Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
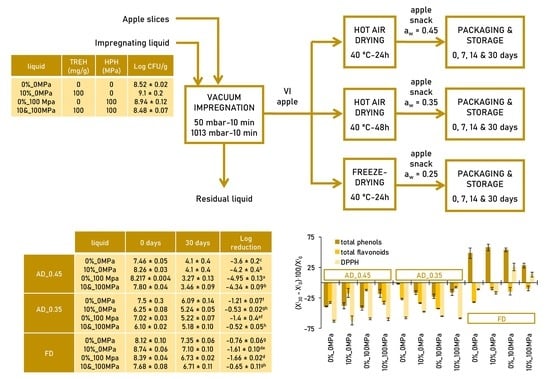
2.2. Snack Manufacturing Process and Storage
2.3. Analytical Determinations
2.3.1. Moisture Content and Water Activity
2.3.2. Antioxidant Properties
2.3.3. Color Measurements
2.3.4. Mechanical Properties
2.3.5. Microbial Counts
2.3.6. Statistical Analysis
3. Results and Discussion
3.1. Survival of Lactobacillus salivarius spp. Salivarius during the Snack Manufacturing Process
3.2. Antioxidant Properties Affected by the Snack Manufacturing Process
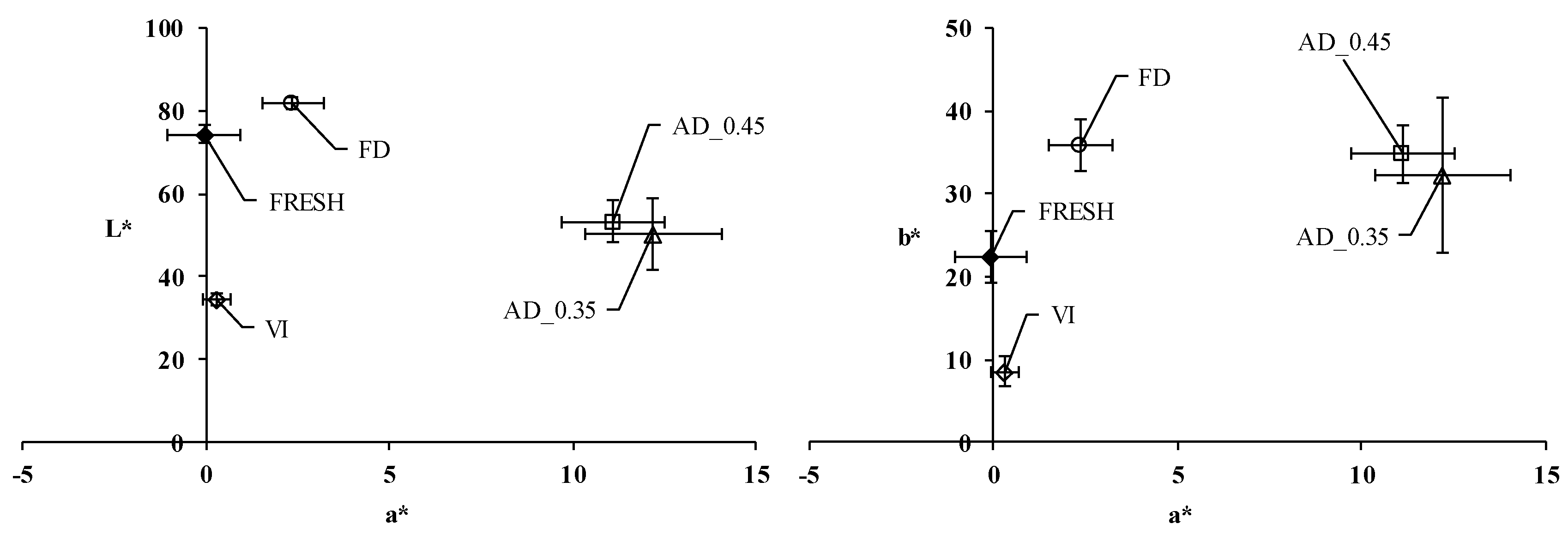
3.3. Color Properties as Affected by the Snack Manufacturing Process
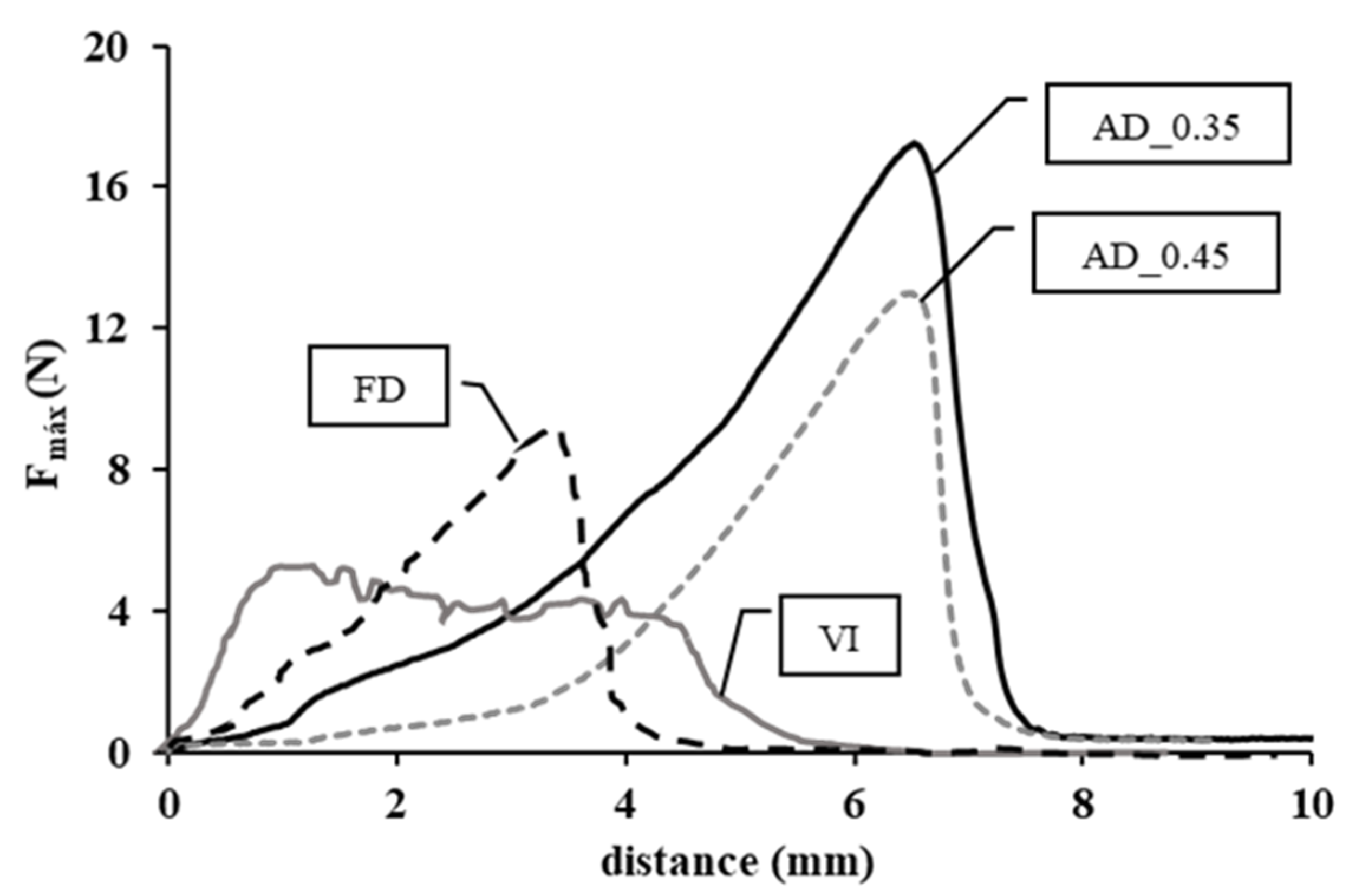
3.4. Mechanical Properties as Affected by the Snack Manufacturing Process
3.5. Survival of Lactobacillus salivarius spp. Salivarius during Snack Storage
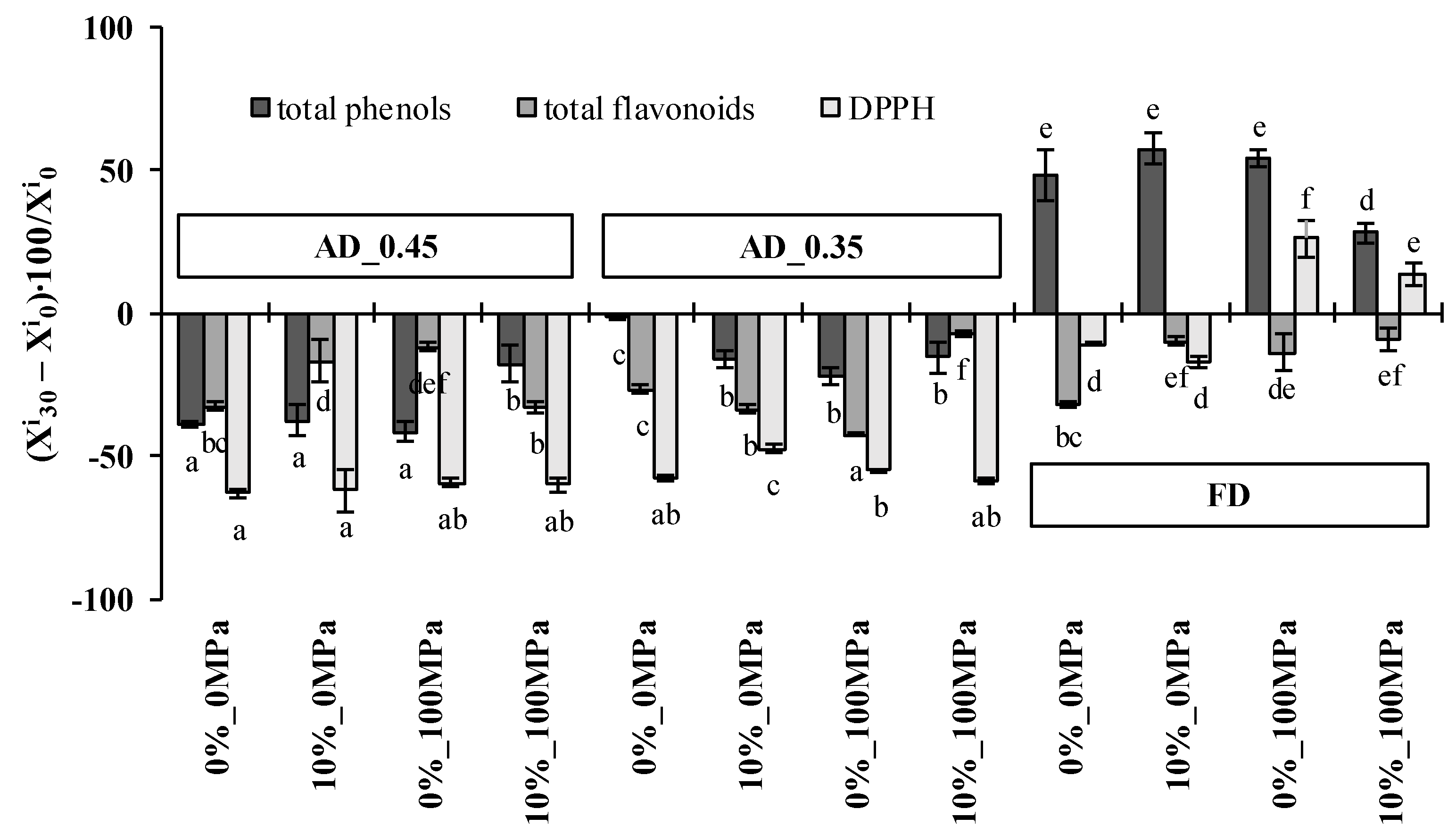
3.6. Antioxidant Properties Change during Snack Storage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Day, L.; Seynour, R.B.; Pitts, K.F.; Konczak, I.; Lumdin, L. Incorporation of functional ingredients into foods. Trends Food Sci. Technol. 2009, 20, 388–395. [Google Scholar] [CrossRef]
- Boyer, L.; Liu, R. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Fito, P.; Chiralt, A.; Betoret, N.; Gras, M.; Cháfer, M.; Martínez-Monzó, J.; Andrés, A.; Vidal., D. Vacuum impregnation and osmotic dehydration in matrix engineering. Application in functional fresh food development. J. Food Eng. 2001, 49, 175–183. [Google Scholar] [CrossRef]
- Assis, F.R.; Rodrigues, L.G.G.; Tribuzi, G.; De Souza, P.G.; Carciofi, B.A.M.; Laurindo, J.B. Fortified apple (Malus spp., var. Fuji) snacks by vacuum impregnation of calcium lactate and convective drying. Lwt-Food Sci. Technol. 2019, 113, 108298. [Google Scholar] [CrossRef]
- Genevois, C.; De Escalada Pla, M.; Flores, S. Novel strategies for fortifying vegetable matrices with iron and Lactobacillus casei simultaneously. Lwt-Food Sci. Technol. 2017, 79, 34–41. [Google Scholar] [CrossRef]
- Betoret, E.; Sentandreu, E.; Betoret, N.; Codoñer-Franch, P.; Valls-Bellés, V.; Fito., P. Technological development and functional properties of an apple snack rich in flavonoid from mandarin juice. Innov. Food Sci. Emerg. Technol. 2012, 16, 298–304. [Google Scholar] [CrossRef]
- Akman, P.K.; Uysal, E.; Ozkaya, G.U.; Tornuk, F.; Durak, M.Z. Development of probiotic carrier dried apples for consumption as snack food with the impregnation of Lactobacillus paracasei. Lwt-Food Sci. Technol. 2019, 103, 60–68. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Arilla, A.; Bennár, M.; Barrera, C.; Codoñer, P.; Fito, P. No invasive methodology to produce a probiotic low humid apple snack with potential effect against Helicobacter pylori. J. Food Eng. 2012, 110, 289–293. [Google Scholar] [CrossRef]
- Cui, L.; Niu, L.Y.; Li, D.J.; Liu, C.Q.; Liu, Y.P.; Liu, C.J.; Song, J.F. Effects of different drying methods on quality, bacterial viability and storage stability of probiotic enriched apple snacks. J. Integr. Agr. 2018, 17, 247–255. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; Food and Agriculture Organization of the United Nations. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; FAO: Rome, Italy, 2006; Volume 85. [Google Scholar]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Passot, S.; Cenard, S.; Douania, I.; Tréléa, I.C.; Fonseca, F. Critical water activity and amorphous state for optimal preservation of lyophilised lactic acid bacteria. Food Chem. 2012, 132, 1699–1705. [Google Scholar] [CrossRef]
- Vesterlund, S.; Salminen, K.; Salminen, S. Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed. Int. J. Food Microbiol. 2012, 157, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Golowczyc, M.A.; Gerez, C.L.; Silva, J.; Abraham, A.G.; De Antoni, G.L.; Paula Teixeira, P. Survival of spray-dried Lactobacillus kefir is affected by different protectants and storage conditions. Biotechnol. Lett 2011, 33, 681–686. [Google Scholar] [CrossRef] [Green Version]
- Nualkaekul, S.; Charalampopoulos, D. Survival of Lactobacillus plantarum in model solutions and fruit juices. Int. J. Food Microbiol. 2011, 146, 117–118. [Google Scholar] [CrossRef]
- Weinbreck, F.; Bodnár, I.; Marco, M.L. Can encapsulation lengthen the shelf-life of probiotic bacteria in dry products? Int. J. Food Microbiol. 2010, 136, 364–367. [Google Scholar] [CrossRef]
- Miao, S.; Mills, S.; Stanton, C.; Fitzgerald, G.F.; Roos, Y.; Ross, R.P. Effect of disaccharides on survival during storage of freeze dried probiotics. Dairy Sci. Technol. 2008, 88, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Betoret, E.; Betoret, N.; Calabuig-Jiménez, L.; Patrignani, F.; Barrera, C.; Lanciotti, R.; Dalla Rosa, M. Probiotic survival and in vitro digestion of L. salivarius spp. salivarius encapsulated by high homogenization pressures and incorporated into a fruit matrix. Lwt-Food Sci. Technol. 2019, 111, 883–888. [Google Scholar] [CrossRef]
- Bagad, M.; Pande, R.; Dubey, V.; Ghosh, A.R. Survivability of freeze-dried probiotic Pediococcus pentosaceus strains GS4, GS17 and Lactobacillus gasseri (ATCC 19992) during storage with commonly used pharmaceutical excipients within a period of 120 days. Asian Pac. J. Trop Med. 2017, 7, 921–929. [Google Scholar] [CrossRef]
- Lapsiri, W.; Bhandari, B.; Wanchaitanawong, P. Viability of Lactobacillus plantarum TISTR 2075 in Different Protectants during Spray Drying and Storage. Dry. Technol. 2012, 30, 1407–1412. [Google Scholar] [CrossRef]
- Oldenhof, H.; Wolkers, W.F.; Fonseca, F.; Passot, S.; Marin, M. Effect of Sucrose and Maltodextrin on the Physical Properties and Survival of Air-Dried Lactobacillus Bulgaricus: An in Situ Fourier Transform Infrared Spectroscopy Study. Biotechnol. Prog. 2005, 21, 885–892. [Google Scholar] [CrossRef]
- Barrera, C.; Burca, C.; Betoret, E.; García-Hernández, J.; Hernández, M.; Betoret, N. Improving antioxidant properties and probiotic effect of clementine juice inoculated with Lactobacillus salivarius spp. salivarius (CECT 4063) by trehalose addition and/or sublethal homogenisation. Int. J. Food Sci Technol. 2019, 54, 2109–2122. [Google Scholar] [CrossRef]
- Betoret, E.; Calabuig-Jiménez, L.; Patrignani, F.; Lanciotti, R.; Dalla Rosa, M. Effect of high pressure processing and trehalose addition on functional properties of mandarin juice enriched with probiotic microorganisms. Lwt-Food Sci Technol 2017, 85, 418–422. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists; Horwitz, W. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, WV, USA, 2005; Volume 222. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol Viticult 1965, 16, 144–158. [Google Scholar]
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A.; Zbarsky, V.; Datla, K.; Dexter, D.; Aruoma, O. Characterization of the antioxidant functions of flavonoids and proanthocyanicins in Muritian black teas. Food Res. Int 2005, 38, 357–367. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt-Food Sci Technol 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Chen, Y.S.; Srionnual, S.; Onda, T.; Yanagida, F. Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett. App. Microbiol. 2007, 45, 190–193. [Google Scholar] [CrossRef]
- Li, C.; Liu, L.B.; Liu, N. Effects of carbon sources and lipids on freeze-drying survival of Lactobacillus bulgaricus in growth media. Ann. Microbiol. 2012, 62, 949–956. [Google Scholar] [CrossRef]
- Betoret, N.; Puente, L.; Díaz, M.J.; Pagán, M.J.; García, M.J.; Gras, M.L.; Martínez-Monzó, J.; Fito, P. Development of probiotic-enriched dried fruits by vacuum impregnation. J. Food Eng. 2003, 56, 273–277. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol. Progr. 2007, 23, 302–315. [Google Scholar] [CrossRef]
- Zayed, G.; Roos, Y.H. Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process. Biochem 2004, 39, 1081–1086. [Google Scholar] [CrossRef]
- Betoret, E.; Betoret, N.; Rocculi, P.; Dalla Rosa, M. Strategies to improve food functionality: Structure–property relationships on high pressures homogenization, vacuum impregnation and drying technologies. Trends Food Sci. Technol. 2015, 46, 1–12. [Google Scholar] [CrossRef]
- Kets, E.; Teunissen, P.; De Bont, J. Effect of compatible solutes on survival of lactic acid bacteria subjected to drying. Appl. Env. Microbiol. 1996, 62, 259–261. [Google Scholar] [CrossRef] [Green Version]
- Betoret, E.; Betoret, N.; Carbonell, J.V.; Fito, P. Effects of pressure homogenization on particle size and the functional properties of citrus juices. J. Food Eng. 2009, 92, 18–23. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. Isrn. Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef] [Green Version]
- Vuthijumnok, J.; Molan, A.L.; Heyes, J.A. Effect of freeze-drying and extraction solvents on the total phenolic contents, total flavonoids and antioxidant activity of different Rabbiteye blueberry genotypes grown in New Zealand. Iosr J. Pharm. Biol. Sci. 2013, 8, 42–48. [Google Scholar] [CrossRef]
- Heredia, A.; Peinado, I.; Barrera, C.; Andrés, A. Influence of process variables on colour changes, carotenoids retention and cellular tissue alteration of cherry tomato during osmotic dehydration. J. Food Compos. Anal. 2009, 22, 285–294. [Google Scholar] [CrossRef]
- Seguí, L.; Calabuig-Jiménez, L.; Betoret, N.; Fito, P. Physicochemical and antioxidant properties of non-refined sugarcane alternatives to white sugar. Food Sci. Technol. 2015, 50, 2579–2588. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; Di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Kurtmann, L.; Carlsen, C.U.; Risbo, J.; Skibsted, L.H. Storage stability of freeze-dried Lactobacillus acidophilus (La-5) in relation to water activity and presence of oxygen and ascorbate. Cryobiology 2009, 58, 175–180. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Teixeira, P. Influence of sub-lethal stresses on the survival of lactic acid bacteria after spray-drying in orange juice. Food Microbiol. 2015, 52, 77–83. [Google Scholar] [CrossRef]
- Lim, E.M.; Ehrlich, S.D.; Maguin, E. Identification of stress-inducible proteins in Lactobacillus delbrueckii subsp. Bulg. Electrophor. 2000, 21, 2557–2561. [Google Scholar] [CrossRef]
- Kilstrup, M.; Jacobsen, S.; Hammer, K.; Vogensen, F.K. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Env. Microbiol. 1997, 63, 1826–1837. [Google Scholar] [CrossRef] [Green Version]
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piretti, M.V.; Gallerani, G.; Brodnik, U. Polyphenol polymerisation involvement in apple superficial scald. Postharvest Biol Technol. 1996, 8, 11–18. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, H. Characterisation and comparison of phenols, flavonoids and isoflavones of soy milk and their correlations with antioxidant activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
| Sample | TRE%_HPH | Log CFU/g | |
|---|---|---|---|
| Experimental | Log Reduction | ||
| VI LIQ | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 8.52 ± 0.02fgh 9.1 ± 0.2i 8.94 ± 0.12hi 8.48 ± 0.07fg | - |
| VI APP xw = 85.3 ± 1.2 g w/100 g | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 7.839 ± 0.009de 8.20 ± 0.11ef 8.1 ± 0.2ef 7.7 ± 0.5cd | 0.10 ± 0.02j −0.09 ± 0.04i −0.08 ± 0.04i −0.05 ± 0.02i |
| FD xw = 5.0 ± 1.0 g w/100 g | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 8.12 ± 0.10ef 8.74 ± 0.06ghi 8.39 ± 0.04fg 7.68 ± 0.08cd | −0.53 ± 0.02g −0.22 ± 0.04h −0.64 ± 0.08g −0.9 ± 0.3f |
| AD_0.45 xw = 11.4 ± 1.5 g w/100 g | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 7.46 ± 0.05c 8.26 ± 0.03ef 8.217 ± 0.004ef 7.80 ± 0.04cde | −1.167 ± 0.009e −1.46 ± 0.03cd −1.60 ± 0.07c −1.25 ± 0.02de |
| AD_0.35 xw = 9.2 ± 0.8 g w/100 g | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 7.5 ± 0.3cd 6.25 ± 0.08a 7.02 ± 0.03b 6.10 ± 0.02a | −1.337 ± 0.009de −2.68 ± 0.03a −2.10 ± 0.07b −2.63 ± 0.02a |
| Sample | TRE%_HPH | Total Phenols (mg GAE/g dw) | Total Flavonoids (mg QE/g dw) | Antioxidant Activity (mg TE/g dw) |
|---|---|---|---|---|
| VI APP aw = 0.983 ± 0.002 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 5.4 ± 0.7a 5.7 ± 1.2abc 5.7 ± 0.5ab 5.3 ± 1.5a | 1.24 ± 0.15abc 1.07 ± 0.06a 1.3 ± 0.3abc 1.2 ± 0.4ab | 7.0 ± 0.9bcdef 7 ± 3bcde 6.0 ± 0.9bc 6.4 ± 1.8bcd |
| FD aw = 0.25 ± 0.02 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 5.4 ± 0.3ab 8.39 ± 0.03f 6.3 ± 0.3abcd 6.6 ± 0.5abcde | 1.584 ± 0.013cd 1.187 ± 0.013abc 1.76 ± 0.12de 1.74 ± 0.03de | 8.64 ± 0.07efghi 10.3 ± 0.4i 4.9 ± 0.3ab 3.6 ± 0.5a |
| AD_0.45 aw = 0.42 ± 0.02 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 10.6 ± 0.3g 6.7 ± 0.2bcde 7.6 ± 0.2def 7.1 ± 0.5cdef | 2.47 ± 0.09g 1.51 ± 0.06bcd 2.13 ± 0.09efg 1.53 ± 0.02cd | 10.2 ± 0.4i 9 ± 2ghi 6.9 ± 0.2bcdefg 8.5 ± 0.4defghi |
| AD_0.35 aw = 0.36 ± 0.03 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 8.1 ± 0. 2ef 6.6 ± 0.5abcde 7.8 ± 0.4def 5.867 ± 0.014abc | 1.990 ± 0.006ef 1.876 ± 0.012de 2.36 ± 0.07fg 1.044 ± 0.005a | 9.2 ± 0.3fghi 7.68 ± 0.15cdefgh 6.20 ± 0.07bcde 9.5 ± 0.3hi |
| Sample | TRE%_HPH | Storage Time | Log Reduction | ||
|---|---|---|---|---|---|
| 7 Days | 15 Days | 30 Days | |||
| FD aw = 0.25 ± 0.02 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 7.80 ± 0.05c 8.23 ± 0.06d 7.75 ± 0.04c 7.84 ± 0.02c | 7.66 ± 0.11fg 7.84 ± 0.04g 7.46 ± 0.12f 7.76 ± 0.06g | 7.35 ± 0.06h 7.10 ± 0.10gh 6.73 ± 0.02fg 6.71 ± 0.11g | −0.76 ± 0.06g −1.61 ± 0.10de −1.66 ± 0.02d −0.65 ± 0.11gh |
| AD_0.45 aw = 0.42 ± 0.02 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 5.24 ± 0.09a 6.05 ± 0.04b 6.11 ± 0.02b 5.2 ± 0.4a | 5.1 ± 0.7b 5.7 ± 0.2d 5.4 ± 0.4cd 4.2 ± 0.4a | 4.1 ± 0.4bc 4.1 ± 0.4c 3.27 ± 0.13a 3.46 ± 0.09ab | −3.6 ± 0.2c −4.2 ± 0.4b −4.95 ± 0.13a −4.34 ± 0.09b |
| AD_0.35 aw = 0.36 ± 0.03 | 0%_0MPa 10%_0MPa 0%_100MPa 10%_100MPa | 6.32 ± 0.07b 5.72 ± 0.02a 6.00 ± 0.08a 5.83 ± 0.05b | 6.261 ± 0.012e 5.64 ± 0.10d 5.889 ± 0.012e 5.58 ± 0.05c | 6.09 ± 0.14ef 5.24 ± 0.05d 5.22 ± 0.07de 5.18 ± 0.10d | −1.21 ± 0.07f −0.53 ± 0.02gh −1.4 ± 0.4ef −0.52 ± 0.05h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; Betoret, E.; Barrera, C. Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization. Microorganisms 2020, 8, 1095. https://doi.org/10.3390/microorganisms8081095
Burca-Busaga CG, Betoret N, Seguí L, Betoret E, Barrera C. Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization. Microorganisms. 2020; 8(8):1095. https://doi.org/10.3390/microorganisms8081095
Chicago/Turabian StyleBurca-Busaga, Cristina Gabriela, Noelia Betoret, Lucía Seguí, Ester Betoret, and Cristina Barrera. 2020. "Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization" Microorganisms 8, no. 8: 1095. https://doi.org/10.3390/microorganisms8081095
APA StyleBurca-Busaga, C. G., Betoret, N., Seguí, L., Betoret, E., & Barrera, C. (2020). Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization. Microorganisms, 8(8), 1095. https://doi.org/10.3390/microorganisms8081095