Leishmania Immunity: Advancing Immunotherapy and Vaccine Development
Abstract
1. Introduction
2. Immunity to Leishmaniasis
2.1. The Innate Immune Response in Leishmaniasis
2.1.1. Neutrophils
2.1.2. Macrophages
2.1.3. Monocytes
2.1.4. Dendritic Cells
2.1.5. Natural Killer (NK) Cells
2.2. The Adaptive Immune Response in Leishmaniasis
2.2.1. CD4+ T Cells
2.2.2. CD8+ T Cells
2.2.3. B Cells
3. Advances in Vaccination against Leishmaniasis
4. Advances in Immunotherapy against Leishmaniasis
4.1. Leishmania Antigens
4.2. Cytokines and Chemokines
4.3. Immune Checkpoint and Anti-Inflammatory Cytokine Inhibitors
4.4. Inhibitors of Signalling Pathways
4.5. Modulation of Host Molecules
4.6. Combination Therapies
5. Perspective and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A Review. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5464238/ (accessed on 14 July 2020).
- WHO. Leishmaniasis. Available online: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/ (accessed on 5 February 2020).
- Ready, P.D. Epidemiology of Visceral Leishmaniasis. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4014360/ (accessed on 28 February 2019).
- Thakur, L.; Singh, K.K.; Shanker, V.; Negi, A.; Jain, A.; Matlashewski, G.; Jain, M. Atypical leishmaniasis: A global perspective with emphasis on the Indian subcontinent. PLoS Negl. Trop. Dis. 2018, 9, e0006659. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, F.; Harandi, A.M.; Rafati, S. Biomarkers of Cutaneous Leishmaniasis. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00222/full (accessed on 14 July 2020).
- Cuba, C.C.; Llanos-Cuentas, E.A.; Barreto, A.C.; Magalhães, A.V.; Lago, E.L.; Reed, S.G.; Marsden, P.D. Human Mucocutaneous Leishmaniasis in Três Braços, Bahia-Brazil: An Area of Leishmania Braziliensis Braziliensis Transmission. I. Laboratory Diagnosis. Available online: http://www.scielo.br/scielo.php?script=sci_abstract&pid=S0037-86821984000400002&lng=en&nrm=iso&tlng=en (accessed on 14 July 2020).
- Bray, R.S.; Ashford, R.W.; Bray, M.A. The Parasite Causing Cutaneous Leishmaniasis in Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 1973, 67, 345–348. [Google Scholar] [CrossRef]
- Bates, P.A. Revising Leishmania’s Life Cycle. Available online: https://www.nature.com/articles/s41564-018-0154-2 (accessed on 14 July 2020).
- Reiner, S.L.; Locksley, R.M. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 1995, 13, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.; Gull, K. Shape, Form, Function and Leishmania Pathogenicity: From Textbook Descriptions to Biological Understanding. Available online: https://royalsocietypublishing.org/doi/10.1098/rsob.170165 (accessed on 14 July 2020).
- Kamhawi, S. The biological and immunomodulatory properties of sand fly saliva and its role in the establishment of Leishmania infections. Microbes Infect. 2000, 2, 1765–1773. [Google Scholar] [CrossRef]
- Scott, P.; Artis, D.; Uzonna, J.; Zaph, C. The development of effector and memory T cells in cutaneous leishmaniasis: The implications for vaccine development. Immunol. Rev. 2004, 201, 318–338. [Google Scholar] [CrossRef]
- Keynan, Y.; Larios, O.E.; Wiseman, M.C.; Plourde, M.; Ouellette, M.; Rubinstein, E. Use of oral miltefosine for cutaneous leishmaniasis in Canadian soldiers returning from Afghanistan. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 394–396. [Google Scholar] [CrossRef]
- Novais, F.O.; Santiago, R.C.; Báfica, A.; Khouri, R.; Afonso, L.; Borges, V.M.; de Oliveira, C.I. Neutrophils and macrophages cooperate in host resistance against Leishmania braziliensis infection. J. Immunol. 2009, 183, 8088–8098. [Google Scholar] [CrossRef]
- Badaro, R.; Falcoff, E.; Badaro, F.S.; Carvalho, E.M.; Pedral-Sampaio, D.; Barral, A.; Bina, J.C. Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. New Engl. J. Med. 1990, 322, 16–21. [Google Scholar] [CrossRef]
- Sundar, S.; Olliaro, P.L. Miltefosine in the Treatment of Leishmaniasis: Clinical Evidence for Informed Clinical Risk Management. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2376078/ (accessed on 14 July 2020).
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and Treatment of Leishmaniasis: Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5239701/ (accessed on 14 July 2020).
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Ouattara, G.M.; Pécoul, B.; Rijal, S.; Rode, J.; et al. Recent Development of Visceral Leishmaniasis Treatments: Successes, Pitfalls, and Perspectives. Available online: https://cmr.asm.org/content/31/4/e00048-18 (accessed on 14 July 2020).
- Balasegaram, M.; Ritmeijer, K.; Lima, M.A.; Burza, S.; Ortiz Genovese, G.; Milani, B.; Gaspani, S.; Potet, J.; Chappuis, F. Liposomal Amphotericin B as a Treatment for Human Leishmaniasis. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3518293/ (accessed on 5 March 2019).
- Goyonlo, V.M.; Vosoughi, E.; Kiafar, B.; Nahidi, Y.; Momenzadeh, A.; Taheri, A.R. Efficacy of Intralesional Amphotericin B for the Treatment of Cutaneous Leishmaniasis. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4248523/ (accessed on 29 May 2019).
- Berman, J.J. Treatment of leishmaniasis with miltefosine: 2008 status. Expert Opin. Drug. Metab. Toxicol. 2008, 4, 1209–1216. [Google Scholar] [CrossRef]
- Sunyoto, T.; Potet, J.; Boelaert, M. Why Miltefosine—A Life-Saving Drug for Leishmaniasis—Is Unavailable to People Who Need It the Most. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5935166/ (accessed on 14 July 2020).
- Uzun, S.; Durdu, M.; Culha, G.; Allahverdiyev, A.M.; Memisoglu, H.R. Clinical features, epidemiology, and efficacy and safety of intralesional antimony treatment of cutaneous leishmaniasis: Recent experience in Turkey. J. Parasitol. 2004, 90, 853–859. [Google Scholar] [CrossRef]
- Peniche, A.G.; Bonilla, D.L.; Palma, G.I.; Melby, P.C.; Travi, B.L.; Osorio, E.Y. A Secondary Wave of Neutrophil Infiltration Causes Necrosis and Ulceration in Lesions of Experimental American Cutaneous Leishmaniasis. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0179084 (accessed on 30 May 2019).
- Lopes, M.F.; Costa-da-Silva, A.C.; DosReis, G.A. Innate Immunity to Leishmania Infection: Within Phagocytes. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4119695/ (accessed on 7 May 2020).
- Rossi, M.; Fasel, N. How to Master the Host Immune System? Leishmania Parasites have the Solutions! Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5892169/ (accessed on 14 July 2020).
- Nylén, S.; Gautam, S. Immunological Perspectives of Leishmaniasis. 2010. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2889653/ (accessed on 14 July 2020).
- Hurrell, B.P.; Regli, I.B.; Tacchini-Cottier, F. Different Leishmania Species Drive Distinct Neutrophil Functions. Trends Parasitol. 2016, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Oualha, R.; Barhoumi, M.; Marzouki, S.; Harigua-Souiai, E.; Ben Ahmed, M.; Guizani, I. Infection of Human Neutrophils with Leishmania infantum or Leishmania Major Strains Triggers Activation and Differential Cytokines Release. 2019. Available online: https://www.frontiersin.org/articles/10.3389/fcimb.2019.00153/full (accessed on 14 July 2020).
- Wardini, A.B.; Pinto-da-Silva, L.H.; Nadaes, N.R.; Nascimento, M.T.; Roatt, B.M.; Reis, A.B.; Viana, K.F.; Giunchetti, R.C.; Saraiva, E.M. Neutrophil Properties in Healthy and Leishmania Infantum-Naturally Infected Dogs. 2019. Available online: https://www.nature.com/articles/s41598-019-42687-9 (accessed on 14 July 2020).
- Liew, F.Y.; Millott, S.; Parkinson, C.; Palmer, R.M.; Moncada, S. Macrophage Killing of Leishmania Parasite In Vivo Is Mediated by Nitric Oxide from L-Arginine. 1990. Available online: https://www.jimmunol.org/content/144/12/4794 (accessed on 21 June 2019).
- Carneiro, P.P.; Conceição, J.; Macedo, M.; Magalhães, V.; Carvalho, E.M.; Bacellar, O. The Role of Nitric Oxide and Reactive Oxygen Species in the Killing of Leishmania braziliensis by Monocytes from Patients with Cutaneous Leishmaniasis. 2016. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4739692/ (accessed on 14 July 2020).
- Acuña, S.M.; Aoki, J.I.; Laranjeira-Silva, M.F.; Zampieri, R.A.; Fernandes, J.C.R.; Muxel, S.M.; Floeter-Winter, L.M. Arginase Expression Modulates Nitric Oxide Production in Leishmania (Leishmania) Amazonensis. 2017. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0187186 (accessed on 14 July 2020).
- Vouldoukis, I.; Riveros-Moreno, V.; Dugas, B.; Ouaaz, F.; Bécherel, P.; Debré, P.; Moncada, S.; Mossalayi, M.D. The Killing of Leishmania Major by Human Macrophages Is Mediated by Nitric Oxide Induced after Ligation of the Fc Epsilon RII/CD23 Surface Antigen. 1995. Available online: https://www.pnas.org/content/92/17/7804 (accessed on 14 July 2020).
- Charmoy, M.; Megnekou, R.; Allenbach, C.; Zweifel, C.; Perez, C.; Monnat, K.; Breton, M.; Ronet, C.; Launois, P.; Tacchini-Cottier, F. Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J. Leukoc. Biol. 2007, 82, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Faber, W.R.; Oskam, L.; van Gool, T.; Kroon, N.C.M.; Knegt-Junk, K.J.; Hofwegen, H.; van der Wal, A.C.; Kager, P.A. Value of diagnostic techniques for cutaneous leishmaniasis. J. Am. Acad. Dermatol. 2003, 49, 70–74. [Google Scholar] [CrossRef]
- Von Stebut, E.; Metz, M.; Milon, G.; Knop, J.; Maurer, M. Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1alpha /beta released from neutrophils recruited by mast cell-derived TNFalpha. Blood 2003, 101, 210–215. [Google Scholar] [CrossRef]
- Van Zandbergen, G.; Hermann, N.; Laufs, H.; Solbach, W.; Laskay, T. Leishmania Promastigotes Release a Granulocyte Chemotactic Factor and Induce Interleukin-8 Release but Inhibit Gamma Interferon-Inducible Protein 10 Production by Neutrophil Granulocytes. Infect. Immun. 2002, 70, 4177–4184. [Google Scholar] [CrossRef]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003, 11, 210–214. [Google Scholar] [CrossRef]
- Peters, N.C.; Egen, J.G.; Secundino, N.; Debrabant, A.; Kimblin, N.; Kamhawi, S.; Lawyer, P.; Fay, M.P.; Germain, R.N.; Sacks, D. In vivo imaging reveals an essential role for neutrophils in Leishmaniasis transmitted by sand flies. Science 2008, 321, 970–974. [Google Scholar] [CrossRef]
- Soulat, D.; Bogdan, C. Function of Macrophage and Parasite Phosphatases in Leishmaniasis. Front Immunol. 2017. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2017.01838/full (accessed on 14 July 2020).
- Tomiotto-Pellissier, F.; Bortoleti, B.T.d.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. 2018. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6220043/ (accessed on 14 July 2020).
- Jayakumar, A.; Widenmaier, R.; Ma, X.; McDowell, M.A. Transcriptional Inhibition of Interleukin-12 Promoter Activity in Leishmania spp.-Infected Macrophages. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2665708/ (accessed on 14 July 2020).
- Costa, S.F.; Gomes, V.O.; dos Santos Maciel, M.O.; Melo, L.M.; Venturin, G.L.; Bragato, J.P.; Rebech, G.T.; de Oliveira Santos, C.; de Oliveira, B.M.N.; de Sá Oliveira, G.G.; et al. Combined in vitro IL-12 and IL-15 stimulation promotes cellular immune response in dogs with visceral leishmaniasis. PLoS Negl. Trop. Dis. 2020, 14, e0008021. [Google Scholar] [CrossRef] [PubMed]
- Park, A.Y.; Hondowicz, B.D.; Scott, P. IL-12 Is Required to Maintain a Th1 Response during Leishmania major Infection. J. Immunol. 2000, 165, 896–902. [Google Scholar] [CrossRef]
- Park, A.Y.; Hondowicz, B.; Kopf, M.; Scott, P. The role of IL-12 in maintaining resistance to Leishmania major. J. Immunol. 2002, 168, 5771–5777. [Google Scholar] [CrossRef]
- Wanderley, J.L.M.; Deolindo, P.; Carlsen, E.; Portugal, A.B.; DaMatta, R.A.; Barcinski, M.A.; Soong, L. CD4+ T Cell-Dependent Macrophage Activation Modulates Sustained PS Exposure on Intracellular Amastigotes of Leishmania amazonensis. Front. Cell. Infect. Microbiol. 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C.; Arendse, B.; Schwegmann, A.; Myburgh, E.; Brombacher, F. Impairment of Alternative Macrophage Activation Delays Cutaneous Leishmaniasis in Nonhealing BALB/c Mice. J. Immunol. 2006, 176, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur, H.; Prina, E.; Rosazza, T.; Kokou, K.; N’Diaye, P.; Aulner, N.; Varet, H.; Bussotti, G.; Xing, Y.; Milon, G.; et al. Targeting Macrophage Histone H3 Modification as a Leishmania Strategy to Dampen the NF-κB/NLRP3-Mediated Inflammatory Response. Cell Rep. 2020, 30, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y.; Li, Y.; Millott, S. Tumour necrosis factor (TNF-alpha) in leishmaniasis. II. TNF-alpha-induced macrophage leishmanicidal activity is mediated by nitric oxide from L-arginine. Immunology 1990, 71, 556–559. [Google Scholar]
- Gregory, D.J.; Godbout, M.; Contreras, I.; Forget, G.; Olivier, M. A novel form of NF-kappaB is induced by Leishmania infection: Involvement in macrophage gene expression. Eur. J. Immunol. 2008, 38, 1071–1081. [Google Scholar] [CrossRef]
- Bogdan, C.; Röllinghoff, M. How do Protozoan Parasites Survive inside Macrophages? Parasitol. Today 1999, 15, 22–28. [Google Scholar] [CrossRef]
- Wanasen, N.; Soong, L. L-arginine metabolism and its impact on host immunity against Leishmania infection. Immunol. Res. 2008, 41, 15–25. [Google Scholar] [CrossRef]
- Wei, X.Q.; Charles, I.G.; Smith, A.; Ure, J.; Feng, G.J.; Huang, F.P.; Xu, D.; Muller, W.; Moncada, S.; Liew, F.Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 1995, 375, 408–411. [Google Scholar] [CrossRef]
- Gantt, K.R.; Goldman, T.L.; McCormick, M.L.; Miller, M.A.; Jeronimo, S.M.B.; Nascimento, E.T.; Britigan, B.E.; Wilson, M.E. Oxidative Responses of Human and Murine Macrophages During Phagocytosis of Leishmania chagasi. J. Immunol. 2001, 167, 893–901. [Google Scholar] [CrossRef]
- Stenger, S.; Donhauser, N.; Thüring, H.; Röllinghoff, M.; Bogdan, C. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 1996, 183, 1501–1514. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Carneiro, M.B.H.; Doria, N.A.; Roma, E.H.; Ribeiro-Gomes, F.L.; Inbar, E.; Lee, S.H.; Mendez, J.; Paun, A.; Sacks, D.L.; et al. Divergent roles for Ly6C+CCR2+CX3CR1+ inflammatory monocytes during primary or secondary infection of the skin with the intra-phagosomal pathogen Leishmania major. PLoS Pathog. 2017, 13, e1006479. [Google Scholar] [CrossRef]
- Soong, L. Modulation of Dendritic Cell Function by Leishmania Parasites. J. Immunol. 2008, 180, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Feijó, D.; Tibúrcio, R.; Ampuero, M.; Brodskyn, C.; Tavares, N. Dendritic Cells and Leishmania Infection: Adding Layers of Complexity to a Complex Disease. 2016. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4745329/ (accessed on 14 July 2020).
- Tibúrcio, R.; Nunes, S.; Nunes, I.; Rosa Ampuero, M.; Silva, I.B.; Lima, R.; Machado Tavares, N.; Brodskyn, C. Molecular Aspects of Dendritic Cell Activation in Leishmaniasis: An Immunobiological View. 2019. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00227/full (accessed on 14 July 2020).
- Hammami, A.; Abidin, B.M.; Heinonen, K.M.; Stäger, S. HIF-1α hampers dendritic cell function and Th1 generation during chronic visceral leishmaniasis. Sci. Rep. 2018, 8, 3500. [Google Scholar] [CrossRef] [PubMed]
- Contreras, I.; Estrada, J.A.; Guak, H.; Martel, C.; Borjian, A.; Ralph, B.; Shio, M.T.; Fournier, S.; Krawczyk, C.M.; Olivier, M. Impact of Leishmania mexicana Infection on Dendritic Cell Signaling and Functions. PLoS Negl. Trop. Dis. 2014, 8, e3202. [Google Scholar] [CrossRef]
- Alexander, J.; Brombacher, F. T Helper1/T Helper2 Cells and Resistance/Susceptibility to Leishmania Infection: Is This Paradigm Still Relevant? 2012. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2012.00080/full (accessed on 14 July 2020).
- Jaiswal, A.K.; Khare, P.; Joshi, S.; Kushawaha, P.K.; Sundar, S.; Dube, A. Th1 Stimulatory Proteins of Leishmania donovani: Comparative Cellular and Protective Responses of rTriose Phosphate Isomerase, rProtein Disulfide Isomerase and rElongation Factor-2 in Combination with rHSP70 against Visceral Leishmaniasis. PLoS ONE 2014, 9, e108556. [Google Scholar] [CrossRef] [PubMed]
- Miralles, G.D.; Stoeckle, M.Y.; McDermott, D.F.; Finkelman, F.D.; Murray, H.W. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect. Immun. 1994, 62, 1058–1063. [Google Scholar] [CrossRef]
- Dayakar, A.; Chandrasekaran, S.; Kuchipudi, S.V.; Kalangi, S.K. Cytokines: Key Determinants of Resistance or Disease Progression in Visceral Leishmaniasis: Opportunities for Novel Diagnostics and Immunotherapy. 2019. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00670/full (accessed on 14 July 2020).
- Marovich, M.A.; McDowell, M.A.; Thomas, E.K.; Nutman, T.B. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J. Immunol. 2000, 164, 5858–5865. [Google Scholar] [CrossRef]
- Kelsall, B.L.; Stüber, E.; Neurath, M.; Strober, W. Interleukin-12 production by dendritic cells: The role of CD40-CD40L interactions in Th1 T-cell responses. Ann. N. Y. Acad. Sci. 1996, 795, 116–126. [Google Scholar] [CrossRef]
- Okwor, I.; Jia, P.; Uzonna, J.E. Interaction of Macrophage Antigen 1 and CD40 Ligand Leads to IL-12 Production and Resistance in CD40-Deficient Mice Infected with Leishmania major. J. Immunol. 2015, 195, 3218–3226. [Google Scholar] [CrossRef]
- Kamanaka, M.; Yu, P.; Yasui, T.; Yoshida, K.; Kawabe, T.; Horii, T.; Kishimoto, T.; Kikutani, H. Protective Role of CD40 in Leishmania major Infection at Two Distinct Phases of Cell-Mediated Immunity. Immunity 1996, 4, 275–281. [Google Scholar] [CrossRef]
- Hathcock, K.; Laszlo, G.; Pucillo, C.; Linsley, P.; Hodes, R.J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: Expression and function. J. Exp. Med. 1994, 180, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Natural Killer Cells in Experimental and Human Leishmaniasis. 2012. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3417408/ (accessed on 24 June 2020).
- Prajeeth, C.K.; Haeberlein, S.; Sebald, H.; Schleicher, U.; Bogdan, C. Leishmania-Infected Macrophages Are Targets of NK Cell-Derived Cytokines but Not of NK Cell Cytotoxicity. Infect. Immun. 2011, 79, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Cañeda-Guzmán, I.C.; Salaiza-Suazo, N.; Fernández-Figueroa, E.A.; Carrada-Figueroa, G.; Aguirre-García, M.; Becker, I.N.K. Cell Activity Differs between Patients with Localized and Diffuse Cutaneous Leishmaniasis Infected with Leishmania mexicana: A Comparative Study of TLRs and Cytokines. PLoS ONE 2014, 9, e112410. [Google Scholar] [CrossRef]
- Müller, K.; van Zandbergen, G.; Hansen, B.; Laufs, H.; Jahnke, N.; Solbach, W.; Laskay, T. Chemokines, natural killer cells and granulocytes in the early course of Leishmania major infection in mice. Med. Microbiol. Immunol. 2001, 190, 73–76. [Google Scholar] [CrossRef]
- Scharton, T.M.; Scott, P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 1993, 178, 567–577. [Google Scholar] [CrossRef]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Tacchini-Cottier, F.; Zweifel, C.; Belkaid, Y.; Mukankundiye, C.; Vasei, M.; Launois, P.; Milon, G.; Louis, J.A. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J. Immunol. 2000, 165, 2628–2636. [Google Scholar] [CrossRef]
- Gonzalez-Lombana, C.; Gimblet, C.; Bacellar, O.; Oliveira, W.W.; Passos, S.; Carvalho, L.P.; Goldschmidt, M.; Carvalho, E.M.; Scott, P. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog. 2013, 9, e1003243. [Google Scholar] [CrossRef]
- Nascimento, M.S.L.; Carregaro, V.; Lima-Júnior, D.S.; Costa, D.L.; Ryffel, B.; Duthie, M.S.; de Jesus, A.; de Almeida, R.P.; da Silva, J.S. Interleukin 17A acts synergistically with interferon γ to promote protection against Leishmania infantum infection. J. Infect. Dis. 2015, 211, 1015–1026. [Google Scholar] [CrossRef]
- Pitta, M.G.R.; Romano, A.; Cabantous, S.; Henri, S.; Hammad, A.; Kouriba, B.; Argiro, L.; el Kheir, M.; Bucheton, B.; Mary, C.; et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J. Clin. Invest. 2009, 119, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Piccirillo, C.A.; Mendez, S.; Shevach, E.M.; Sacks, D.L. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 2002, 420, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Mandell, M.A.; Beverley, S.M. Continual renewal and replication of persistent Leishmania major parasites in concomitantly immune hosts. Proc. Natl. Acad. Sci. USA 2017, 114, E801–E810. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Sun, C.M.; Bouladoux, N. Parasites and immunoregulatory T cells. Curr. Opin. Immunol. 2006, 18, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Mendez, S.; Sacks, D.L. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J. Immunol. 2005, 174, 2934–2941. [Google Scholar] [CrossRef]
- Mendez, S.; Reckling, S.K.; Piccirillo, C.A.; Sacks, D.; Belkaid, Y. Role for CD4+ CD25+ regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J. Exp. Med. 2004, 200, 201–210. [Google Scholar] [CrossRef]
- Belkaid, Y.; Von Stebut, E.; Mendez, S.; Lira, R.; Caler, E.; Bertholet, S.; Udey, M.C.; Sacks, D. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 2002, 168, 3992–4000. [Google Scholar] [CrossRef]
- Huber, M.; Timms, E.; Mak, T.W.; Röllinghoff, M.; Lohoff, M. Effective and long-lasting immunity against the parasite Leishmania major in CD8-deficient mice. Infect. Immun. 1998, 66, 3968–3970. [Google Scholar] [CrossRef]
- Uzonna, J.E.; Joyce, K.L.; Scott, P. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon gamma-producing CD8+ T cells. J. Exp. Med. 2004, 199, 1559–1566. [Google Scholar] [CrossRef]
- Okwor, I.B.; Jia, P.; Mou, Z.; Onyilagha, C.; Uzonna, J.E. CD8+ T cells Are Preferentially Activated during Primary Low Dose Leishmania major Infection but Are Completely Dispensable during Secondary Anti-Leishmania Immunity. PLoS Negl. Trop. Dis. 2014, 8, e3300. [Google Scholar] [CrossRef]
- Méndez, S.; Belkaid, Y.; Seder, R.A.; Sacks, D. Optimization of DNA vaccination against cutaneous leishmaniasis. Vaccine 2002, 20, 3702–3708. [Google Scholar] [CrossRef]
- Gurunathan, S.; Stobie, L.; Prussin, C.; Sacks, D.L.; Glaichenhaus, N.; Fowell, D.J.; Locksley, R.M.; Chang, J.T.; Wu, C.-Y.; Seder, R.A. Requirements for the Maintenance of Th1 Immunity In Vivo Following DNA Vaccination: A Potential Immunoregulatory Role for CD8+ T Cells. J. Immunol. 2000, 165, 915–924. [Google Scholar] [CrossRef]
- Deak, E.; Jayakumar, A.; Cho, K.W.; Goldsmith-Pestana, K.; Dondji, B.; Lambris, J.D.; McMahon-Pratt, D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur. J. Immunol. 2010, 40, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G.; Hale, C.; Liew, F.Y. Immunological regulation of experimental cutaneous leishmaniasis. IV. Prophylactic effect of sublethal irradiation as a result of abrogation of suppressor T cell generation in mice genetically susceptible to Leishmania tropica. J. Exp. Med. 1981, 153, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.F.; Handman, E. Leishmania tropica major in mice: Vaccination against cutaneous leishmaniasis in mice of high genetic susceptibility. Aust. J. Exp. Biol. Med. Sci. 1983, 61, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Woelbing, F.; Kostka, S.L.; Moelle, K.; Belkaid, Y.; Sunderkoetter, C.; Verbeek, S.; Waisman, A.; Nigg, A.P.; Knop, J.; Udey, M.C.; et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J. Exp. Med. 2006, 203, 177–188. [Google Scholar] [CrossRef]
- Wylie, C.E.; Carbonell-Antoñanzas, M.; Aiassa, E.; Dhollander, S.; Zagmutt, F.J.; Brodbelt, D.C.; Solano-Gallego, L. A systematic review of the efficacy of prophylactic control measures for naturally-occurring canine leishmaniosis, part I: Vaccinations. Prev. Vet. Med. 2014, 117, 7–18. [Google Scholar] [CrossRef]
- Srivastava, S.; Shankar, P.; Mishra, J.; Singh, S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasit Vectors 2016, 9, 277. [Google Scholar] [CrossRef]
- Moreno, J.; Vouldoukis, I.; Martin, V.; McGahie, D.; Cuisinier, A.-M.; Gueguen, S. Use of a LiESP/QA-21 Vaccine (CaniLeish) Stimulates an Appropriate Th1-Dominated Cell-Mediated Immune Response in Dogs. PLoS Negl. Trop. Dis. 2012, 6, e1683. [Google Scholar] [CrossRef]
- Fernández Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L.C.; Alonso, C. A large-scale field randomized trial demonstrates safety and efficacy of the vaccine LetiFend® against canine leishmaniosis. Vaccine 2018, 36, 1972–1982. [Google Scholar] [CrossRef]
- Petitdidier, E.; Pagniez, J.; Papierok, G.; Vincendeau, P.; Lemesre, J.-L.; Bras-Gonçalves, R. Recombinant Forms of Leishmania amazonensis Excreted/Secreted Promastigote Surface Antigen (PSA) Induce Protective Immune Responses in Dogs. PLoS Negl. Trop. Dis. 2016, 10, e0004614. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; Aguiar-Soares, R.D.d.O.; Vitoriano-Souza, J.; Coura-Vital, W.; Braga, S.L.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; de Lana, M.; Gontijo, N.F.; et al. Performance of LBSap Vaccine after Intradermal Challenge with L. infantum and Saliva of Lu. longipalpis: Immunogenicity and Parasitological Evaluation. PLoS ONE 2012, 7, e49780. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.A.; Aguiar-Soares, R.D. de O.; Gama-Ker, H.; Roatt, B.M.; Mendonça, L.Z. de; Alves, M.L.R.; Silveira-Lemos, D. da; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Araújo, M.S.S.; et al. Impact of LbSapSal Vaccine in Canine Immunological and Parasitological Features before and after Leishmania chagasi-Challenge. PLoS ONE 2016, 11, e0161169. [Google Scholar]
- Mou, Z.; Li, J.; Boussoffara, T.; Kishi, H.; Hamana, H.; Ezzati, P.; Hu, C.; Yi, W.; Liu, D.; Khadem, F.; et al. Identification of broadly conserved cross-species protective Leishmania antigen and its responding CD4+ T cells. Sci. Transl. Med. 2015, 7, 310ra167. [Google Scholar] [CrossRef]
- Louis, L.; Clark, M.; Wise, M.C.; Glennie, N.; Wong, A.; Broderick, K.; Uzonna, J.; Weiner, D.B.; Scott, P. Intradermal Synthetic DNA Vaccination Generates Leishmania-Specific T Cells in the Skin and Protection against Leishmania major. Infect. Immun. 2019, 87, e00227-e19. [Google Scholar] [CrossRef] [PubMed]
- Chamakh-Ayari, R.; Bras-Gonçalves, R.; Bahi-Jaber, N.; Petitdidier, E.; Markikou-Ouni, W.; Aoun, K.; Moreno, J.; Carrillo, E.; Salotra, P.; Kaushal, H.; et al. In Vitro Evaluation of a Soluble Leishmania Promastigote Surface Antigen as a Potential Vaccine Candidate against Human Leishmaniasis. PLoS ONE 2014, 9, e92708. [Google Scholar] [CrossRef]
- Osman, M.; Mistry, A.; Keding, A.; Gabe, R.; Cook, E.; Forrester, S.; Wiggins, R.; Di Marco, S.; Colloca, S.; Siani, L.; et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: First-in-human trial of ChAd63-KH. PLoS Negl. Trop. Dis. 2017, 11, e0005527. [Google Scholar] [CrossRef]
- Roatt, B.M.; Aguiar-Soares RD de, O.; Reis, L.E.S.; Cardoso JM de, O.; Mathias, F.A.S.; de Brito, R.C.F.; da Silva, S.M.; Gontijo, N.D.F.; Ferreira, S.d.A.; Valenzuela, J.G.; et al. A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden. Front. Immunol. 2017, 8, 217. [Google Scholar] [CrossRef]
- Mayrink, W.; Magalhaes, P.A.; Michalick, M.S.; da Costa, C.A.; Lima, A.d.O.; Melo, M.N.; Toledo, V.P.; Nascimento, E.; Dias, M.; Genaro, O. Immunotherapy as a treatment of American cutaneous leishmaniasis: Preliminary studies in Brazil. Parassitologia 1992, 34, 159–165. [Google Scholar]
- Jamshidi, S.; Avizeh, R.; Mohebali, M.; Bokaie, S. Immunotherapy Using Autoclaved, L. major Antigens and M. vaccae with Meglumine Antimoniate, for the Treatment of Experimental Canine Visceral Leishmaniasis. Iran. J. Parasitol. 2011, 6, 26–34. [Google Scholar]
- Toepp, A.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Leal-Lima, A.; Anderson, B.; Parrish, M.; Anderson, M.; Fowler, H.; et al. Randomized, controlled, double-blinded field trial to assess Leishmania vaccine effectiveness as immunotherapy for canine leishmaniosis. Vaccine 2018, 36, 6433–6441. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, F.P.; Schoenhaut, D.S.; Rerko, R.M.; Rosser, L.E.; Gately, M.K. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 1993, 177, 1505–1509. [Google Scholar] [CrossRef]
- Montakhab-Yeganeh, H.; Abdossamadi, Z.; Zahedifard, F.; Taslimi, Y.; Badirzadeh, A.; Saljoughian, N.; Taheri, T.; Taghikhani, M.; Rafati, S. Leishmania tarentolae expressing CXCL-10 as an efficient immunotherapy approach against Leishmania major-infected BALB/c mice. Parasite Immunol. 2017, 39, e12461. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, A.; Chandrasekaran, S.; Veronica, J.; Bharadwaja, V.; Maurya, R. Leptin regulates Granzyme-A, PD-1 and CTLA-4 expression in T cell to control visceral leishmaniasis in BALB/c Mice. Sci. Rep. 2017, 7, 14664. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca-Martins, A.M.; Ramos, T.D.; Pratti, J.E.S.; Firmino-Cruz, L.; Gomes, D.C.O.; Soong, L.; Saraiva, E.M.; de Guedes, H.L.d.M. Immunotherapy using anti-PD-1 and anti-PD-L1 in Leishmania amazonensis -infected BALB/c mice reduce parasite load. Sci. Rep. 2019, 9, 20275. [Google Scholar] [CrossRef] [PubMed]
- Quirino, G.F.S.; Nascimento, M.S.L.; Davoli-Ferreira, M.; Sacramento, L.A.; Lima, M.H.F.; Almeida, R.P.; Carregaro, V.; Silva, J.S. Interleukin-27 (IL-27) Mediates Susceptibility to Visceral Leishmaniasis by Suppressing the IL-17–Neutrophil Response. Infect. Immun. 2016, 84, 2289–2298. [Google Scholar] [CrossRef]
- Bodas, M.; Jain, N.; Awasthi, A.; Martin, S.; Loka, R.K.P.; Dandekar, D.; Mitra, D.; Saha, B. Inhibition of IL-2 Induced IL-10 Production as a Principle of Phase-Specific Immunotherapy. J. Immunol. 2006, 177, 4636–4643. [Google Scholar] [CrossRef]
- Gautam, S.; Kumar, R.; Maurya, R.; Nylén, S.; Ansari, N.; Rai, M.; Sundar, S.; Sacks, D. IL-10 Neutralization Promotes Parasite Clearance in Splenic Aspirate Cells From Patients With Visceral Leishmaniasis. J. Infect. Dis. 2011, 204, 1134–1137. [Google Scholar] [CrossRef]
- Khadem, F.; Jia, P.; Mou, Z.; Feiz Barazandeh, A.; Liu, D.; Keynan, Y.; Uzonna, J.E. Pharmacological inhibition of p110δ subunit of PI3K confers protection against experimental leishmaniasis. J. Antimicrob. Chemother. 2017, 72, 467–477. [Google Scholar] [CrossRef]
- Khadir, F.; Shaler, C.R.; Oryan, A.; Rudak, P.T.; Mazzuca, D.M.; Taheri, T.; Dikeakos, J.D.; Haeryfar, S.M.M.; Rafati, S. Therapeutic control of leishmaniasis by inhibitors of the mammalian target of rapamycin. PLoS Negl. Trop. Dis. 2018, 12, e0006701. [Google Scholar] [CrossRef]
- Baranowski, M. Biological role of liver x receptors. J. Physiol. Pharmacol. 2008, 59, 31–55. [Google Scholar] [PubMed]
- Bruhn, K.W.; Marathe, C.; Maretti-Mira, A.C.; Nguyen, H.; Haskell, J.; Tran, T.A.; Vanchinathan, V.; Gaur, U.; Wilson, M.E.; Tontonoz, P.; et al. LXR Deficiency Confers Increased Protection against Visceral Leishmania Infection in Mice. PLoS Negl. Trop. Dis. 2010, 4, e886. [Google Scholar] [CrossRef] [PubMed]
- Aryl Hydrocarbon Receptor-Signaling Regulates Early Leishmania Major-Induced Cytokine Expression—Abstract—Europe PMC [Internet]. Available online: https://europepmc.org/article/PMC/6843081 (accessed on 7 May 2020).
- Gupta, G.; Mou, Z.; Jia, P.; Sharma, R.; Zayats, R.; Viana, S.; Shan, L.; Barral, A.; Boaventura, V.; Murooka, T.; et al. The Long Pentraxin 3 (PTX3) Suppresses Immunity to Cutaneous Leishmaniasis by Negatively Regulating Th17 Response. Cell Rep. 2020. in revision. [Google Scholar]
- Ikeogu, N.; Edechi, C.; Akaluka, G.; Feiz-Barazandeh, A.; Zayats, R.; Salako, E.; Onyilagha, C.; Jia, P.; Mou, Z.; Shan, L.; et al. Semaphorin 3E promotes susceptibility to Leishmania major infection in mice by suppressing CD4+ Th1 cell response. J. Immunol. 2020. in revision. [Google Scholar]
- Nascimento, L.F.M.; Miranda, D.F.H.; Moura, L.D.; Pinho, F.A.; Werneck, G.L.; Khouri, R.; Reed, S.G.; Duthie, M.S.; Barral, A.; Barral-Netto, M.; et al. Allopurinol therapy provides long term clinical improvement, but additional immunotherapy is required for sustained parasite clearance, in L. infantum-infected dogs. Vaccine X. 2020, 4, 100048. [Google Scholar] [CrossRef]
- Ghosh, M.; Pal, C.; Ray, M.; Maitra, S.; Mandal, L.; Bandyopadhyay, S. Dendritic cell-based immunotherapy combined with antimony-based chemotherapy cures established murine visceral leishmaniasis. J. Immunol. 2003, 170, 5625–5629. [Google Scholar] [CrossRef] [PubMed]
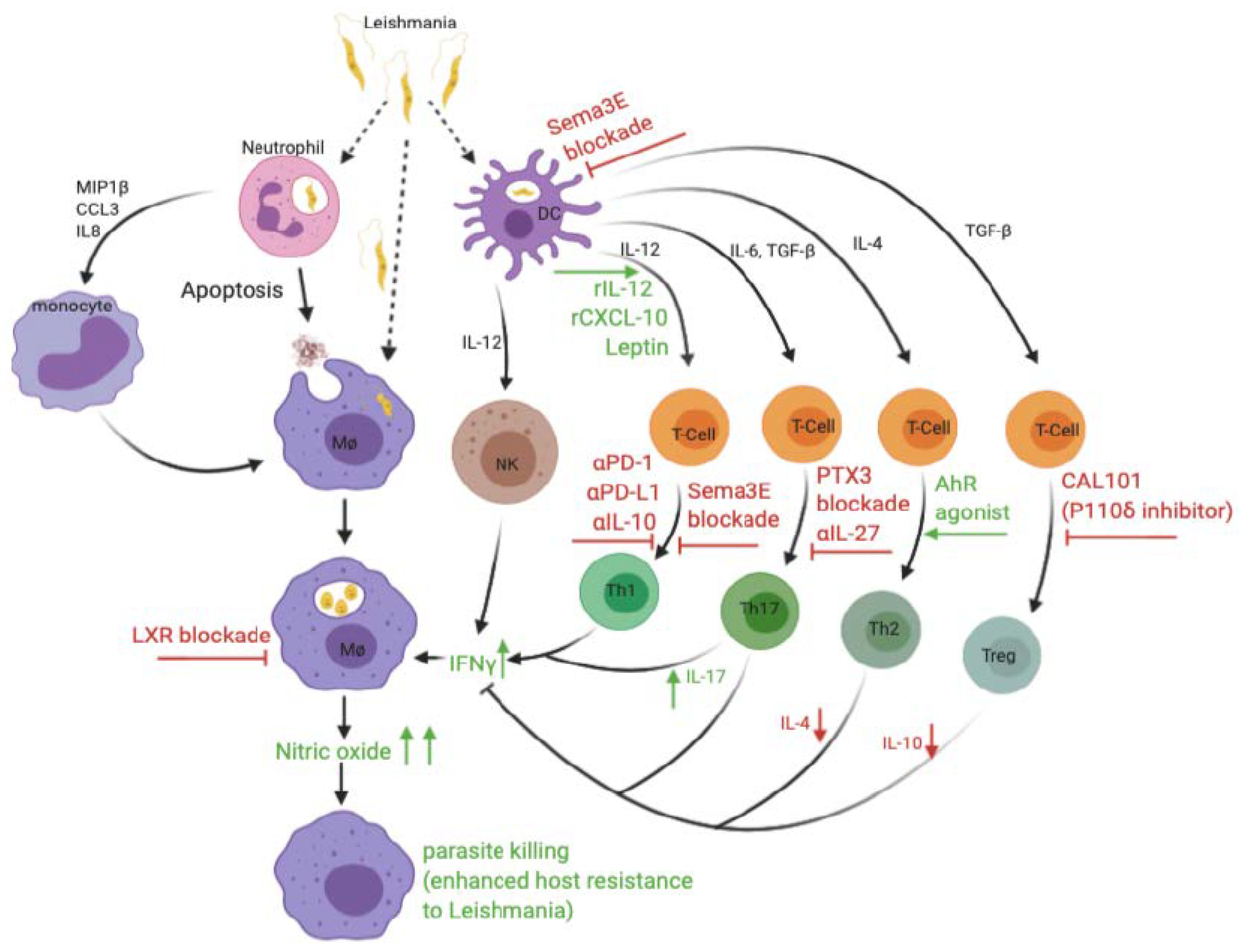
| Vaccine Candidate | Type of Vaccine | Quality of Protection/Species | Host | Reference(s) |
|---|---|---|---|---|
| CaniLeish® | Fractionated | Good | Dogs | [98] |
| Leishmune® | Fractionated | Good | Dogs | [98,99] |
| LiESP/QA-21 | Good | Dogs | [100] | |
| LetiFend® | Recombinant protein | Good/L. infantum | Dogs | [101] |
| ES LaPSA-38S | Recombinant protein | Good prophylactic vaccine | Dogs | [102] |
| LBSap vaccine (L. brazilliensis antigen & saponin adjuvant) | Fractionated | Good/cross-protection (L. infantum) | Dogs | [103] |
| LbSapSal vaccine (L. brazilliensis antigen + saponin + lutzomiyia longpalpis salivary gland extract) | Fractionated | Good/cross protection (L. chagasi) | Dogs | [104] |
| Leishmania glycosomal PEPCK | Recombinant protein | Good/cross protection (L. major, L. donovani) | Mice | [105,106] |
| Promastigote Surface Antigen (PSA) from L. amazonensis | Recombinant protein | Good/L. amazonensis | Human T cells | [107] |
| ChAd63-KH | DNA | Good/L. donovani | Human (Clinical trial) | [108] |
| L. bralizilliensis antigen + monophosphoryl lipid A (MPL) adjuvant | Subunit | Good/L. infantum | Dogs | [109] |
| Agent | Type of Therapy | Protection | Host | Reference(s) |
|---|---|---|---|---|
| Leishmania + M. vaccae + Meglumine antimoniate | Immunotherapy | Effective treatment of cutaneous leishmaniasis; not as effective as glucantime | Dogs | [111] |
| Recombinant IL-12 | Immunotherapy | Promoted parasite clearance and induced protective immunity against L. major challenge | Mice | [113] |
| Recombinant CXCL-10 | Immunotherapy | Significantly decreased parasite burden, increased NO production and Th1 response | Mice | [114] |
| Anti-PD-1 and anti-PD-L1 monoclonal antibodies | Immunotherapy | Increased induction of protective immune cells resulting in lower parasite burden | Mice | [116] |
| IL-2 and IL-10 blockade by monoclonal antibody treatment | Immunotherapy | Effectively restored the host’s T cell functions leading to reduced parasite burdens | Mice | [118,119] |
| CAL-101 (p110δ- inhibitor) | Chemotherapy | Reduced parasite burden in cutaneous lesions, spleens, and liver | Mice | [120] |
| GSK-2126458 and rapamycin (m-Tor inhibitor) | Chemotherapy | Controlled disease progression and reduced parasite burden | Mice | [121] |
| Liver X receptors deletion | Immunotherapy | Reduced parasite burden in liver | Mice | [123] |
| Pentraxin-3 (PTX-3) gene deletion | Immunotherapy | Reduced cutaneous lesion and parasite burden by enhancing Th17/I17 response | Mice | [125] |
| Semaphorin-3E gene deletion | Immunotherapy | Reduced cutaneous lesion and parasite burden by increasing Th1 response | Mice | [126] |
| Allopurinol & Leishmania vaccine | Immunotherapy + chemotherapy | Clearance of L. infantum and long-lasting immunity | Mice | [127] |
| Soluble L. donovani antigen (SLDA) pulsed-BMDCs & sodium antimony gluconate | Immunotherapy + chemotherapy | Complete parasite clearance from liver and spleens | Mice | [128] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeogu, N.M.; Akaluka, G.N.; Edechi, C.A.; Salako, E.S.; Onyilagha, C.; Barazandeh, A.F.; Uzonna, J.E. Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms 2020, 8, 1201. https://doi.org/10.3390/microorganisms8081201
Ikeogu NM, Akaluka GN, Edechi CA, Salako ES, Onyilagha C, Barazandeh AF, Uzonna JE. Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms. 2020; 8(8):1201. https://doi.org/10.3390/microorganisms8081201
Chicago/Turabian StyleIkeogu, Nnamdi M., Gloria N. Akaluka, Chidalu A. Edechi, Enitan S. Salako, Chukwunonso Onyilagha, Aida F. Barazandeh, and Jude E. Uzonna. 2020. "Leishmania Immunity: Advancing Immunotherapy and Vaccine Development" Microorganisms 8, no. 8: 1201. https://doi.org/10.3390/microorganisms8081201
APA StyleIkeogu, N. M., Akaluka, G. N., Edechi, C. A., Salako, E. S., Onyilagha, C., Barazandeh, A. F., & Uzonna, J. E. (2020). Leishmania Immunity: Advancing Immunotherapy and Vaccine Development. Microorganisms, 8(8), 1201. https://doi.org/10.3390/microorganisms8081201





