Isolation and Propagation of Laboratory Strains and a Novel Flea-Derived Field Strain of Wolbachia in Tick Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Cell Lines
2.2. Wolbachia Strains
2.3. Preparation of Cell-Free Wolbachia Suspensions and Inoculation of Tick Cell Lines
2.4. Preparation of Homogenate from Field-Collected Fleas
2.5. Examination of Wolbachia-infected Cultures by Microscopy
2.6. Molecular Confirmation of Wolbachia and Host Cell Identity
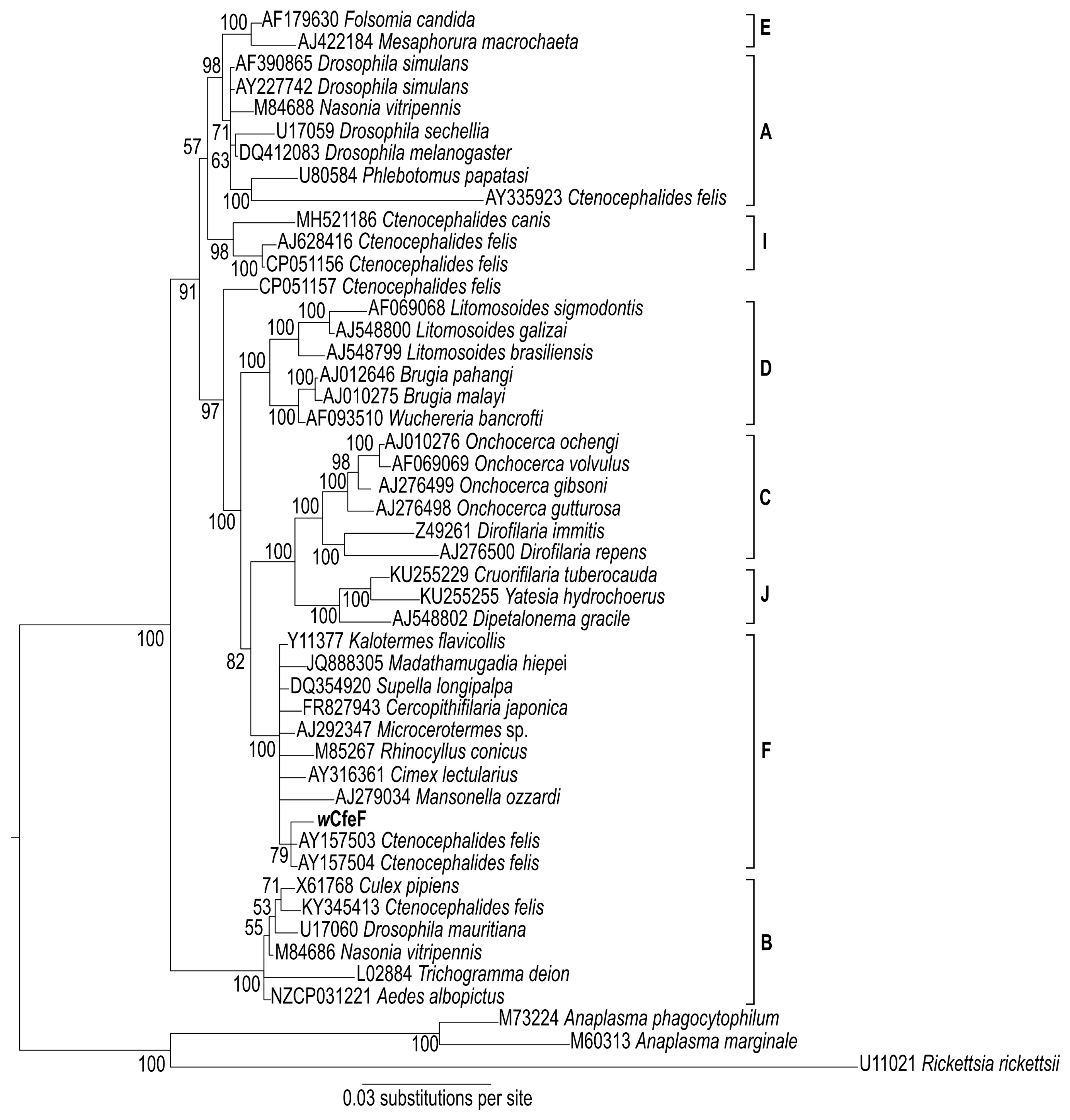
2.7. Sequence and Phylogenetic Analyses
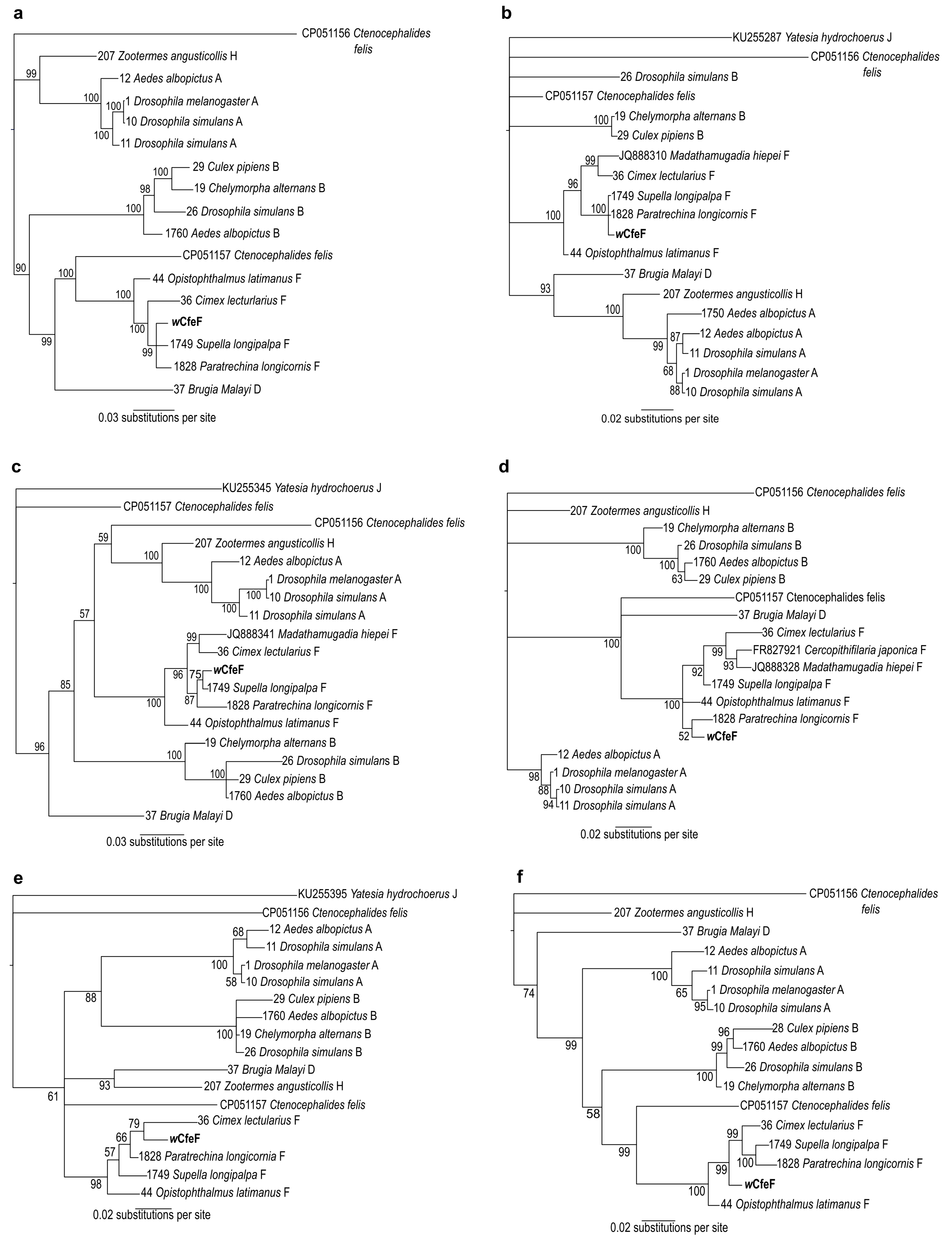
3. Results
3.1. Propagation of Wolbachia Strains wStri and wAlb1 in Ixodes spp. Cell Lines
3.2. Propagation of Wolbachia Strain wAlbB in a R. microplus Cell Line
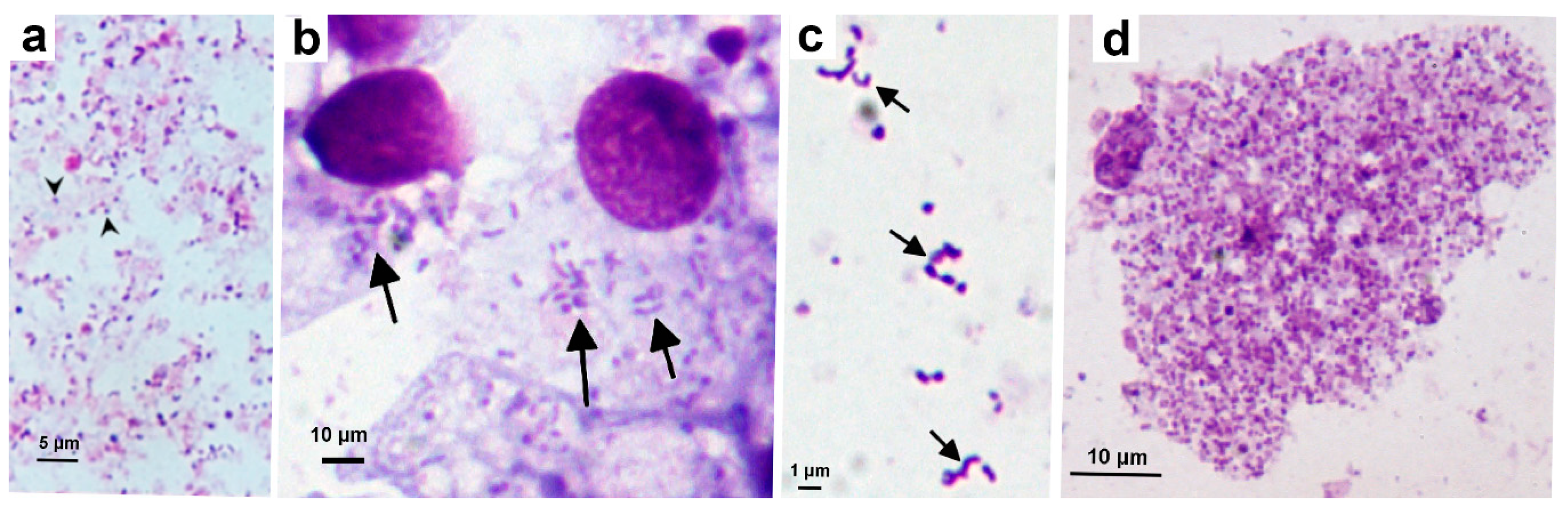
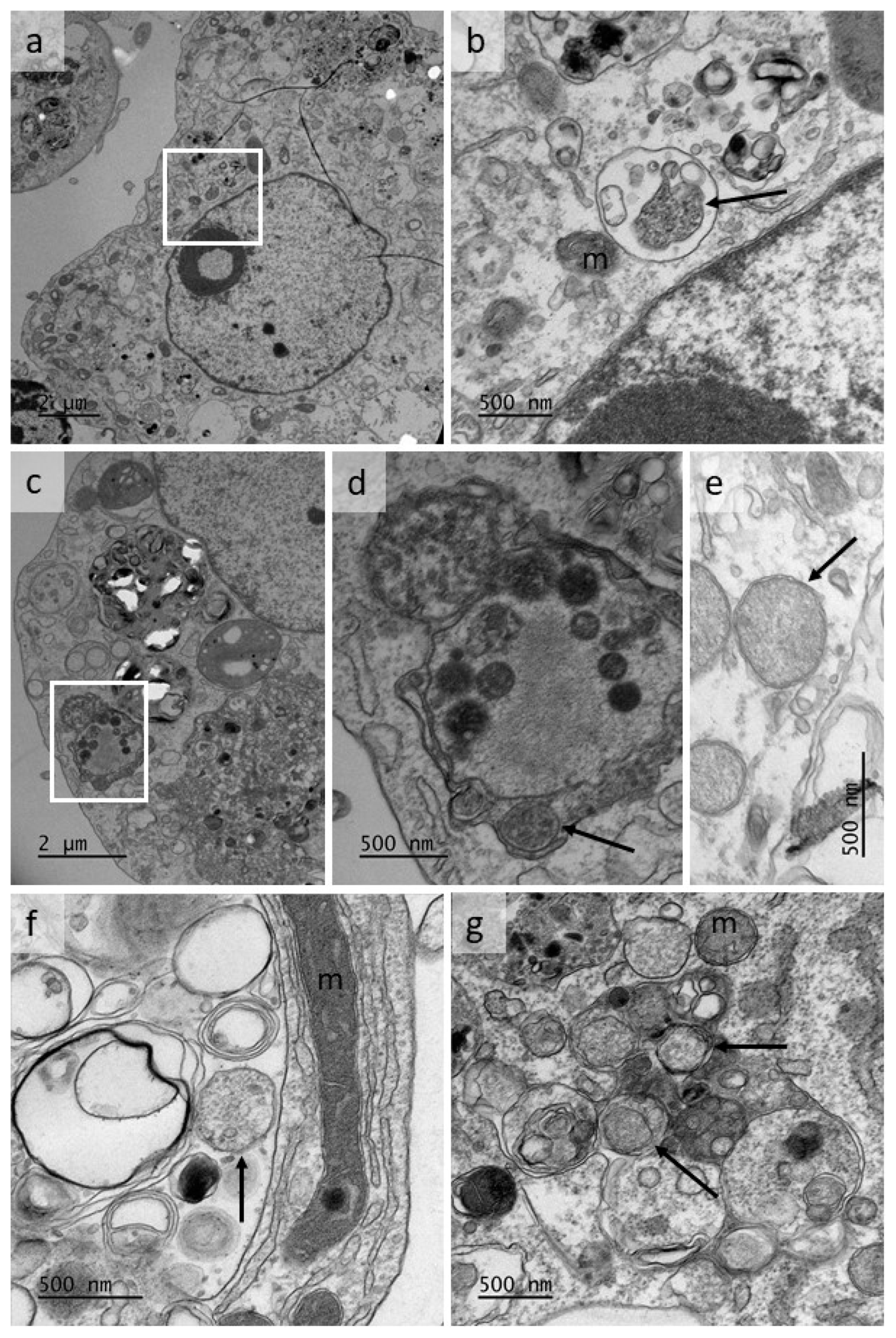
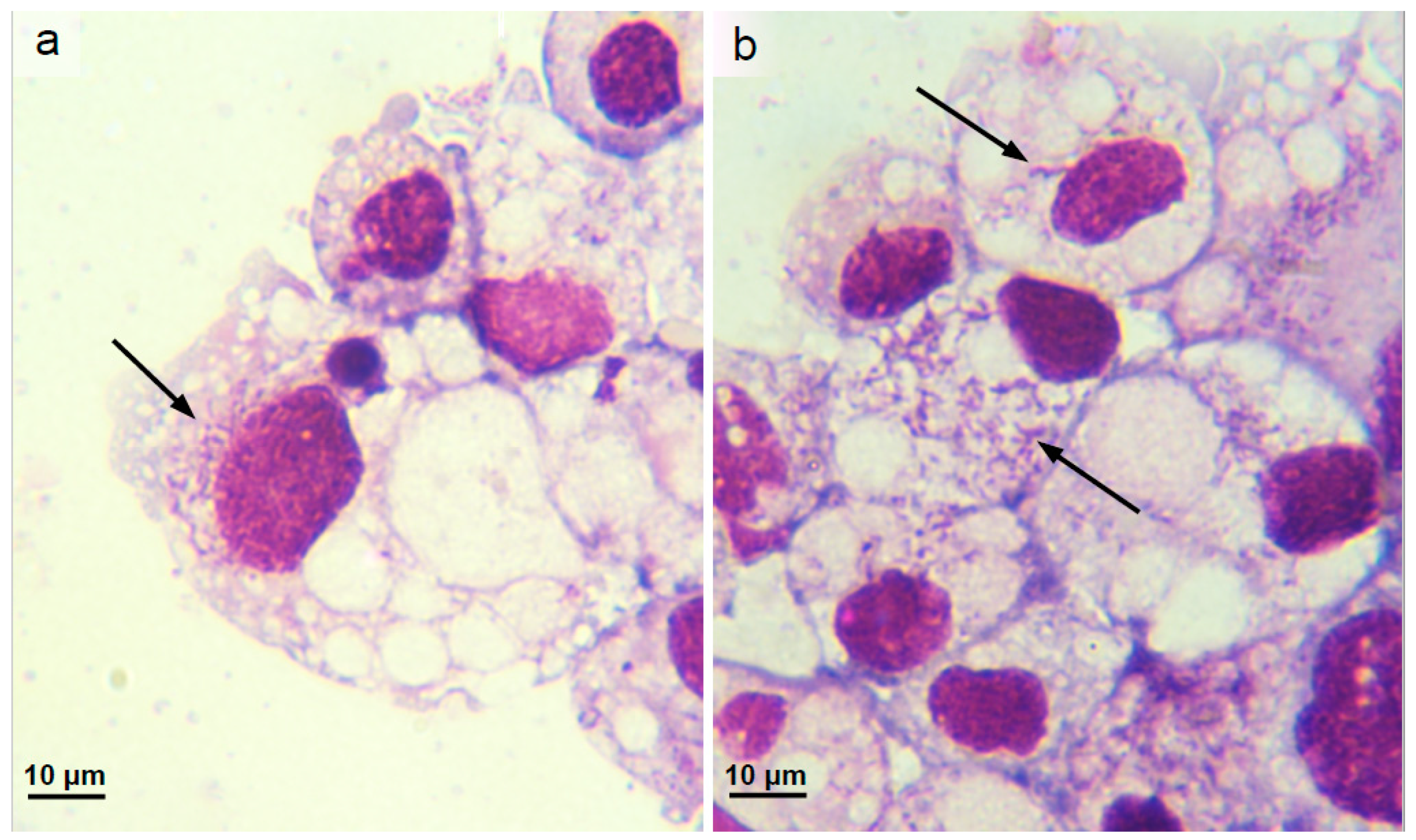
3.3. Isolation and Propagation of a Novel Wolbachia Strain from Malaysian Cat Fleas
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hertig, M. The rickettsia, Wolbachia pipientis (gen. et sp.n.) and associated inclusions of the mosquito, Culex pipiens. Parasitology 1936, 28, 453–486. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; Neill, S.O. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 1998, 265, 509–515. [Google Scholar] [CrossRef]
- Baldo, L.; Dunning Hotopp, J.C.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.J.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098. [Google Scholar] [CrossRef] [PubMed]
- Lefoulon, E.; Clark, T.; Borveto, F.; Perriat-Sanguinet, M.; Moulia, C.; Slatko, B.; Gavott, L. Pseudoscorpion Wolbachia symbionts: Diversity and evidence for a new supergroup S. BMC Microbiol. 2020, 20, 188. [Google Scholar] [CrossRef]
- Chung, M.; Munro, J.B.; Tettelin, H.; Hotopp, J.C.D. Using core genome alignments to assign bacterial species. Ecol. Evol. Sci. 2018, 3, e00236-18. [Google Scholar] [CrossRef]
- Weeks, A.R.; Breeuwer, J.A.J. Wolbachia–induced parthenogenesis in a genus of phytophagous mites. Proc. R. Soc. Lond. B 2001, 268, 2245–2251. [Google Scholar] [CrossRef] [PubMed]
- Hornett, E.A.; Duplouy, A.M.R.; Davies, N.; Roderick, G.K.; Wedell, N.; Hurst, G.D.D.; Charlat, S. You can’t keep a good parasite down: Evolution of a male-killer suppressor uncovers cytoplasmic incompatibility. Evolution 2008, 62, 1258–1263. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Kern, P.; Cook, J.M.; Kageyama, D.; Riegler, M. Double trouble: Combined action of meiotic drive and Wolbachia feminization in Eurema butterflies. Biol. Lett. 2015, 11, 20150095. [Google Scholar] [CrossRef]
- Gerth, M.; Gansauge, M.-T.; Weigert, A.; Bleidorn, C. Phylogenomic analyses uncover origin and spread of the Wolbachia pandemic. Nat. Commun. 2014, 5, 5117. [Google Scholar] [CrossRef]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B 2015, 282, 20150249. [Google Scholar] [CrossRef] [PubMed]
- Fenn, K.; Blaxter, M. Wolbachia genomes: Revealing the biology of parasitism and mutualism. Trends Parasitol. 2006, 22, 60–65. [Google Scholar] [CrossRef]
- Pike, N.; Kingcombe, R. Antibiotic treatment leads to the elimination of Wolbachia endosymbionts and sterility in the diplodiploid collembolan Folsomia candida. BMC Biol. 2009, 7, 54. [Google Scholar] [CrossRef]
- Fischer, P.; Schmetz, C.; Bandi, C.; Bonow, I.; Mand, S.; Fischer, K.; Büttner, D.W. Tunga penetrans: Molecular identification of Wolbachia endobacteria and their recognition by antibodies against proteins of endobacteria from filarial parasites. Exp. Parasitol. 2002, 102, 201–211. [Google Scholar] [CrossRef]
- Gorham, C.; Fang, Q.; Durden, L. Wolbachia endosymbionts in fleas (Siphonaptera). J. Parasitol. 2003, 89, 283–289. [Google Scholar] [CrossRef]
- Dittmar, K.; Whiting, M. New Wolbachia endosymbiont from Nearctic and Neotropical fleas (Siphonaptera). J. Parasitol. 2004, 90, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Kyei-Poku, G.K.; Colwell, D.D.; Coghlin, P.; Benkel, B.; Floate, K.D. On the ubiquity and phylogeny of Wolbachia in lice. Mol. Ecol. 2005, 14, 285–294. [Google Scholar] [CrossRef]
- Espino, C.I.; Gomez, T.; Gonzalez, G.; Brazil do Santos, M.F.; Solano, J.; Sousa, O.; Moreno, N.; Windsor, D.; Ying, A.; Vilchez, S.; et al. Detection of Wolbachia bacteria in multiple organs and feces of the triatomine insect Rhodnius pallescens (Hemiptera, Reduviidae). Appl. Environ. Microbiol. 2009, 75, 547–550. [Google Scholar] [CrossRef]
- Crainey, J.L.; Wilson, M.D.; Post, R.J. Phylogenetically distinct Wolbachia gene and pseudogene sequences obtained from the African onchocerciasis vector Simulium squamosum. Int. J. Parasitol. 2010, 40, 569–578. [Google Scholar] [CrossRef]
- Hosokawa, T.; Koga, R.; Kikuchi, Y.; Meng, X.-Y.; Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl. Acad. Sci. USA 2010, 107, 769–774. [Google Scholar] [CrossRef]
- Doudoumis, V.; Alam, U.; Aksoy, E.; Abd-Alla, A.M.M.; Tsiamis, G.; Brelsfoard, C.; Aksoy, S.; Bourtzis, K. Tsetse-Wolbachia symbiosis: Comes of age and has great potential for pest and disease control. J. Invertebr. Pathol. 2013, 112, S94–S103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tay, S. Wolbachia endosymbionts, Rickettsia felis and Bartonella species, in Ctenocephalides felis fleas in a tropical region. J. Vector Ecol. 2013, 38, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Kittipong, C.; McGarry, J.W.; Morand, S.; Makepeace, B.L. Symbiosis in an overlooked microcosm: A systematic review of the bacterial flora of mites. Parasitology 2015, 142, 1152–1162. [Google Scholar] [CrossRef]
- Onder, Z.; Ciloglu, A.; Duzlu, O.; Yildirim, A.; Okur, M.; Yetismis, G.; Inci, A. Molecular detection and identification of Wolbachia endosymbiont in fleas (Insecta: Siphonaptera). Folia Microbiol. 2019, 64, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Sicard, M.; Bonneau, M.; Weill, M. Wolbachia prevalence, diversity, and ability to induce cytoplasmic incompatibility in mosquitoes. Curr. Opin. Insect Sci. 2019, 34, 12–20. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Verhoeve, V.I.; Gillespie, J.J.; Johnston, J.S.; Guillotte, M.L.; Rennoll-Bankert, K.E.; Rahman, M.S.; Hagen, D.; Elsik, C.G.; Macaluso, K.R.; et al. A chromosome-level assembly of the cat flea genome uncovers rampant gene duplication and genome size plasticity. BMC Biol. 2020, 18, 70. [Google Scholar] [CrossRef]
- Yen, J.H.; Barr, A.R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 1971, 232, 657–658. [Google Scholar] [CrossRef]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito–Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef]
- Bouchery, T.; Lefoulon, E.; Karadjian, G.; Nieguitsila, A.; Martin, C. The symbiotic role of Wolbachia in Onchocercidae and its impact on filariasis. Clin. Microbiol. Infect. 2013, 19, 131–140. [Google Scholar] [CrossRef]
- Hughes, G.L.; Rasgon, J.L. Transinfection: A method to investigate Wolbachia–host interactions and control arthropod-borne disease. Insect Mol. Biol. 2014, 23, 141–151. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Makepeace, B.L.; Benjamin, L.; Baylis, M.; Solomon, T. The potential role of Wolbachia in controlling the transmission of emerging human arboviral infections. Curr. Opin. Infect. Dis. 2017, 30, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, H.; Raoult, D.; Brouqui, P. Detection of Ehrlichia platys DNA in brown dog ticks (Rhipicephalus sanguineus) in Okinawa Island, Japan. J. Clin. Microbiol. 2000, 38, 4219–4221. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.J.; Gawronski, J.D.; Eveleigh, D.E.; Benson, D.R. Intracellular symbionts and other bacteria associated with deer ticks (Ixodes scapularis) from Nantucket and Wellfleet, Cape Cod, Massachusetts. Appl. Environ. Microbiol. 2004, 70, 616–620. [Google Scholar] [CrossRef]
- Hartelt, K.; Oehme, R.; Frank, H.; Brockmann, S.O.; Hassler, D.; Kimmig, P. Pathogens and symbionts in ticks: Prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int. J. Med. Microbiol. 2004, 293 (Suppl. S37), 86–92. [Google Scholar] [CrossRef]
- Sarih, M.; M’Ghirbi, Y.; Bouattour, A.; Gern, L.; Baranton, G.; Postic, D. Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. J. Clin. Microbiol. 2005, 43, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- van Overbeek, L.; Gassner, L.; van der Plas, C.L.; Kastelein, P.; Nunes-da Rocha, U.; Takken, W. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. FEMS Microbiol. Ecol. 2008, 66, 72–84. [Google Scholar] [CrossRef]
- Andreotti, R.; de Leon, A.A.P.; Dowd, S.E.; Guerrero, F.D.; Bendele, K.G.; Scoles, G.A. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011, 11, 6. [Google Scholar] [CrossRef]
- Carpi, G.; Cagnacci, F.; Wittenkindt, N.E.; Zhao, F.; Qi, J.; Tomsho, L.P.; Drautz, D.I.; Rizzoli, A.; Schuster, S.C. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS ONE 2011, 6, e25604. [Google Scholar] [CrossRef]
- Zhang, X.; Norris, D.E.; Rasgon, J.L. Distribution and molecular characterization of Wolbachia endosymbionts and filarial nematodes in Maryland populations of the lone star tick (Amblyomma americanum). FEMS Microbiol. Ecol. 2011, 77, 50–56. [Google Scholar] [CrossRef]
- Khoo, J.-J.; Chen, F.; Kho, K.L.; Shanizza, A.I.A.; Lim, F.-S.; Tan, K.-K.; Chang, L.-Y.; AbuBakar, S. Bacterial community in Haemaphysalis ticks of domesticated animals from the Orang Asli communities in Malaysia. Ticks Tick Borne Dis. 2016, 7, 929–937. [Google Scholar] [CrossRef]
- Hirunkanokpun, S.; Ahantarig, A.; Baimai, V.; Trinachartvanit, W. A new record of Wolbachia in the elephant ticks from Thailand. ScienceAsia 2018, 44S, 44–47. [Google Scholar] [CrossRef]
- Muñoz-Leal, S.; Macedo, C.; Gonçalves, T.C.; Barreira, J.D.; Labruna, M.B.; Sampaio de Lemos, E.R.; Ogrzewalska, M. Detected microorganisms and new geographic records of Ornithodoros rietcorreai (Acari: Argasidae) from northern Brazil. Ticks Tick Borne Dis. 2019, 10, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Nebbak, A.; Dahmana, H.; Almeeras, L.; Raoult, D.; Boulanger, N.; Jaulhac, B.; Mediannikov, O.; Parola, P. Co-infection of bacteria and protozoan parasites in Ixodes ricinus nymphs collected in the Alsace region, France. Ticks Tick Borne Dis. 2019, 10, 101241. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Zhang, Y.; Allen, M.S. Bacterial microbiomes of Ixodes scapularis ticks collected from Massachusetts and Texas, USA. BMC Microbiol. 2019, 19, 138. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.-Y.; Liu, G.-H.; Cheng, T.-Y. Microbiome analysis of the saliva and midgut from partially or fully engorged female adult Dermacentor silvarum ticks in China. Exp. Appl. Acarol. 2020, 80, 543–558. [Google Scholar] [CrossRef]
- Hu, R.; Hyland, K.E.; Oliver, J.H., Jr. A review on the use of Ixodiphagus wasps (Hymenoptera: Encyrtidae) as natural enemies for the control of ticks (Acari: Ixodidae). Syst. Appl. Acarol. 1998, 3, 19–28. [Google Scholar] [CrossRef]
- Tijsse-Klasen, E.; Braks, M.; Scholte, E.-J.; Sprong, H. Parasites of vectors—Ixodiphagus hookeri and its Wolbachia symbionts in ticks in The Netherlands. Parasites Vectors 2011, 4, 228. [Google Scholar] [CrossRef]
- Plantard, O.; Bouju-Albert, A.; Malard, M.-A.; Hermouet, A.; Capron, G.; Verheyden, H. Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the Hymenoptera endoparasitoid Ixodiphagus hookeri. PLoS ONE 2012, 7, e30692. [Google Scholar] [CrossRef]
- Kurtti, T.J.; Munderloh, U.G.; Andreadis, T.G.; Magnarelli, L.A.; Mather, T.N. Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. J. Invert. Pathol. 1996, 67, 318–321. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Liu, Y.; Wang, M.; Chen, C.; Kurtti, T.J. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J. Parasitol. 1994, 80, 533–543. [Google Scholar] [CrossRef]
- Simser, J.A.; Palmer, A.T.; Fingerle, V.; Wilske, B.; Kurtti, T.J.; Munderloh, U.G. Rickettsia monacensis sp. nov., a spotted fever group rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl. Environ. Microbiol. 2002, 68, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Burkhardt, N.Y.; Felsheim, R.F.; Kurtti, T.L.; Munderloh, U.G. Motility characteristics are altered for Rickettsia bellii transformed to overexpress a heterologous rickA gene. Appl. Environ. Microbiol. 2014, 80, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Munderloh, U.G.; Kurtti, T.J. Formulation of medium for tick cell culture. Exp. Appl. Acarol. 1989, 7, 219–229. [Google Scholar] [CrossRef]
- Alberdi, M.P.; Nijhof, A.M.; Jongejan, F.; Bell-Sakyi, L. Tick cell culture isolation and growth of Rickettsia raoultii from Dutch Dermacentor reticulatus ticks. Ticks Tick Borne Dis. 2012, 3, 349–354. [Google Scholar] [CrossRef]
- Mitsuhashi, J. A new continuous cell line from larvae of the mosquito Aedes albopictus (Diptera, Culicidae). Biomed. Res. 1981, 2, 599–606. [Google Scholar] [CrossRef]
- Noda, H.; Miyoshi, T.; Koizumi, Y. In vitro cultivation of Wolbachia in insect and mammalian cell lines. In Vitro Cell. Dev. Biol. Anim. 2002, 38, 423–427. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Pettigrew, M.M.; Sinkins, S.P.; Braig, H.R.; Andreadis, T.G.; Tesh, R.B. In vitro cultivation of Wolbachia pipientis in Aedes albopictus cell line. Insect Mol. Biol. 1997, 6, 33–39. [Google Scholar] [CrossRef]
- Igarashi, A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Kurtti, T.J.; Burkhardt, N.Y.; Heu, C.C.; Munderloh, U.G. Fluorescent protein expressing Rickettsia buchneri and Rickettsia peacockii for tracking symbiont-tick interactions. Vet. Sci. 2016, 3, 34. [Google Scholar] [CrossRef]
- Pratt, H.; Stojanovich, C. Fleas: Illustrated Key to Species Found During Plague Investigations. In CDC Pictorial Keys, Arthropods, Reptiles, Birds and Mammals of Public Health Significance; US Department of Health Education and Welfare, Public Health Service, Communicable Disease Center: Atlanta, GA, USA, 1966; pp. 171–174. [Google Scholar]
- Bell-Sakyi, L.; Mohd Jaafar, F.; Monsion, B.; Luu, L.; Denison, E.; Carpenter, S.; Attoui, H.; Mertens, P.P.C. Continuous cell lines from the European biting midge Culicoides nubeculosus (Meigen, 1830). Microorganisms 2020, 8, 825. [Google Scholar] [CrossRef]
- Palomar, A.M.; Premchand-Branker, S.; Alberdi, P.; Belova, O.; Moniuszko-Malinowska, A.; Kahl, O.; Bell-Sakyi, L. Isolation of known and potentially pathogenic tick-borne microorganisms from European ixodid ticks using tick cell lines. Ticks Tick Borne Dis. 2019, 10, 628–638. [Google Scholar] [CrossRef]
- Makepeace, B.L.; Rodgers, L.; Trees, A.J. Rate of elimination of Wolbachia pipientis by doxycycline in vitro increases following drug withdrawal. Antimicrob. Agents Chemother. 2006, 50, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, A.M.; Clegg, S.R.; Pinder, A.C.; Luu, L.; Hansford, K.M.; Seelig, F.; Dinnis, R.E.; Margos, G.; Medlock, J.M.; Feil, E.J.; et al. Multi-locus sequence typing of Ixodes ricinus and its symbiont Candidatus Midichloria mitochondrii across Europe reveals evidence of local co-cladogenesis in Scotland. Ticks Tick Borne Dis. 2019, 10, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molec. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Wolbachia MLST Databases. Available online: https://pubmlst.org/wolbachia/ (accessed on 8 April 2020).
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Posada, D. Jmodeltest: Phylogenetic model averaging. Molec. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, A.M.; Fong, A.W.C.; Voronin, D.A.; Iturbe-Ormaetxe, I.; Yamada, R.; McGraw, E.A.; O’Neill, S.L. Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl. Environ. Microbiol. 2008, 74, 6963. [Google Scholar] [CrossRef]
- Fischer, K.; Beatty, W.L.; Weil, G.J.; Fischer, P.U. High pressure freezing/freeze substitution fixation improves the ultrastructural assessment of Wolbachia endosymbiont—filarial nematode host interaction. PLoS ONE 2014, 9, e86383. [Google Scholar] [CrossRef]
- Strunov, A.; Kiseleva, E. Drosophila melanogaster brain invasion: Pathogenic Wolbachia in central nervous system of the fly. Insect Sci. 2016, 23, 253–264. [Google Scholar] [CrossRef]
- Fallon, A.M.; Baldridge, G.D.; Higgins, L.A.; Witthuhn, B.A. Wolbachia from the planthopper Laodelphax striatellus establishes a robust, persistent, streptomycin-resistant infection in clonal mosquito cells. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 66–73. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Blouin, E.F.; Kocan, K.M.; Ge, N.L.; Edwards, W.L.; Kurtti, T.J. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J. Med. Entomol. 1996, 33, 656–664. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Jauron, S.D.; Fingerle, V.; Leitritz, L.; Hayes, S.F.; Hautman, J.M.; Nelson, C.M.; Huberty, B.W.; Kurtti, T.J.; Ahlstrand, G.G.; et al. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 1999, 37, 2518–2524. [Google Scholar] [CrossRef]
- Morimoto, S.; Kurtti, T.J.; Noda, H. In vitro cultivation and antibiotic susceptibility of a Cytophaga-like intracellular symbiote isolated from the tick Ixodes scapularis. Curr. Microbiol. 2006, 52, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Ewing, S.A.; Munderloh, U.G.; Blouin, E.F.; Kocan, K.M.; Kurtti, T.J. Ehrlichia canis in tick cell culture. In Proceedings of the 76th Conference of Research Workers in Animal Diseases, Chicago, IL, USA, 13–14 November 1995; Iowa State University Press: Ames, IA, USA, 1995. abstract no. 165. [Google Scholar]
- Bell-Sakyi, L.; Paxton, E.A.; Munderloh, U.G.; Sumption, K.J. Growth of Cowdria ruminantium, the causative agent of heartwater, in a tick cell line. J. Clin. Microbiol. 2000, 38, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Ferrolho, J.; Simpson, J.; Hawes, P.; Zweygarth, E.; Bell-Sakyi, L. Growth of Ehrlichia canis, the causative agent of canine monocytic ehrlichiosis, in vector and non-vector ixodid tick cell lines. Ticks Tick Borne Dis. 2016, 7, 631–637. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Yabsley, M.J.; Murphy, S.M.; Luttrell, M.P.; Howerth, E.W. Isolation and establishment of the raccoon Ehrlichia-like agent in tick cell culture. Vector-Borne Zoonot. Dis. 2007, 7, 418–425. [Google Scholar] [CrossRef]
- Wass, L.; Grankvist, A.; Bell-Sakyi, L.; Bergström, M.; Ulfhammer, E.; Lingblom, C.; Wennerås, C. Cultivation of the causative agent of human neoehrlichiosis from clinical isolates identifies vascular endothelium as a target of infection. Emerg. Microbes Infect. 2019, 8, 413–425. [Google Scholar] [CrossRef]
- Ferreira, J.S.; Oliveira, D.A.S.; Santos, J.P.; Ribeiro, C.C.D.U.; Baêta, B.A.; Teixeira, R.C.; Neumann, A.S.; Rosa, P.S.; Pessolani, M.C.V.; Moraes, M.O.; et al. Ticks as potential vectors of Mycobacterium leprae: Use of tick cell lines to culture the bacilli and generate transgenic strains. PLoS Negl. Trop. Dis. 2018, 12, e0007001. [Google Scholar] [CrossRef]
- Kurtti, T.J.; Simser, J.A.; Baldridge, G.D.; Palmer, A.T.; Munderloh, U.G. Factors influencing in vitro infectivity and growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae). J. Invert. Pathol. 2005, 90, 177–186. [Google Scholar] [CrossRef]
- Pornwiroon, W.; Pourciau, S.S.; Foil, L.D.; Macaluso, K.R. Rickettsia felis from cat fleas: Isolation and culture in a tick-derived cell line. Appl. Environ. Microbiol. 2006, 72, 5589–5595. [Google Scholar] [CrossRef]
- Kurtti, T.J.; Felsheim, R.F.; Burkhardt, N.Y.; Oliver, J.D.; Heu, C.C.; Munderloh, U.G. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int. J. Syst. Evol. Microbiol. 2015, 65, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Bell-Sakyi, L.; Palomar, A.M.; Kazimirova, M. Isolation and propagation of a Spiroplasma sp. from Slovakian Ixodes ricinus ticks in Ixodes spp. cell lines. Ticks Tick Borne Dis. 2015, 6, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Noda, H.; Munderloh, U.G.; Kurtti, T.J. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 1997, 63, 3926–3932. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.A.; Nalbantoglu, U.; Sayood, K.; Zentz, E.B.; Cer, R.Z.; Iwen, P.C.; Francesconi, S.C.; Bishop-Lilly, K.A.; Mokashi, V.P.; Sjöstedt, A.; et al. Reclassification of Wolbachia persica as Francisella persica comb. nov. and emended description of the family Francisellaceae. Int. J. Syst. Evol. Microbiol. 2016, 66, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.S.; Dias, F.A.; Ferreira, J.S.; Fontes, A.N.B.; Rosa, P.S.; Macedo, R.E.; Oliveira, J.H.; Texeira, R.L.F.; Pessolani, M.C.V.; Moraes, M.O.; et al. Experimental infection of Rhodnius prolixus (Hemiptera, Triatominae) with Mycobacterium leprae indicates potential for leprosy transmission. PLoS ONE 2016, 11, e0156037. [Google Scholar] [CrossRef]
- Bell-Sakyi, L.; Kohl, A.; Bente, D.A.; Fazakerley, J.F. Tick cell lines for study of Crimean-Congo hemorrhagic fever virus and other arboviruses. Vector-Borne Zoonot. Dis. 2012, 12, 769–781. [Google Scholar] [CrossRef]
- Lu, P.; Bian, G.; Pan, X.; Xi, Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 2012, 6, e1754. [Google Scholar] [CrossRef]
- Sunyakumthorn, P.; Bourchookarn, A.; Pornwiroon, W.; David, C.; Barker, S.A.; Macaluso, K.R. Characterization and growth of polymorphic Rickettsia felis in a tick cell line. Appl. Environ. Microbiol. 2008, 74, 3151–5158. [Google Scholar] [CrossRef][Green Version]
- Casiraghi, M.; Bordenstein, S.R.; Baldo, L.; Lo, N.; Beninati, T.; Wernegreen, J.J.; Werren, J.H.; Bandi, C. Phylogeny of Wolbachia pipientis based on glta, groel and ftsz gene sequences: Clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology 2005, 151, 4015–4022. [Google Scholar] [CrossRef]
- Ferri, E.; Bain, O.; Barbuto, M.; Martin, C.; Lo, N.; Uni, S.; Landmann, F.; Baccei, S.G.; Guerrero, R.; de Souza Lima, S.; et al. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS ONE 2011, 6, e20843. [Google Scholar] [CrossRef]
- Flatau, R.; Segoli, M.; Khokhlova, I.; Hawlena, H. Wolbachia’s role in mediating its flea’s reproductive success differs according to flea origin. FEMS Microbiol. Ecol. 2018, 94, fiy157. [Google Scholar] [CrossRef] [PubMed]
- Ahantarig, A.; Trinachartvanit, W.; Kittayapong, P. Relative Wolbachia density of field-collected Aedes albopictus mosquitoes in Thailand. J. Vector Ecol. 2008, 33, 173–177. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Vythilingam, I.; Lim, Y.A.L.; Zabari, N.Z.A.M.; Lee, H.L. Detection of Wolbachia in Aedes albopictus and their effects on Chikungunya virus. Am. J. Trop. Med. Hyg. 2017, 96, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, T.M.; Hashimoto, K.; Harnandika, R.K.; Amalin, D.M.; Watanabe, K. Detection of Wolbachia in field-collected Aedes aegypti mosquitoes in metropolitan Manila, Philippines. Parasites Vectors 2019, 12, 361. [Google Scholar] [CrossRef] [PubMed]
- Kittayapong, P.; Milne, J.R.; Tigvattananont, S.; Baimai, V. Distribution of the reproduction-modifying bacteria, Wolbachia, in natural populations of tephritid fruit flies in Thailand. ScienceAsia 2000, 26, 93–103. [Google Scholar] [CrossRef]
- Charlat, S.; Hornett, E.A.; Dyson, E.A.; Ho, P.P.Y.; Loc, N.T.; Schilthuizen, M.; Davies, N.; Roderick, G.K.; Hurst, G.D.D. Prevalence and penetrance variation of male-killing Wolbachia across Indo-Pacific populations of the butterfly Hypolimnas bolina. Molec. Ecol. 2005, 14, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-L.; Lee, W.-C.; Xia, J.; Zhang, G.; Razali, R.; Anwar, A.; Fong, M.-Y. Draft genome of Brugia pahangi: High similarity between B. pahangi and B. malayi. Parasites Vectors 2015, 8, 451. [Google Scholar] [CrossRef]
- Uni, S.; Mat Udin, A.S.; Agatsuma, T.; Junker, K.; Saijuntha, W.; Bunchom, N.; Fukuda, M.; Martin, C.; Lefoulon, E.; Labat, A.; et al. Description, molecular characteristics and Wolbachia endosymbionts of Onchocerca borneensis Uni, Mat Udin & Takaoka n. sp. (Nematoda: Filarioidea) from the Bornean bearded pig Sus barbatus Müller (Cetartiodactyla: Suidae) of Sarawak, Malaysia. Parasites Vectors 2020, 13, 50. [Google Scholar] [CrossRef]
- Tseng, S.; Hsu, P.; Lee, C.; Wetterer, J.K.; Hugel, S.K.; Wu, L.; Lee, C.; Yoshimura, T.; Yang, C.S. Evidence for common horizontal transmission of Wolbachia among ants and ant crickets: Kleptoparasitism added to the list. Microorganisms 2020, 8, 805. [Google Scholar] [CrossRef]
- Vaishampayan, P.A.; Dhotre, D.P.; Gupta, R.P.; Lalwani, P.; Ghate, H.; Patole, M.S.; Shouche, Y.S. Molecular evidence and phylogenetic affiliations of Wolbachia in cockroaches. Molec. Phylogenet. Evol. 2007, 44, 1346–1351. [Google Scholar] [CrossRef]
- Lawrence, A.L.; Webb, C.E.; Clark, N.J.; Halajian, A.; Mihalca, A.D.; Miret, J.; D’Amico, G.; Brown, G.; Kumsa, B.; Modry, D.; et al. Out-of-Africa, human-mediated dispersal of the common cat flea, Ctenocephalides felis: The hitchhiker’s guide to world domination. Int. J. Parasitol. 2019, 49, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Ashwini, M.S.; Puttalakshmamma, G.C.; Mamatha, G.S.; Chandranaik, B.M.; Thimmareddy, P.M.; Placid, E.; Jalali, S.K.; Venkatshan, T. Studies on morphology and molecular characterization of oriental cat flea infesting small ruminants by barcoding. J. Entomol. Zool. Stud. 2017, 5, 301–305. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoo, J.J.; Kurtti, T.J.; Husin, N.A.; Beliavskaia, A.; Lim, F.S.; Zulkifli, M.M.S.; Al-Khafaji, A.M.; Hartley, C.; Darby, A.C.; Hughes, G.L.; et al. Isolation and Propagation of Laboratory Strains and a Novel Flea-Derived Field Strain of Wolbachia in Tick Cell Lines. Microorganisms 2020, 8, 988. https://doi.org/10.3390/microorganisms8070988
Khoo JJ, Kurtti TJ, Husin NA, Beliavskaia A, Lim FS, Zulkifli MMS, Al-Khafaji AM, Hartley C, Darby AC, Hughes GL, et al. Isolation and Propagation of Laboratory Strains and a Novel Flea-Derived Field Strain of Wolbachia in Tick Cell Lines. Microorganisms. 2020; 8(7):988. https://doi.org/10.3390/microorganisms8070988
Chicago/Turabian StyleKhoo, Jing Jing, Timothy J. Kurtti, Nurul Aini Husin, Alexandra Beliavskaia, Fang Shiang Lim, Mulya Mustika Sari Zulkifli, Alaa M. Al-Khafaji, Catherine Hartley, Alistair C. Darby, Grant L. Hughes, and et al. 2020. "Isolation and Propagation of Laboratory Strains and a Novel Flea-Derived Field Strain of Wolbachia in Tick Cell Lines" Microorganisms 8, no. 7: 988. https://doi.org/10.3390/microorganisms8070988
APA StyleKhoo, J. J., Kurtti, T. J., Husin, N. A., Beliavskaia, A., Lim, F. S., Zulkifli, M. M. S., Al-Khafaji, A. M., Hartley, C., Darby, A. C., Hughes, G. L., AbuBakar, S., Makepeace, B. L., & Bell-Sakyi, L. (2020). Isolation and Propagation of Laboratory Strains and a Novel Flea-Derived Field Strain of Wolbachia in Tick Cell Lines. Microorganisms, 8(7), 988. https://doi.org/10.3390/microorganisms8070988




