Cooperative Regulation of Campylobacter jejuni Heat-Shock Genes by HspR and HrcA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Media
2.2. Molecular Biology Procedures
2.3. Overexpression and Purification of Recombinant Proteins
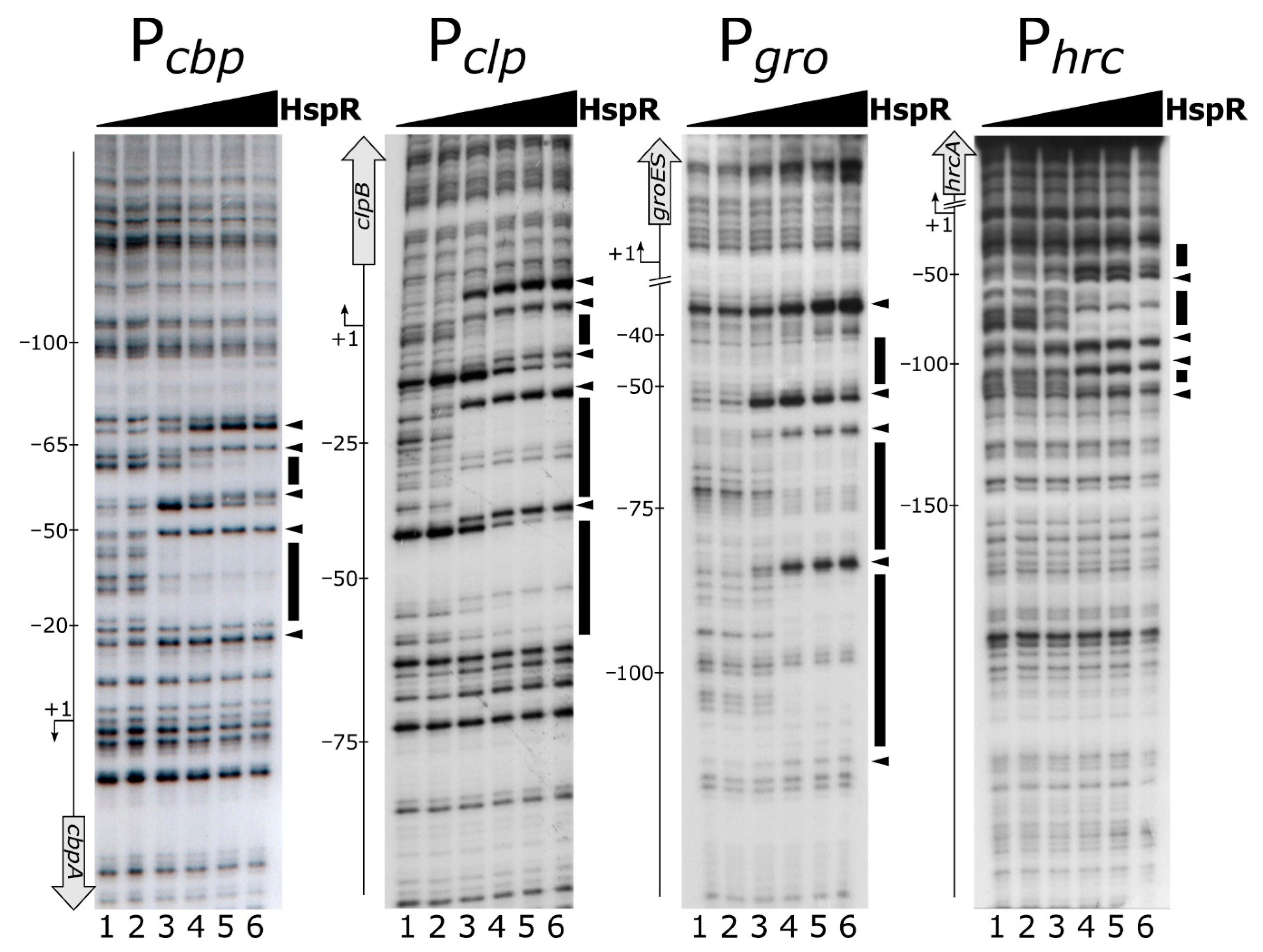
2.4. DNase I Footprinting
2.5. GST-Pull-Down Assay
2.5.1. Protein Overexpression and Slurry Interaction
2.5.2. GST Pull-Down and SDS-PAGE or Western Blot Analyses
3. Results
3.1. The HspR Repressor Directly Binds to Extended DNA Regions Mapping within Several C. jejuni Heat-Shock Promoters
3.2. Sequence Analysis of HspR Binding Sites
3.3. High-Affinity HAIR-Like Motifs are Necessary to Enhance HspR-DNA Interaction to Flanking Low-Affinity Sites
3.4. The HrcA Repressor Directly Binds to CIRCE-Like Sequences Mapping within Pgro and Phrc Heat-Shock Promoters
3.5. Cooperative Interaction of HspR and HrcA on the Co-Regulated Pgro Promoter
3.6. HrcA Directly Interacts with HspR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Narberhaus, F. Negative regulation of bacterial heat shock genes. Mol. Microbiol. 1999, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mogk, A.; Tomoyasu, T.; Goloubinoff, P.; Rüdiger, S.; Röder, D.; Langen, H.; Bukau, B. Identification of thermolabile Escherichia coli proteins: Prevention and reversion of aggregation by DnaK and ClpB. EMBO J. 1999, 18, 6934–6949. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, C. Toothpicks, serendipity and the emergence of the Escherichia coli DnaK (Hsp70) and GroEL (Hsp60) chaperone machines. Genetics 2006, 174, 1699–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncarati, D.; Scarlato, V. Regulation of heat-shock genes in bacteria: From signal sensing to gene expression output. FEMS Microbiol. Rev. 2017, 41, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Blaser, M.J. Epidemiologic and clinical features of Campylobacter jejuni infections. J. Infect. Dis. 1997, 176 (Suppl. 2), S103–S105. [Google Scholar] [CrossRef] [Green Version]
- Nyati, K.K.; Nyati, R. Role of Campylobacter jejuni infection in the pathogenesis of Guillain-Barré syndrome: An update. Biomed Res. Int. 2013, 2013, 852195. [Google Scholar] [CrossRef] [Green Version]
- Young, K.T.; Davis, L.M.; Dirita, V.J. Campylobacter jejuni: Molecular biology and pathogenesis. Nat. Rev. Microbiol. 2007, 4, 665–679. [Google Scholar] [CrossRef]
- Apel, D.; Ellermeier, J.; Pryjma, M.; Dirita, V.J.; Gaynor, E.C. Characterization of Campylobacter jejuni RacRS reveals roles in the heat shock response, motility, and maintenance of cell length homogeneity. J. Bacteriol. 2012, 194, 2342–2354. [Google Scholar] [CrossRef] [Green Version]
- Parkhill, J.; Wren, B.W.; Mungall, K.; Ketley, J.M.; Churcher, C.; Basham, D.; Chillingworth, T.; Davies, R.M.; Feltwell, T.; Holroyd, S.; et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 2000, 403, 665–668. [Google Scholar] [CrossRef] [Green Version]
- Gundogdu, O.; Bentley, S.D.; Holden, M.T.; Parkhill, J.; Dorrell, N.; Wren, B.W. Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence. BMC Genom. 2007, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.T.; Brøndsted, L.; Pearson, B.M.; Mulholland, F.; Parker, M.; Pin, C.; Wells, J.M.; Ingmer, H. Diverse roles for HspR in Campylobacter jejuni revealed by the proteome, transcriptome and phenotypic characterization of an hspR mutant. Microbiology 2005, 151 Pt 3, 905–915. [Google Scholar] [CrossRef] [Green Version]
- Holmes, C.W.; Penn, C.W.; Lund, P.A. The hrcA and hspR regulons of Campylobacter jejuni. Microbiology 2010, 156 Pt 1, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Roncarati, D.; Danielli, A.; Spohn, G.; Delany, I.; Scarlato, V. Transcriptional regulation of stress response and motility functions in Helicobacter pylori is mediated by HspR and HrcA. J. Bacteriol. 2007, 189, 7234–7243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepe, S.; Pinatel, E.; Fiore, E.; Puccio, S.; Peano, C.; Brignoli, T.; Vannini, A.; Danielli, A.; Scarlato, V.; Roncarati, D. The Helicobacter pylori heat-shock repressor HspR: Definition of its direct regulon and characterization of the cooperative DNA-binding mechanism on its own promoter. Front. Microbiol. 2018, 9, 1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucca, G.; Ferina, G.; Puglia, A.M.; Smith, C.P. The dnaK operon of Streptomyces coelicolor encodes a novel heat-shock protein which binds to the promoter region of the operon. Mol. Microbiol. 1995, 17, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Bucca, G.; Hindle, Z.; Smith, C.P. Regulation of the dnaK operon of Streptomyces coelicolor A3 is governed by HspR, an autoregulatory repressor protein. J. Bacteriol. 1997, 179, 5999–6004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandvalet, C.; Servant, P.; Mazodier, P. Disruption of hspR, the repressor gene of the dnaK operon in Streptomyces albus G. Mol. Microbiol. 1997, 23, 77–84. [Google Scholar] [CrossRef]
- Zuber, U.; Schumann, W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J. Bacteriol. 1994, 176, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Thies, F.L.; Karch, H.; Hartung, H.P.; Giegerich, G. Cloning and expression of the dnaK gene of Campylobacter jejuni and antigenicity of heat shock protein 70. Infect. Immun. 1999, 67, 1194–1200. [Google Scholar] [CrossRef] [Green Version]
- Battesti, A.; Bouveret, E. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods 2012, 58, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Roncarati, D.; Danielli, A.; Scarlato, V. The HrcA repressor is the thermosensor of the heat-shock regulatory circuit in the human pathogen Helicobacter pylori. Mol. Microbiol. 2014, 92, 910–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncarati, D.; Spohn, G.; Tango, N.; Danielli, A.; Delany, I.; Scarlato, V. Expression, purification and characterization of the membrane-associated HrcA repressor protein of Helicobacter pylori. Protein Expr. Purif. 2007, 51, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yamamoto, T.; Suzuki, Y. Renaturation of Bacillus thermoglucosidasius HrcA repressor by DNA and thermostability of the HrcA-DNA complex in vitro. J. Bacteriol. 2001, 183, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reischl, S.; Wiegert, T.; Schumann, W. Isolation and analysis of mutant alleles of the Bacillus subtilis HrcA repressor with reduced dependency on GroE function. J. Biol. Chem. 2002, 277, 32659–32667. [Google Scholar] [CrossRef] [Green Version]
- Pelliciari, S.; Pinatel, E.; Vannini, A.; Peano, C.; Puccio, S.; De Bellis, G.; Danielli, A.; Scarlato, V.; Roncarati, D. Insight into the essential role of the Helicobacter pylori HP1043 orphan response regulator: Genome-wide identification and characterization of the DNA-binding sites. Sci. Rep. 2017, 7, 41063. [Google Scholar] [CrossRef]
- Zhou, P.; Wagner, G. Overcoming the solubility limit with solubility-enhancement tags: Successful applications in biomolecular NMR studies. J. Biomol. NMR 2010, 46, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Roncarati, D.; Danielli, A.; Scarlato, V. CbpA acts as a modulator of HspR repressor DNA binding activity in Helicobacter pylori. J. Bacteriol. 2011, 193, 5629–5636. [Google Scholar] [CrossRef] [Green Version]
- Dugar, G.; Herbig, A.; Förstner, K.U.; Heidrich, N.; Reinhardt, R.; Nieselt, K.; Sharma, C.M. High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates. PLoS Genet. 2013, 9, e1003495. [Google Scholar] [CrossRef]
- Grandvalet, C.; De Crécy-Lagard, V.; Mazodier, P. The ClpB ATPase of Streptomyces albus G belongs to the HspR heat shock regulon. Mol. Microbiol. 1999, 31, 521–532. [Google Scholar] [CrossRef]
- Stewart, G.R.; Wernisch, L.; Stabler, R.; Mangan, J.A.; Hinds, J.; Laing, K.G.; Young, D.B.; Butcher, P.D. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology 2002, 148 Pt 10, 3129–3138. [Google Scholar] [CrossRef] [Green Version]
- Chastanet, A.; Prudhomme, M.; Clavery, J.P.; Msadek, T. Regulation of Streptococcus pneumoniae clp genes and their role in competence development and stress survival. J. Bacteriol. 2001, 183, 7295–7307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastanet, A.; Fert, J.; Msadek, T. Comparative genomics reveal novel heat shock regulatory mechanisms in Staphylococcus aureus and other Gram-positive bacteria. Mol. Microbiol. 2003, 47, 1061–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Anil Kumar, V.; Das, A.K.; Bansal, R.; Sarkar, D. A transcriptional co-repressor regulatory circuit controlling the heat-shock response of Mycobacterium tuberculosis. Mol. Microbiol. 2014, 94, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Sevalkar, R.R.; Arora, D.; Singh, P.R.; Singh, R.; Nandicoori, V.K.; Karthikeyan, S.; Sarkar, D. Functioning of Mycobacterial heat shock repressors requires the master virulence regulator PhoP. J. Bacteriol. 2019, 201, e00013-19. [Google Scholar] [CrossRef] [Green Version]
- Spohn, G.; Danielli, A.; Roncarati, D.; Delany, I.; Rappuoli, R.; Scarlato, V. Dual control of Helicobacter pylori heat shock gene transcription by HspR and HrcA. J. Bacteriol. 2004, 186, 2956–2965. [Google Scholar] [CrossRef] [Green Version]
- Roncarati, D.; Scarlato, V. The interplay between two transcriptional repressors and chaperones orchestrates Helicobacter pylori heat-shock response. Int. J. Mol. Sci. 2018, 19, 1702. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palombo, M.; Scarlato, V.; Roncarati, D. Cooperative Regulation of Campylobacter jejuni Heat-Shock Genes by HspR and HrcA. Microorganisms 2020, 8, 1161. https://doi.org/10.3390/microorganisms8081161
Palombo M, Scarlato V, Roncarati D. Cooperative Regulation of Campylobacter jejuni Heat-Shock Genes by HspR and HrcA. Microorganisms. 2020; 8(8):1161. https://doi.org/10.3390/microorganisms8081161
Chicago/Turabian StylePalombo, Marta, Vincenzo Scarlato, and Davide Roncarati. 2020. "Cooperative Regulation of Campylobacter jejuni Heat-Shock Genes by HspR and HrcA" Microorganisms 8, no. 8: 1161. https://doi.org/10.3390/microorganisms8081161
APA StylePalombo, M., Scarlato, V., & Roncarati, D. (2020). Cooperative Regulation of Campylobacter jejuni Heat-Shock Genes by HspR and HrcA. Microorganisms, 8(8), 1161. https://doi.org/10.3390/microorganisms8081161






