of nitrogen and phosphorus in a fringing reef lagoon of Ishigaki Island, northwestern Pacific. Mar. Ecol. Prog. Ser. 2007, 341, 45–57. [Google Scholar] [CrossRef]
- Godinot, C.; Ferrier-Pagès, C.; Sikorski, S.; Grover, R. Alkaline phosphatase activity of reef building corals. Limnol. Oceanogr. 2013, 58, 227–234. [Google Scholar] [CrossRef]
- Tanaka, Y.; Suzuki, A.; Sakai, K. The stoichiometry of coral-dinoflagellate symbiosis: Carbon and nitrogen cycles are balanced in the recycling and double translocation system. ISME J. 2018, 12, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.C.; Kleypas, J. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Fordyce, A.J.; Ainsworth, T.D.; Heron, S.F.; Leggat, W. Marine heatwave hotspots in coral reef environments: Physical drivers, ecophysiological outcomes and impact upon structural complexity. Front. Mar. Sci. 2019, 6, 498. [Google Scholar] [CrossRef]
- Lapointe, B.E. Nutrient thresholds for bottom-up control of macroalgal blooms on coral reefs in Jamaica and southeast Florida. Limnol. Oceanogr. 1997, 42, 1119–1131. [Google Scholar] [CrossRef]
- Fabricius, K.E.; De’ath, G.; McCook, L.; Turak, E.; Williams, D.M. Changes in algal, coral and fish assemblages along water quality gradients on the inshore Great Barrier Reef. Mar. Pollut. Bull. 2005, 51, 384–398. [Google Scholar] [CrossRef]
- Rosset, S.; Wiedenmann, J.; Reed, A.J.; D’Angelo, C. Phosphate deficiency promotes coral bleaching and is reflected by the ultrastructure of symbiotic dinoflagellates. Mar. Pollut. Bull. 2017, 118, 180–187. [Google Scholar] [CrossRef]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.-E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Chang. 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Chumun, P.K.; Casareto, B.E.; Higuchi, T.; Irikawa, A.; Bhagooli, R.; Ishikawa, Y.; Suzuki, Y. High nitrate levels exacerbate thermal photo-physiological stress of zooxanthellae in the reef-building coral Pocillopora damicornis. Eco-Engineering 2013, 25, 75–83. [Google Scholar] [CrossRef]
- Reymond, C.E.; Lloyd, A.; Kline, D.I.; Dove, S.G.; Pandolfi, J.M. Decline in growth of foraminifer Marginopora rossi under eutrophication and ocean acidification scenarios. Glob. Chang. Biol. 2013, 19, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.R.F.; Elmetri, I.; Lapointe, B.E. Evidence of large-scale chronic eutrophication in the Great Barrier Reef: Quantification of chlorophyll a thresholds for sustaining coral reef communities. Ambio 2014, 43, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Vega Thurber, R.L.; Burkepile, D.E.; Fuchs, C.; Shantz, A.A.; McMinds, R.; Zaneveld, J.R. Chronic nutrient enrichment increases prevalence and severity of coral disease and bleaching. Glob. Chang. Biol. 2014, 20, 544–554. [Google Scholar] [CrossRef]
- Borell, E.M.; Bischof, K. Feeding sustains photosynthetic quantum yield of a scleractinian coral during thermal stress. Oecologia 2008, 157, 593–601. [Google Scholar] [CrossRef]
- Edmunds, P.J. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 2011, 56, 2402–2410. [Google Scholar] [CrossRef]
- Connolly, S.R.; Lopez-Yglesias, M.A.; Anthony, K.R.N. Food availability promotes rapid recovery from thermal stress in a scleractinian coral. Coral Reefs 2012, 31, 951–960. [Google Scholar] [CrossRef]
- Camp, E.F.; Nitschke, M.R.; Rodolfo-Metalpa, R.; Houlbreque, F.; Gardner, S.G.; Smith, D.J.; Zampighi, M.; Suggett, D.J. Reef-building corals thrive within hot-acidified and deoxygenated waters. Sci. Rep. 2017, 7, 2434. [Google Scholar] [CrossRef]
- Kürten, B.; Al-Aidaroos, A.M.; Struck, U.; Khomayis, H.S.; Gharbawi, W.Y.; Sommer, U. Influence of environmental gradients on C and N stable isotope ratios in coral reef biota of the Red Sea, Saudi Arabia. J. Sea Res. 2014, 85, 379–394. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Manuel, S.A.; Coates, K.A.; Kenworthy, W.J.; Boyer, J.N. Water quality, isoscapes and stoichioscapes of seagrasses indicate general P limitation and unique N cycling in shallow water benthos of Bermuda. Biogeosciences 2015, 12, 6235–6249. [Google Scholar] [CrossRef]
- Thibault, M.; Duprey, N.; Gillikin, D.P.; Thébault, J.; Douillet, P.; Chauvaud, L.; Amice, E.; Munaron, J.M.; Lorrain, A. Bivalve δ15N isoscapes provide a baseline for urban nitrogen footprint at the edge of a World Heritage coral reef. Mar. Pollut. Bull. 2020, 152, 110870. [Google Scholar] [CrossRef] [PubMed]
- Yamano, H.; Hata, H.; Miyajima, T.; Nozaki, K.; Kato, K.; Negishi, A.; Tamura, M.; Kayanne, H. Water circulation in a fringing reef and implications for coral distribution and resilience. In Integrative Observations and Assessments; Nakano, S., Yahara, T., Nakashizuka, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 275–293. [Google Scholar]
- Umezawa, Y.; Miyajima, T.; Kayanne, H.; Koike, I. Significance of groundwater nitrogen discharge into coral reefs at Ishigaki Island, southwest of Japan. Coral Reefs 2002, 21, 346–356. [Google Scholar] [CrossRef]
- Umezawa, Y.; Miyajima, T.; Yamamuro, M.; Kayanne, H.; Koike, I. Fine-scale mapping of land-derived nitrogen in coral reefs by δ15N in macroalgae. Limnol. Oceanogr. 2002, 47, 1405–1416. [Google Scholar] [CrossRef]
- Kayanne, H.; Harii, S.; Ide, Y.; Akimoto, F. Recovery of coral populations after the 1998 bleaching on Shiraho Reef, in the southern Ryukyus, NW Pacific. Mar. Ecol. Prog. Ser. 2002, 239, 93–103. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kayanne, H. Relationship of species composition of tropical seagrass meadows to multiple physical environmental factors. Ecol. Res. 2007, 22, 87–96. [Google Scholar] [CrossRef]
- Miyajima, T.; Tanaka, Y.; Koike, I.; Yamano, H.; Kayanne, H. Evaluation of spatial correlation between nutrient exchange rates and benthic biota in a reef-flat ecosystem by GIS-assisted flow-tracking. J. Oceanogr. 2007, 63, 643–659. [Google Scholar] [CrossRef]
- Harii, S.; Hongo, C.; Ishihara, M.; Ide, Y.; Kayanne, H. Impacts of multiple disturbances on coral communities at Ishigaki Island, Okinawa, Japan, during a 15 year survey. Mar. Ecol. Prog. Ser. 2014, 509, 171–180. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Muscatine, L.; Goiran, C.; Siggaard, D.; Marion, G. Nutrient-induced perturbations to δ13C and δ15N in symbiotic dinoflagellates and their coral hosts. Mar. Ecol. Prog. Ser. 2004, 280, 105–114. [Google Scholar] [CrossRef]
- Muller-Parker, G.; Cook, C.B.; D’Elia, C.F. Elemental composition of the coral Pocillopora damicornis exposed to elevated seawater ammonium. Pac. Sci. 1994, 48, 234–246. [Google Scholar]
- Tanaka, Y.; Grottoli, A.G.; Matsui, Y.; Suzuki, A.; Sakai, K. Effects of nitrate and phosphate availability on the tissues and carbonate skeleton of scleractinian corals. Mar. Ecol. Prog. Ser. 2017, 570, 101–112. [Google Scholar] [CrossRef]
- Berner, T.; Izhaki, I. Effect of exogenous nitrogen levels on ultrastructure of zooxanthellae from the hermatypic coral Pocillopora damicornis. Pac. Sci. 1994, 48, 254–262. [Google Scholar]
- Patton, J.S.; Abraham, S.; Benson, A.A. Lipogenesis in the intact coral Pocillopora capitata and its isolated zooxanthellae: Evidence for a light-driven carbon cycle between symbiont and host. Mar. Biol. 1977, 44, 235–247. [Google Scholar] [CrossRef]
- Rodrigues, L.J.; Grottoli, A.G.; Pease, T.K. Lipid class composition of bleached and recovering Porites compressa Dana, 1846 and Montipora capitata Dana, 1846 corals from Hawaii. J. Exp. Mar. Biol. Ecol. 2008, 358, 136–143. [Google Scholar] [CrossRef]
- Treignier, C.; Grover, R.; Ferrier-Pages, C.; Tolosa, I. Effect of light and feeding on the fatty acid and sterol composition of zooxanthellae and host tissue isolated from the scleractinian coral Turbinaria reniformis. Limnol. Oceanogr. 2008, 53, 2702–2710. [Google Scholar] [CrossRef]
- Anthony, K.; Connolly, S.R.; Hoegh-Guldberg, O. Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol. Oceanogr. 2007, 52, 716–726. [Google Scholar] [CrossRef]
- Tagliafico, A.; Rudd, D.; Rangel, M.S.; Kelaher, B.P.; Christidis, L.; Cowden, K.; Scheffers, S.R.; Benkendorff, K. Lipid-enriched diets reduce the impacts of thermal stress in corals. Mar. Ecol. Prog. Ser. 2017, 573, 129–141. [Google Scholar] [CrossRef]
- Weber, J.N.; Woodhead, P.M.J. Diurnal variations in the isotopic composition of dissolved inorganic carbon in seawater from coral reef environments. Geochim. Cosmochim. Acta 1971, 35, 891–902. [Google Scholar] [CrossRef]
- Smith, S.V.; Kroopnick, P. Carbon-13 isotopic fractionation as a measure of aquatic metabolism. Nature 1981, 249, 252–253. [Google Scholar] [CrossRef]
- Carvalho, M.C.; Santos, I.R.; Maher, D.T.; Cyronak, T.; McMahon, A.; Schulz, K.G.; Eyre, B.D. Drivers of carbon isotopic fractionation in a coral reef lagoon: Predominance of demand over supply. Geochim. Cosmochim. Acta 2015, 153, 105–115. [Google Scholar] [CrossRef]
- Bertucci, A.; Moya, A.; Tambutté, S.; Allemand, D.; Supuran, C.T.; Zoccola, D. Carbonic anhydrases in anthozoan corals—A review. Bioorg. Med. Chem. 2013, 21, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Weis, V.M.; Smith, G.J.; Muscatine, L. A “CO2 supply” mechanism in zooxanthellate cnidarians: Role of carbonic anhydrase. Mar. Biol. 1989, 100, 195–202. [Google Scholar] [CrossRef]
- Barott, K.L.; Venn, A.A.; Perez, S.O.; Tambutté, S.; Tresguerres, M. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Tansik, A.L.; Fitt, W.K.; Hopkinson, B.M. Inorganic carbon is scarce for symbionts in scleractinian corals. Limnol. Oceanogr. 2017, 62, 2045–2055. [Google Scholar] [CrossRef]
- Alamaru, A.; Loya, Y.; Brokovich, E.; Yam, R.; Shemesh, A. Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: Insights from stable isotope analysis of total organic material and lipids. Geochim. Cosmochim. Acta 2009, 17, 5333–5342. [Google Scholar] [CrossRef]
- Mendes, J.M.; Risk, M.J.; Schwarcz, H.P.; Woodley, J.D. Stable isotopes of nitrogen as measures of marine pollution: A preliminary assay of coral tissue from Jamaica. In Proceedings of the 8th International Coral Reef Symposium, Panama City, Panama, 24–29 June 1996; Volume 2, pp. 1869–1872. [Google Scholar]
- Heikoop, J.M.; Risk, M.J.; Lazier, A.V.; Edinger, E.N.; Jompa, J.; Limmon, G.V.; Dunn, J.J.; Browne, D.R.; Schwarcz, H.P. Nitrogen-15 signals of anthropogenic nutrient loading in reef corals. Mar. Pollut. Bull. 2000, 40, 628–636. [Google Scholar] [CrossRef]
- Miyajima, T.; Umezawa, Y. Stable isotope composition of nitrogen (δ15N) as a tool for investigating nitrogen cycling in coral reef ecosystems. In Earth, Life, and Isotopes; Ohkouchi, N., Tayasu, I., Koba, K., Eds.; Kyoto University Press: Kyoto, Japan, 2010; pp. 197–222. [Google Scholar]
- Heikoop, J.M.; Dunn, J.J.; Risk, M.J.; Sandeman, I.M.; Schwarcz, H.P.; Waltho, N. Relationship between light and the δ15N of coral tissue: Examples from Jamaica and Zanzibar. Limnol. Oceanogr. 1998, 43, 909–920. [Google Scholar] [CrossRef][Green Version]
- Vander Zanden, M.J.; Rasmussen, J.B. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 1999, 80, 1395–1404. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Peirano, A.; Abbate, M.; Cocito, S.; Negri, A.; Rottier, C.; Riera, P.; Rodolfo-Metalpa, R.; Reynaud, S. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 2011, 56, 1429–1438. [Google Scholar] [CrossRef]
- O’Connell, T.C. ‘Trophic’ and ‘source’ amino acids in trophic estimation: A likely metabolic explanation. Oecologia 2017, 184, 317–326. [Google Scholar] [CrossRef]
- McClelland, J.W.; Montoya, J.P. Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 2002, 83, 2173–2180. [Google Scholar] [CrossRef]
- McCarthy, M.D.; Benner, R.; Lee, C.; Fogel, M.L. Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochim. Cosmochim. Acta 2007, 71, 4727–4744. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Ogawa, N.O.; Kashiyama, Y.; Takano, Y.; Suga, H.; Tomitani, A.; Miyashita, H.; Kitazato, H.; Ohkouchi, N. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Methods 2009, 7, 740–750. [Google Scholar] [CrossRef]
- Lindroth, P.; Mopper, K. High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal. Chem. 1979, 51, 1667–1674. [Google Scholar] [CrossRef]
- Metges, C.C.; Petzke, K.-J.; Hennig, U. Gas chromatography/combustion/isotope ratio mass spectrometric comparison of N-acetyl- and N-pivaloyl amino acid esters to measure 15N isotopic abundances in physiological samples: A pilot study on amino acid synthesis in the upper gastro-intestinal tract of minipigs. J. Mass Spectrom. 1996, 31, 367–376. [Google Scholar] [CrossRef]
- Chikaraishi, Y.; Kashiyama, Y.; Ogawa, N.O.; Kitazato, H.; Ohkouchi, N. Metabolic control of nitrogen isotope composition of amino acids in macroalgae and gastropods: Implications for aquatic food web studies. Mar. Ecol. Prog. Ser. 2007, 342, 85–90. [Google Scholar] [CrossRef]
- Maki, K.; Ohkouchi, N.; Chikaraishi, Y.; Fukuda, H.; Miyajima, T.; Nagata, T. Influence of nitrogen substrates and substrate C: N ratios on the nitrogen isotopic composition of amino acids from the marine bacterium Vibrio harveyi. Geochim. Cosmochim. Acta 2014, 140, 521–530. [Google Scholar] [CrossRef]
- FitzGerald, L.M.; Szmant, A.M. Biosynthesis of ‘essential’ amino acids by scleractinian corals. Biochem. J. 1997, 322, 213–221. [Google Scholar] [CrossRef]
- Kayanne, H.; Hata, H.; Kudo, S.; Yamano, H.; Watanabe, A.; Ikeda, Y.; Nozaki, K.; Kato, K.; Negishi, A.; Saito, H. Seasonal and bleaching-induced changes in coral reef metabolism and CO2 flux. Glob. Biogeochem. Cycles 2005, 19, GB3015. [Google Scholar] [CrossRef]
- Miyajima, T.; Morimoto, N.; Nakamura, T.; Yamamoto, T.; Watanabe, A.; Nadaoka, K. Atmospheric deposition of reactive nitrogen as a regional-scale eutrophication stress on the coral reef ecosystem. In Coral Reefs of the World: Coral Reef Science; Kayanne, H., Ed.; Springer: Tokyo, Japan, 2016; pp. 95–101. [Google Scholar] [CrossRef]
- Finlay, J.C.; Power, M.E.; Cabana, G. Effects of water velocity on algal carbon isotope ratios: Implications for river food web studies. Limnol. Oceanogr. 1999, 44, 1198–1203. [Google Scholar] [CrossRef]
- Trudeau, V.; Rasmussen, J.B. The effect of water velocity on stable carbon and nitrogen isotope signatures of periphyton. Limnol. Oceanogr. 2003, 48, 2194–2199. [Google Scholar] [CrossRef]
- van Dongen, B.E.; Schouten, S.; Sinninghe Damsté, J.S. Carbon isotope variability in monosaccharides and lipids of aquatic algae and terrestrial plants. Mar. Ecol. Prog. Ser. 2002, 232, 83–92. [Google Scholar] [CrossRef]
- Logan, J.M.; Jardine, T.D.; Miller, T.J.; Bunn, S.E.; Cunjak, R.A.; Lutcavage, M.E. Lipid corrections in carbon and nitrogen stable isotope analyses: Comparison of chemical extraction and modelling methods. J. Anim. Ecol. 2008, 77, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P.; Weis, V.M.; Patterson, M.R.; Jokiel, P.L. Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): Diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Biol. Ecol. 1994, 178, 153–179. [Google Scholar] [CrossRef]
- Wanek, W.; Heintel, S.; Richter, A. Preparation of starch and other carbon fractions from higher plant leaves for stable carbon isotope analysis. Rapid Commun. Mass Spectrom. 2001, 15, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.M.; McClelland, J.W.; Sigman, D.M.; Fry, B.; Peterson, B.J. Measuring 15N-NH4+ in marine, estuarine and fresh waters: An adaptation of the ammonia diffusion method for samples with low ammonium concentrations. Mar. Chem. 1998, 60, 235–243. [Google Scholar] [CrossRef]
- Sigman, D.M.; Granger, J.; DiFiore, P.J.; Lehmann, M.M.; Ho, R.; Cane, G.; van Geen, A. Coupled nitrogen and oxygen isotope measurements of nitrate along the eastern North Pacific margin. Glob. Biogeochem. Cycles 2005, 19, GB4022. [Google Scholar] [CrossRef]
- Iwade, T. Influence of Terrestrial Nutrient Inputs on the Health of Coral Reefs in Ishigaki Island, Japan. Bachelor’s Thesis, Faculty of Science, the University of Tokyo, Tokyo, Japan, 2006; 30p. (In Japanese). [Google Scholar]
- Umezawa, Y. Nutrient Dynamics in Tropical and Subtropical Coastal Ecosystems Assessed by δ15N in Macroalgae. Ph.D. Thesis, The University of Tokyo, Tokyo, Japan, 2004; 219p. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyajima, T.; Koike, I.; Hayashibara, T.; Ogawa, H. Translocation and conservation of organic nitrogen within the coral-zooxanthella symbiotic system of Acropora pulchra, as demonstrated by dual-isotope labeling techniques. J. Exp. Mar. Biol. Ecol. 2006, 336, 110–119. [Google Scholar] [CrossRef]
- Tremblay, P.; Grover, R.; Maguer, J.F.; Legendre, L.; Ferrier-Pages, C. Autotrophic carbon budget in coral tissue: A new 13C-based model of photosynthate translocation. J. Exp. Biol. 2012, 215, 1384–1393. [Google Scholar] [CrossRef]
- Muscatine, L.; Pool, R.R. Regulation of numbers of intracellular algae. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1979, 204, 131–139. [Google Scholar] [CrossRef]
- Jones, R.J.; Yellowlees, D. Regulation and control of intracellular algae (= zooxanthellae) in hard corals. Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1997, 352, 457–468. [Google Scholar] [CrossRef]
- Ohkouchi, N.; Chikaraishi, Y.; Close, H.G.; Fry, B.; Larsen, T.; Madigan, D.J.; McCarthy, M.D.; McMahon, K.W.; Nagata, T.; Naito, Y.I.; et al. Advances in the application of amino acid nitrogen isotopic analysis in ecological and biogeochemical studies. Org. Geochem. 2017, 113, 150–174. [Google Scholar] [CrossRef]
- McCarthy, M.D.; Lehman, J.; Kudela, R. Compound-specific amino acid δ15N patterns in marine algae: Tracer potential for cyanobacterial vs. eukaryotic organic nitrogen sources in the ocean. Geochim. Cosmochim. Acta 2013, 103, 104–120. [Google Scholar] [CrossRef]
- Wang, J.T.; Douglas, A.E. Essential amino acid synthesis and nitrogen recycling in an alga–invertebrate symbiosis. Mar. Biol. 1999, 135, 219–222. [Google Scholar] [CrossRef]
- Shinzato, C.; Mungpakdee, S.; Satoh, N.; Shoguchi, E. A genomic approach to coral-dinoflagellate symbiosis: Studies of Acropora digitifera and Symbiodinium minutum. Front. Microbiol. 2014, 5, 10–3389. [Google Scholar] [CrossRef]
- Wang, J.; Douglas, A.E. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J. Exp. Biol. 1998, 201, 2445–2453. [Google Scholar]
- Swanson, R.; Hoegh-Guldberg, O. Amino acid synthesis in the symbiotic sea anemone Aiptasia pulchella. Mar. Biol. 1998, 131, 83–93. [Google Scholar] [CrossRef]
- Rahav, O.; Dubinsky, Z.; Achituv, Y.; Folkowski, P.G. Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proc. R. Soc. Lond. Ser. B 1989, 236, 325–337. [Google Scholar] [CrossRef]
- Grover, R.; Maguer, J.-F.; Allemand, D.; Ferrier-Pagès, C. Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J. Exp. Biol. 2008, 211, 860–865. [Google Scholar] [CrossRef]
- Piniak, G.A.; Lipschultz, F.; McClelland, J. Assimilation and partitioning of prey nitrogen within two anthozoans and their endosymbiotic zooxanthellae. Mar. Ecol. Prog. Ser. 2003, 262, 125–136. [Google Scholar] [CrossRef]
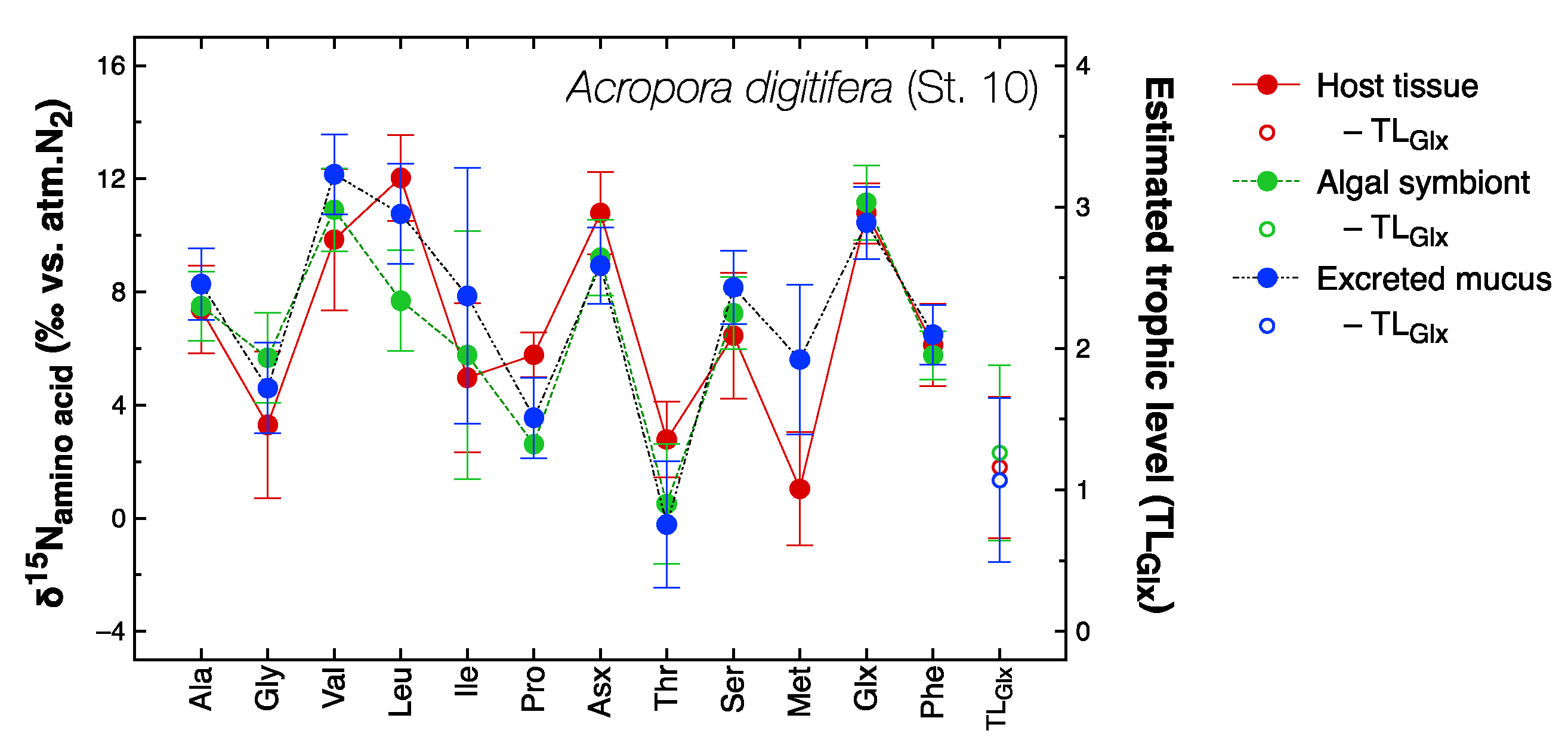
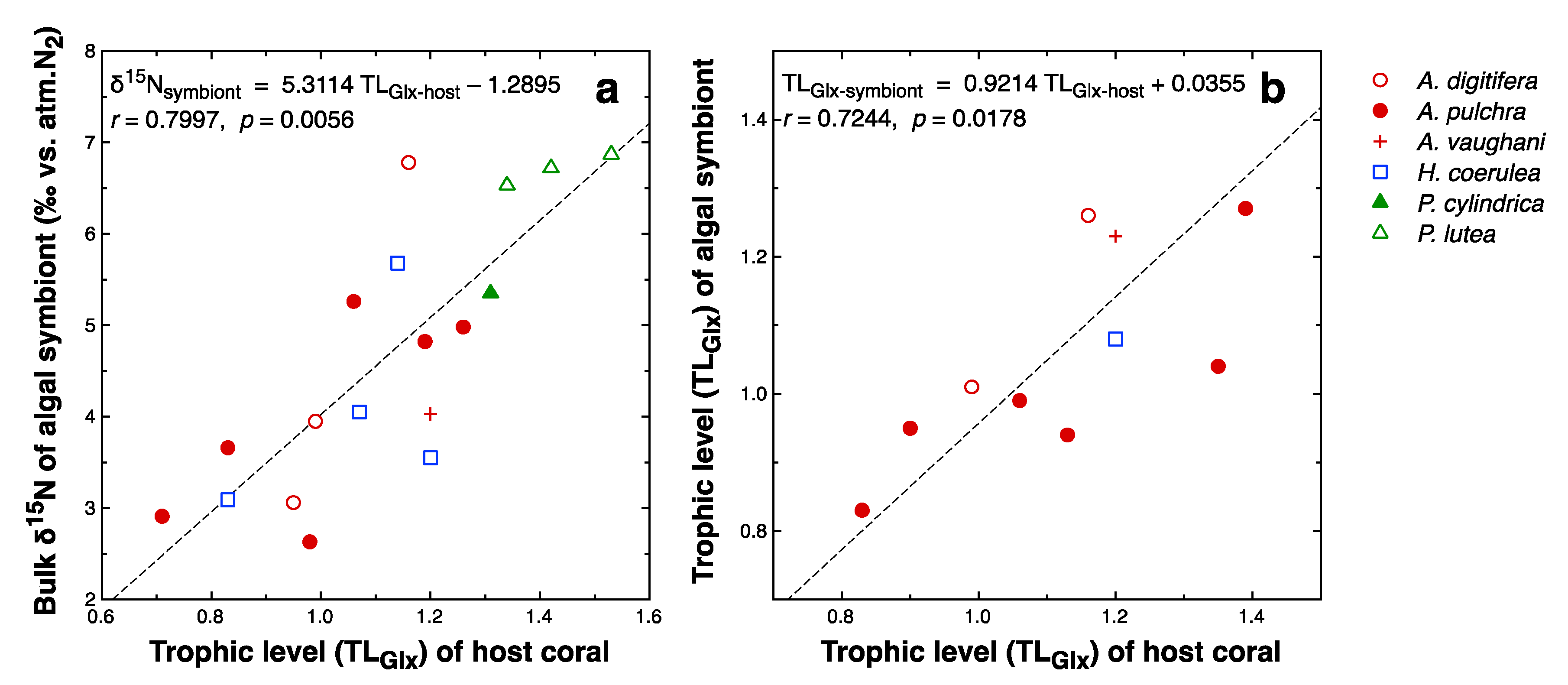
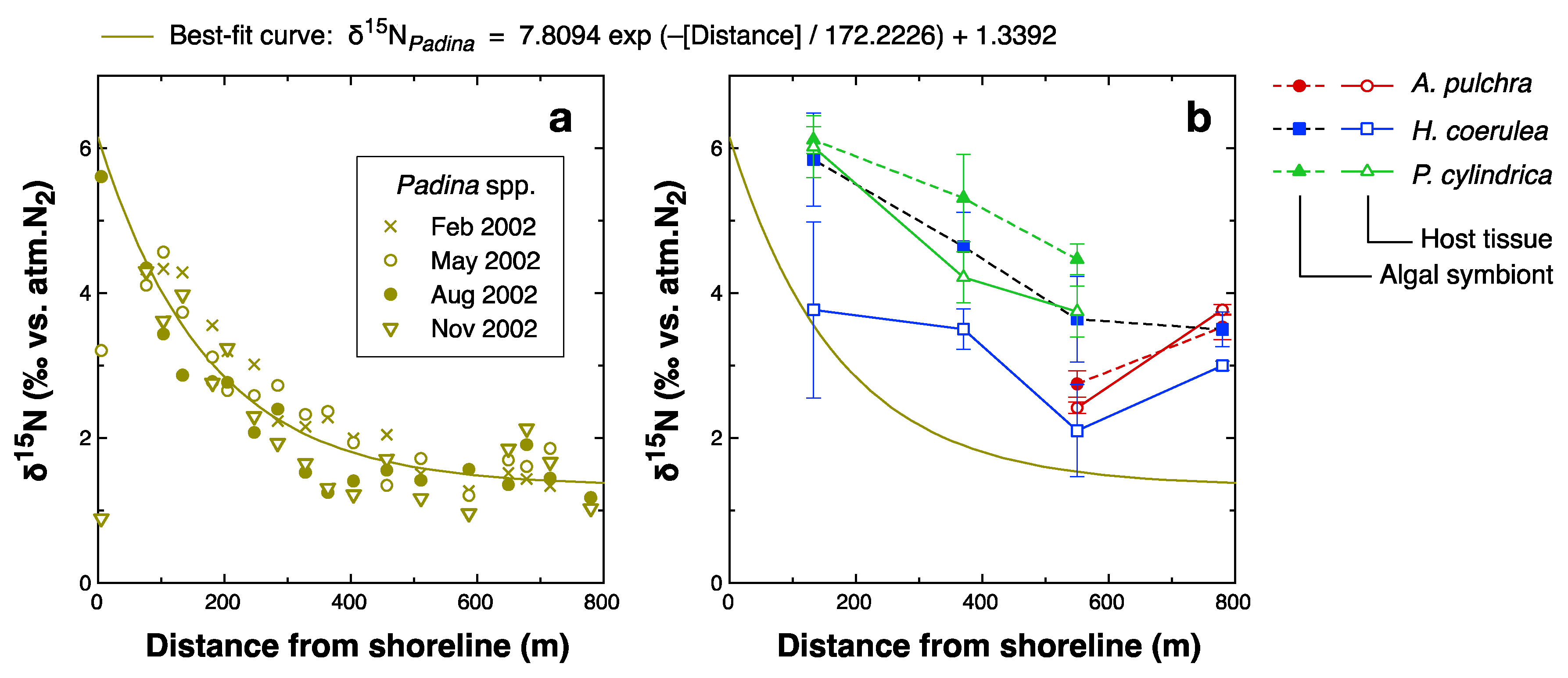
| Parameter | Dependence on | ||
|---|---|---|---|
| Season | Species | Site | |
| C/N ratio | |||
| Algal symbiont | – | +++ | +++ |
| Host coral | ++ | +++ | +++ |
| Difference | ++ | +++ | +++ |
| Bulk δ15N | |||
| Algal symbiont | +++ | +++ | +++ |
| Host coral | + | ++ | +++ |
| Difference | – | +++ | + |
| Bulk δ13C | |||
| Algal symbiont | – | +++ | + |
| Host coral | – | +++ | +++ |
| Difference | +++ | + | – |
| Sample | Most Affected Station | Season | NH4+ | NO3− | ||
|---|---|---|---|---|---|---|
| Conc. (µM) | δ15N | Conc. (µM) | δ15N | |||
| River water | Station 4 | June 2010 | 1.8 | nd | 120 | 7.5 |
| September 2010 | <0.1 | nd | 170 | 7.6 | ||
| January 2012 | 0.7 | nd | 180 | nd | ||
| Groundwater | Station 3 | August 2010 | <0.1 | nd | 300 | 8.8 |
| Station 9 | June 2010 | 1.1 | nd | 380 | 6.4 | |
| September 2010 | <0.1 | nd | 310 | 6.2 | ||
| January 2012 | 0.1 | nd | 310 | nd | ||
| Sewage water | Station 10 | July 2005 | 110–550 | 21.2 | 9–72 | nd |
| Reference | “Non-Fractionating” | “Fractionating” | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asx | Ala | Val | Pro | Thr | Phe | Gly | Ile | Leu | Ser | Lys | Met | |
| This study | −0.14 ± 1.15 | −1.88 ± 1.53 | −0.13 ± 1.93 | (−4.4 ± 3.7) * | −2.21 ± 3.98 | −3.74 ± 1.17 | −4.43 ± 1.01 | −2.98 ± 1.66 | 0.02 ± 1.97 | −2.31 ± 1.45 | nd | −3.82 ± 0.94 |
| Sample number (n) | 10 | 10 | 10 | 6 | 10 | 10 | 10 | 9 | 10 | 10 | 0 | 6 |
| McCarthy et al. [91] | ||||||||||||
| Eukaryotic algae | −1.62 ± 1.34 | −0.19 ± 1.08 | 0.35 ± 1.61 | −0.77 ± 2.88 | −3.02 ± 1.37 | −1.39 ± 1.75 | −8.50 ± 1.58 | −3.86 ± 1.06 | −6.72 ± 0.55 | −3.34 ± 1.90 | −6.30 ± 1.11 | nd |
| Cyanobacteria | −0.16 ± 1.08 | −0.49 ± 2.08 | 0.97 ± 1.62 | 0.97 ± 1.13 | 0.49 ± 1.59 | −2.63 ± 1.54 | −0.84 ± 1.77 | −2.62 ± 0.96 | −2.53 ± 1.28 | −5.36 ± 0.85 | −4.30 ± 2.04 | nd |
| Δδ15Nx–Phe | Statistics | ||
|---|---|---|---|
| x | Slope | Offset | r |
| Ala | 1.19 | −0.18 | 0.720 * |
| Gly | 0.98 | −5.25 | 0.637 * |
| Val | 1.46 | −1.63 | 0.802 ** |
| Leu | 1.78 | −1.07 | 0.830 *** |
| Ile | 1.94 | −6.08 | 0.611 * |
| Pro | 1.72 | −9.49 | 0.587 |
| Asx | 0.86 | 1.24 | 0.841 *** |
| Thr | 0.34 | −2.09 | 0.221 |
| Ser | 0.83 | −2.46 | 0.608 * |
| Met | 0.07 | −0.52 | 0.073 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, T.; Tanaka, Y.; Maki, K.; Saotome, N.; Morimoto, N.; Watanabe, A.; Miyajima, T. Organic Carbon and Nitrogen Isoscapes of Reef Corals and Algal Symbionts: Relative Influences of Environmental Gradients and Heterotrophy. Microorganisms 2020, 8, 1221. https://doi.org/10.3390/microorganisms8081221
Fujii T, Tanaka Y, Maki K, Saotome N, Morimoto N, Watanabe A, Miyajima T. Organic Carbon and Nitrogen Isoscapes of Reef Corals and Algal Symbionts: Relative Influences of Environmental Gradients and Heterotrophy. Microorganisms. 2020; 8(8):1221. https://doi.org/10.3390/microorganisms8081221
Chicago/Turabian StyleFujii, Takanori, Yasuaki Tanaka, Koh Maki, Nobue Saotome, Naoko Morimoto, Atsushi Watanabe, and Toshihiro Miyajima. 2020. "Organic Carbon and Nitrogen Isoscapes of Reef Corals and Algal Symbionts: Relative Influences of Environmental Gradients and Heterotrophy" Microorganisms 8, no. 8: 1221. https://doi.org/10.3390/microorganisms8081221
APA StyleFujii, T., Tanaka, Y., Maki, K., Saotome, N., Morimoto, N., Watanabe, A., & Miyajima, T. (2020). Organic Carbon and Nitrogen Isoscapes of Reef Corals and Algal Symbionts: Relative Influences of Environmental Gradients and Heterotrophy. Microorganisms, 8(8), 1221. https://doi.org/10.3390/microorganisms8081221