Malaria Elimination in Costa Rica: Changes in Treatment and Mass Drug Administration
Abstract
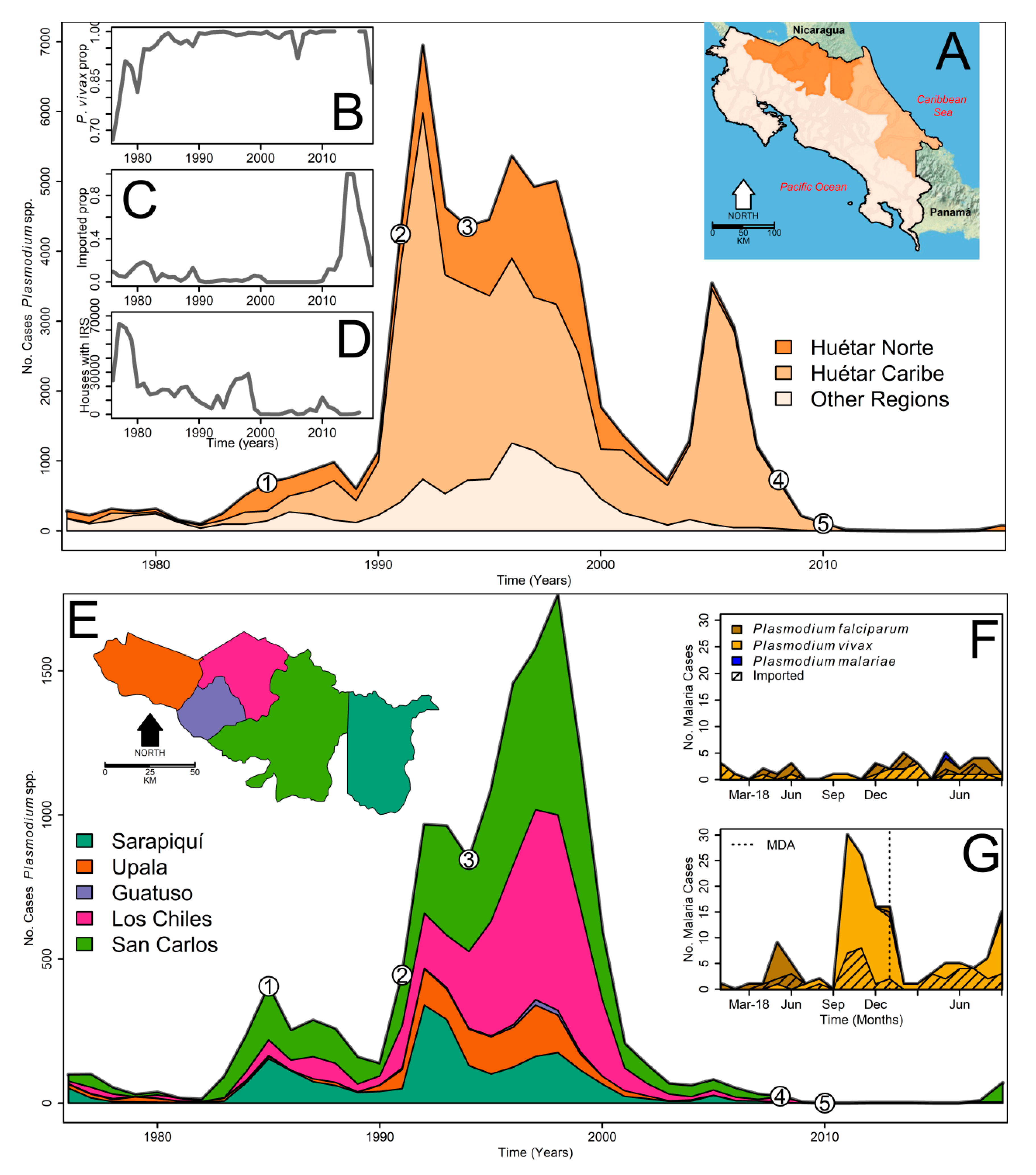
1. Introduction
2. Materials and Methods
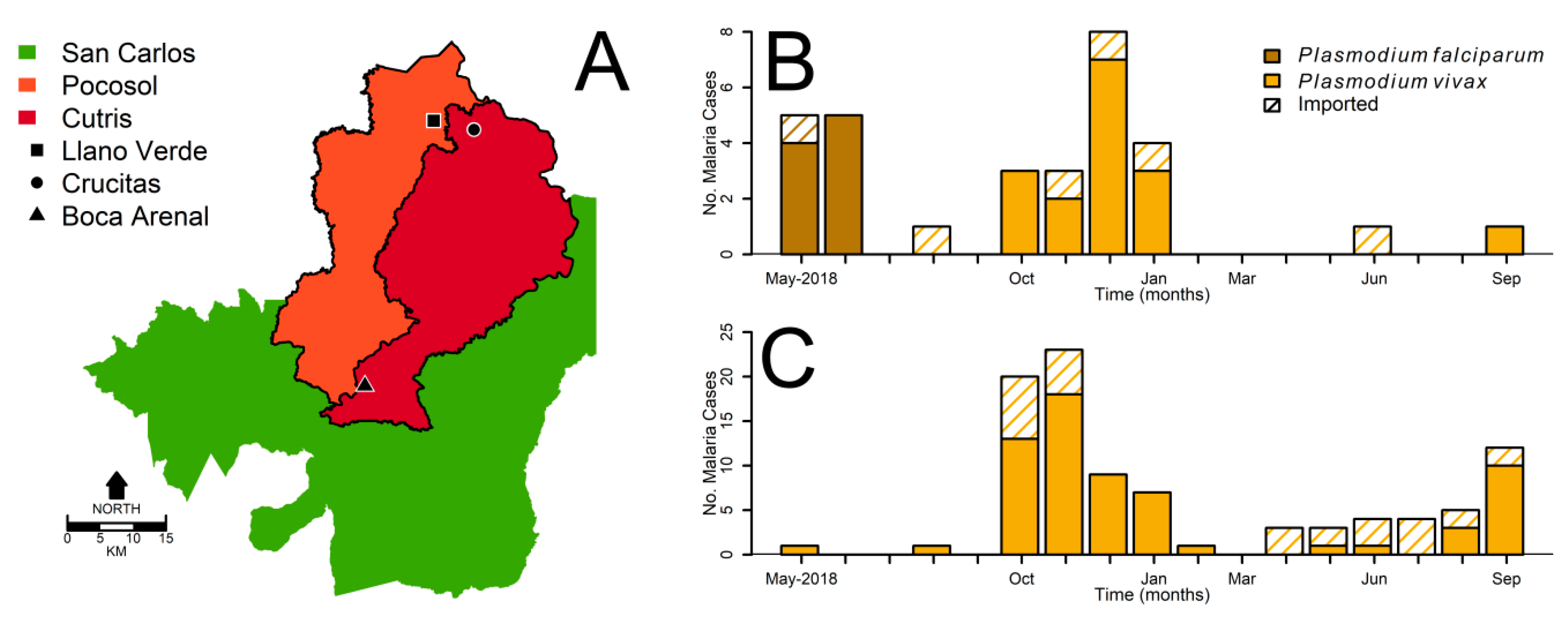
2.1. Surveillance Data
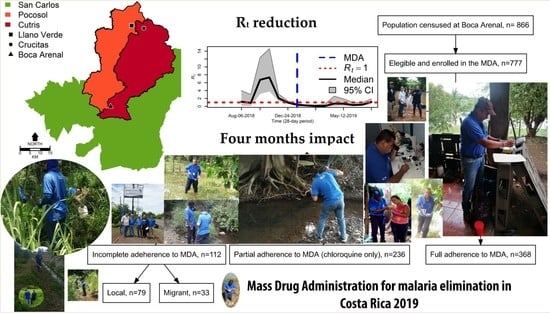
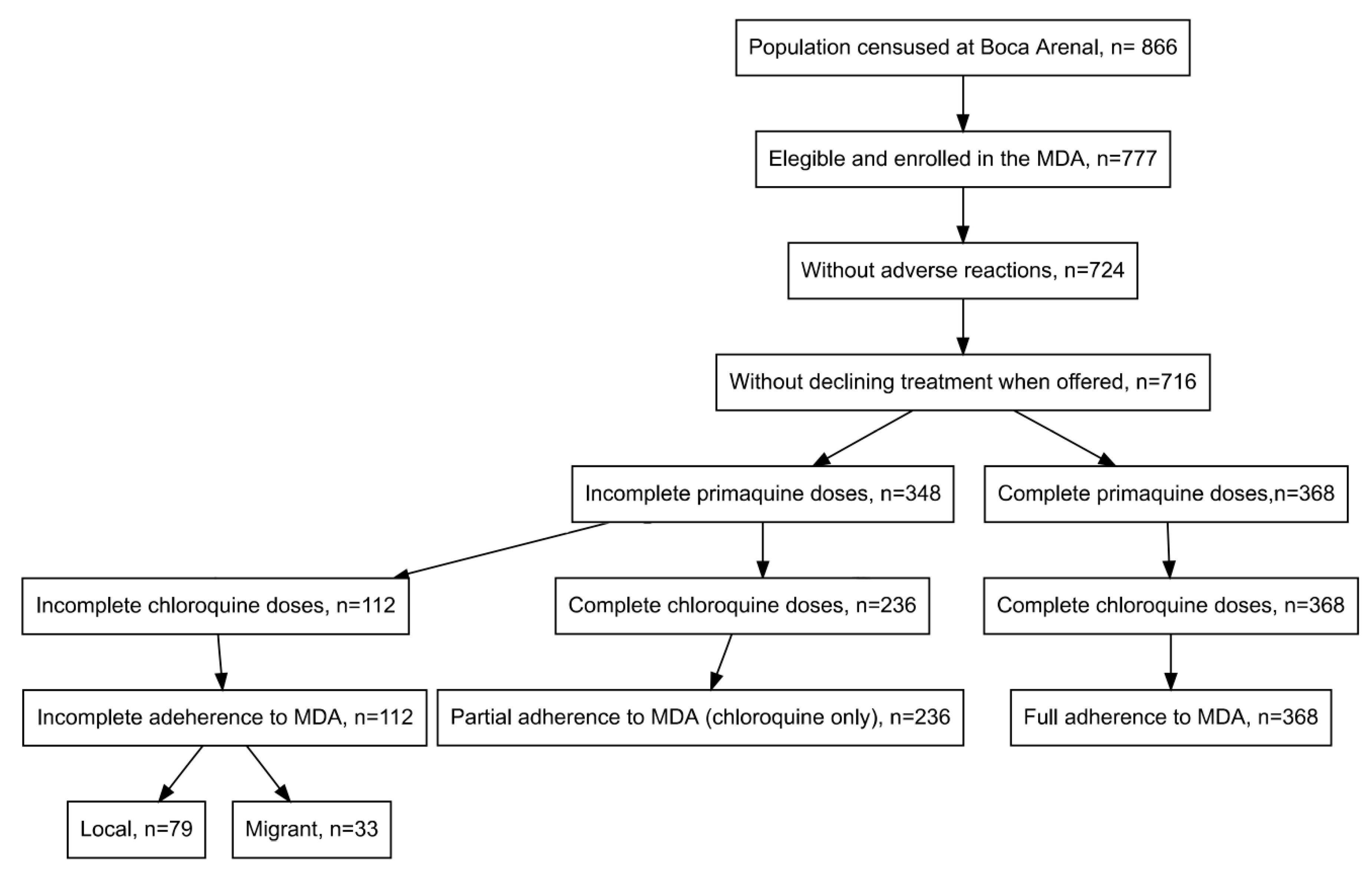
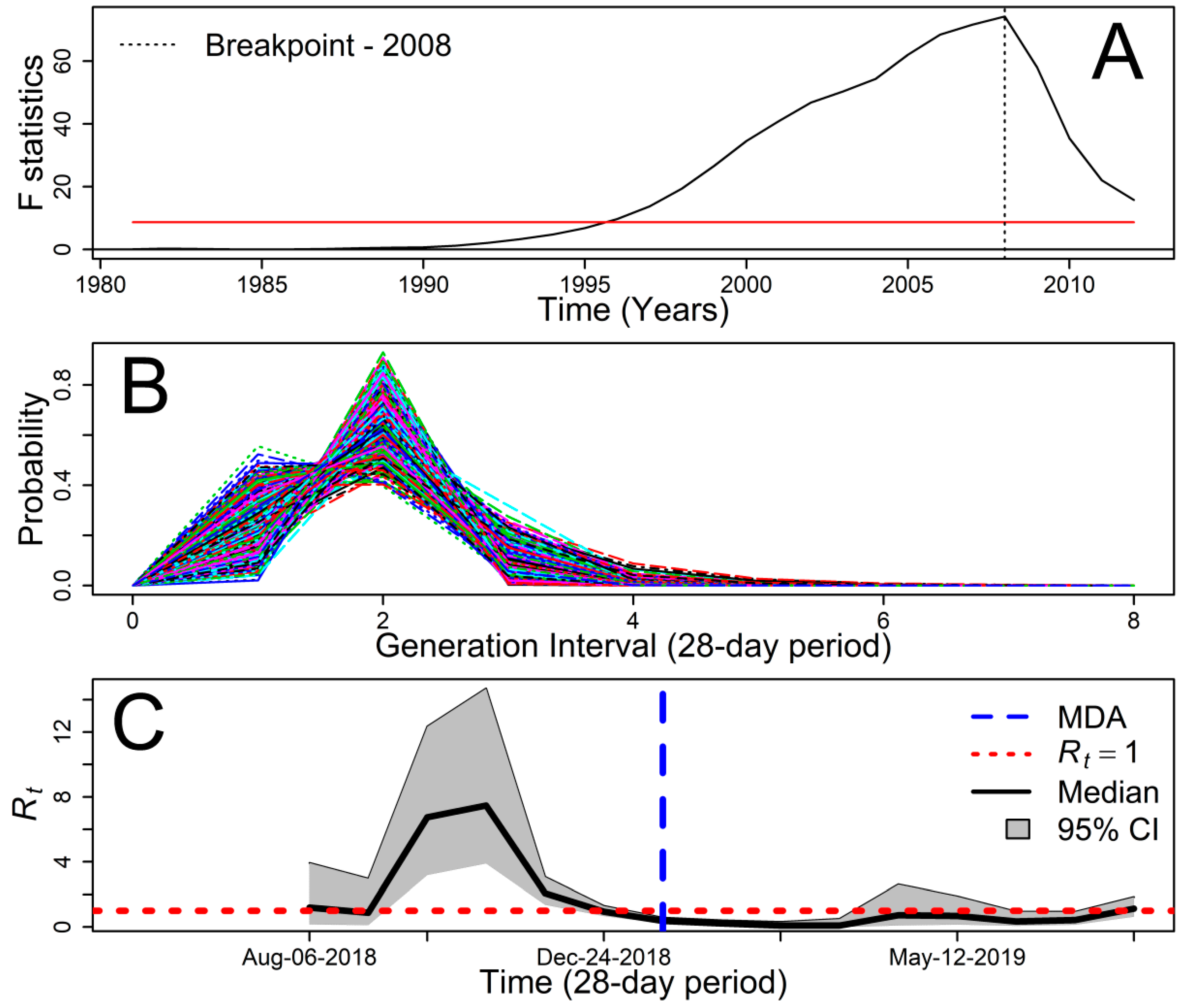
2.2. Mass Drug Administration
2.3. Ethical Clearance
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Chaves, L.F.; Ramírez Rojas, M.; Prado, M.; Garcés, J.L.; Salas Peraza, D.; Marín Rodríguez, R. Health policy impacts on malaria transmission in Costa Rica. Parasitology 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Grupo Técnico Nacional de Enfermedades Vectoriales. Plan de Eliminación de la Malaria en Costa Rica; 2015–2020; Llorca, F., Ed.; Ministerio de Salud de Costa Rica: San José, Costa Rica, 2015; p. 16.
- WHO. Update on the E-2020 Initiative of 21 Malaria-Eliminating Countries; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Ávila-Agüero, M.L. Epidemiología de la malaria en Costa Rica. Acta Médica Costarric. 2008, 50, 72–74. [Google Scholar]
- Carter, K.H.; Singh, P.; Mujica, O.J.; Escalada, R.P.; Ade, M.P.; Castellanos, L.G.; Espinal, M.A. Malaria in the Americas: Trends from 1959 to 2011. Am. J. Trop. Med. Hyg. 2015, 92, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M. Diagnóstico Situacional de la Malaria y el Uso del DDT en Costa Rica; Organización Panamericana de la Salud: San José, Costa Rica, 2001. [Google Scholar]
- Grupo Técnico Nacional de Enfermedades Vectoriales. Norma de Malaria; Ministerio de Salud de Costa Rica: San José, Costa Rica, 2016; p. 60.
- Sáenz, R.; Bissell, R.A.; Paniagua, F. Post-Disaster Malaria in Costa Rica. Prehosp. Disaster Med. 2012, 10, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Castro-Sancho, J.I.; Munguía-Ramírez, M.d.R.; Ávila-Agüero, M.L. Malaria: Una actualización. Acta Médica Costarric. 2002, 44, 107–112. [Google Scholar]
- Marín Rodríguez, R.; Chaves, L.F. Parasite Removal for Malaria Elimination in Costa Rica. Trends Parasitol 2019, 35, 585–588. [Google Scholar] [CrossRef]
- Villalobos, J.A. “El oro que contemplan los gusanos, que lo disfruten los humanos”. Crucitas y la disputa por el desarrollo en Costa Rica. Anu. De Estud. Centroam. 2016, 42, 133–157. [Google Scholar] [CrossRef][Green Version]
- Chaves, L.F.; Hashizume, M.; Satake, A.; Minakawa, N. Regime shifts and heterogeneous trends in malaria time series from Western Kenya Highlands. Parasitology 2012, 139, 14–25. [Google Scholar] [CrossRef]
- Bai, J.; Perron, P. Computation and analysis of multiple structural change models. J. Appl. Econom. 2003, 18, 1–22. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practices of Statistics in Biological Research, 3rd ed.; W. H. Freeman: New York, NY, USA, 1994; p. 880. [Google Scholar]
- Thompson, R.N.; Stockwin, J.E.; van Gaalen, R.D.; Polonsky, J.A.; Kamvar, Z.N.; Demarsh, P.A.; Dahlqwist, E.; Li, S.; Miguel, E.; Jombart, T.; et al. Improved inference of time-varying reproduction numbers during infectious disease outbreaks. Epidemics 2019, 29, 100356. [Google Scholar] [CrossRef]
- Reich, N.G.; Lessler, J.; Cummings, D.A.T.; Brookmeyer, R. Estimating incubation period distributions with coarse data. Stat. Med. 2009, 28, 2769–2784. [Google Scholar] [CrossRef] [PubMed]
- Priestley, M.B. Non-Linear and Non-Stationary Time Series Analysis; Academic Press: London, UK, 1988. [Google Scholar]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.L.C.; Shih, H.H.; Zheng, Q.N.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Huber, J.H.; Johnston, G.L.; Greenhouse, B.; Smith, D.L.; Perkins, T.A. Quantitative, model-based estimates of variability in the generation and serial intervals of Plasmodium falciparum malaria. Malar. J. 2016, 15, 490. [Google Scholar] [CrossRef] [PubMed]
- Breeland, S.G. Studies on the Ecology of Anopheles albimanus. Am. J. Trop. Med. Hyg. 1972, 21, 751–754. [Google Scholar] [CrossRef]
- Hurtado, L.A.; Rigg, C.A.; Calzada, J.E.; Dutary, S.; Bernal, D.; Koo, S.I.; Chaves, L.F. Population Dynamics of Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a Village in a Region Targeted for Malaria Elimination in Panamá. Insects 2018, 9, 164. [Google Scholar] [CrossRef]
- Rigg, C.A.; Hurtado, L.A.; Calzada, J.E.; Chaves, L.F. Malaria infection rates in Anopheles albimanus (Diptera: Culicidae) at Ipetí-Guna, a village within a region targeted for malaria elimination in Panamá. Infect. Genet. Evol. 2019, 69, 216–223. [Google Scholar] [CrossRef]
- Vigilancia de la Salud. Boletín Epidemiológico No. 20; Ministerio de Salud de Costa Rica: San José, Costa Rica, 2019; p. 15.
- WHO. Guidelines for the Treatment of Malaria, 2nd ed.; WHO: Geneva, Switzerland, 2010; p. 194. [Google Scholar]
- Price, R.N.; Douglas, N.M. Expanding the Use of Primaquine for the Radical Cure of Plasmodium vivax. Clin. Infect. Dis. 2018, 67, 1008–1009. [Google Scholar] [CrossRef]
- Garita, R.B. La Reforma de la administración financiero-presupuestaria en Costa Rica. Rev. Nac. De Adm. 2018, 9, 27–45. [Google Scholar]
- Llanos-Cuentas, A.; Lacerda, M.V.; Rueangweerayut, R.; Krudsood, S.; Gupta, S.K.; Kochar, S.K.; Arthur, P.; Chuenchom, N.; Möhrle, J.J.; Duparc, S.; et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): A multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 2014, 383, 1049–1058. [Google Scholar] [CrossRef]
- Hetzel, M.W.; Genton, B. Mass drug administration for malaria elimination: Do we understand the settings well enough? BMC Med. 2018, 16, 239. [Google Scholar] [CrossRef]
- Smith, D.L.; Perkins, T.A.; Reiner, J.R.C.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Domingo, G.J.; Satyagraha, A.W.; Anvikar, A.; Baird, K.; Bancone, G.; Bansil, P.; Carter, N.; Cheng, Q.; Culpepper, J.; Eziefula, C.; et al. G6PD testing in support of treatment and elimination of malaria: Recommendations for evaluation of G6PD tests. Malar. J. 2013, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Slater, H.C.; Pemberton-Ross, P.; Wenger, E.; Maude, R.J.; Ghani, A.C.; Penny, M.A.; Gerardin, J.; White, L.J.; Chitnis, N.; et al. Role of mass drug administration in elimination of Plasmodium falciparum malaria: A consensus modelling study. Lancet Glob. Health 2017, 5, e680–e687. [Google Scholar] [CrossRef]
- Tada, M.S.; Ferreira, R.d.G.M.; Katsuragawa, T.H.; Martha, R.C.D.; Costa, J.D.A.N.; Albrecht, L.; Wunderlich, G.; Silva, L.H.P.d. Asymptomatic infection with Plasmodium falciparum and Plasmodium vivax in the Brazilian Amazon Basin: To treat or not to treat? Memórias Inst. Oswaldo Cruz 2012, 107, 621–629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaneko, A.; Taleo, G.; Kalkoa, M.; Yamar, S.; Kobayakawa, T.; Björkman, A. Malaria eradication on islands. Lancet 2000, 356, 1560–1564. [Google Scholar] [CrossRef]
- Kaneko, A. A community-directed strategy for sustainable malaria elimination on islands: Short-term MDA integrated with ITNs and robust surveillance. Acta Trop. 2010, 114, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Eisele, T.P. Mass drug administration can be a valuable addition to the malaria elimination toolbox. Malar. J. 2019, 18, 281. [Google Scholar] [CrossRef]
- Parker, D.M.; Tun, S.T.T.; White, L.J.; Kajeechiwa, L.; Thwin, M.M.; Landier, J.; Chaumeau, V.; Corbel, V.; Dondorp, A.M.; von Seidlein, L.; et al. Potential herd protection against Plasmodium falciparum infections conferred by mass antimalarial drug administrations. eLife 2019, 8, e41023. [Google Scholar] [CrossRef]
- Kondrashin, A.; Baranova, A.M.; Ashley, E.A.; Recht, J.; White, N.J.; Sergiev, V.P. Mass primaquine treatment to eliminate vivax malaria: Lessons from the past. Malar. J. 2014, 13, 51. [Google Scholar] [CrossRef]
- Lindblade, K.A.; Steinhardt, L.; Samuels, A.; Kachur, S.P.; Slutsker, L. The silent threat: Asymptomatic parasitemia and malaria transmission. Expert Rev. Anti Infect. Ther. 2013, 11, 623–639. [Google Scholar] [CrossRef]
- Herrera, S.; Ochoa-Orozco, S.A.; González, I.J.; Peinado, L.; Quiñones, M.L.; Arévalo-Herrera, M. Prospects for Malaria Elimination in Mesoamerica and Hispaniola. PLoS Negl. Trop. Dis. 2015, 9, e0003700. [Google Scholar] [CrossRef]
- Koepfli, C.; Yan, G. Plasmodium Gametocytes in Field Studies: Do We Measure Commitment to Transmission or Detectability? Trends Parasitol 2018, 34, 378–387. [Google Scholar] [CrossRef]
- Heinemann, S.; Belkin, J.N. Collection records of the project “Mosquitoes of Middle America” 7. Costa Rica (CR). Mosq. Syst. 1977, 9, 237–287. [Google Scholar]
- Poh, K.C.; Chaves, L.F.; Reyna-Nava, M.; Roberts, C.M.; Fredregill, C.; Bueno, R.; Debboun, M.; Hamer, G.L. The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Sci. Total Environ. 2019, 675, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Friberg, M.D.; Moji, K. Synchrony of globally invasive Aedes spp. immature mosquitoes along an urban altitudinal gradient in their native range. Sci. Total Environ. 2020, 139365. [Google Scholar] [CrossRef] [PubMed]
- Obaldia, N.; Baro, N.K.; Calzada, J.E.; Santamaria, A.M.; Daniels, R.; Wong, W.; Chang, H.-H.; Hamilton, E.J.; Arevalo-Herrera, M.; Herrera, S.; et al. Clonal Outbreak of Plasmodium falciparum Infection in Eastern Panama. J. Infect. Dis. 2015, 211, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- The mal, E.R.A.R.C.P.o.C.t.R.; Measuring, T. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017, 14, e1002452. [Google Scholar] [CrossRef]
- Ministerio de Planificación Nacional y Política Económica. Índice de Desarrollo Social 2017; MIDEPLAN: San José, CR, USA, 2018; p. 126.
- PAHO. Interactive Malaria Statistics. Available online: http://www.paho.org/hq/index.php?option=com_content&view=article&id=2632:2010-interactive-malaria-statistics&Itemid=2130&lang=en (accessed on 4 May 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, L.F.; Huber, J.H.; Rojas Salas, O.; Ramírez Rojas, M.; Romero, L.M.; Gutiérrez Alvarado, J.M.; Perkins, T.A.; Prado, M.; Rodríguez, R.M. Malaria Elimination in Costa Rica: Changes in Treatment and Mass Drug Administration. Microorganisms 2020, 8, 984. https://doi.org/10.3390/microorganisms8070984
Chaves LF, Huber JH, Rojas Salas O, Ramírez Rojas M, Romero LM, Gutiérrez Alvarado JM, Perkins TA, Prado M, Rodríguez RM. Malaria Elimination in Costa Rica: Changes in Treatment and Mass Drug Administration. Microorganisms. 2020; 8(7):984. https://doi.org/10.3390/microorganisms8070984
Chicago/Turabian StyleChaves, Luis F., John H. Huber, Obdulio Rojas Salas, Melissa Ramírez Rojas, Luis M. Romero, José M. Gutiérrez Alvarado, T. Alex Perkins, Monica Prado, and Rodrigo Marín Rodríguez. 2020. "Malaria Elimination in Costa Rica: Changes in Treatment and Mass Drug Administration" Microorganisms 8, no. 7: 984. https://doi.org/10.3390/microorganisms8070984
APA StyleChaves, L. F., Huber, J. H., Rojas Salas, O., Ramírez Rojas, M., Romero, L. M., Gutiérrez Alvarado, J. M., Perkins, T. A., Prado, M., & Rodríguez, R. M. (2020). Malaria Elimination in Costa Rica: Changes in Treatment and Mass Drug Administration. Microorganisms, 8(7), 984. https://doi.org/10.3390/microorganisms8070984