Abstract
Prediction parameters of possible outcomes of canine leishmaniasis (CanL) therapy might help with therapeutic decisions and animal health care. Here, we aimed to develop a diagnostic method with predictive value by analyzing two groups of dogs with CanL, those that exhibited a decrease in parasite load upon antiparasitic treatment (group: responders) and those that maintained high parasite load despite the treatment (group: non-responders). The parameters analyzed were parasitic load determined by q-PCR, hemogram, serum biochemistry and immune system-related gene expression signature. A mathematical model was applied to the analysis of these parameters to predict how efficient their response to therapy would be. Responder dogs restored hematological and biochemical parameters to the reference values and exhibited a Th1 cell activation profile with a linear tendency to reach mild clinical alteration stages. Differently, non-responders developed a mixed Th1/Th2 response and exhibited markers of liver and kidney injury. Erythrocyte counts and serum phosphorus were identified as predictive markers of therapeutic response at an early period of assessment of CanL. The results presented in this study are highly encouraging and may represent a new paradigm for future assistance to clinicians to interfere precociously in the therapeutic approach, with a more precise definition in the patient’s prognosis.
1. Introduction
Canine leishmaniasis (CanL) is a severe disease caused by the protozoa Leishmania infantum [1,2,3]. As a zoonotic infection, L. infantum is a major health concern, since it also causes human visceral leishmaniasis, a neglected tropical disease, in the Americas, Europe, North Africa, the Middle East and China [4]. Dogs are susceptible to the development of high parasitic loads, playing a key role in the transmission cycle of L. infantum via phlebotomine sand flies in endemic areas [5,6]. Higher parasite loads in dogs are related to more severe clinical disease [7] and higher infectiousness to sand flies [5]. As full-blown disease is associated with active infection [8], efficacy of CanL treatment should be primarily centered in its capability of reducing L. infantum load.
Treatment of CanL is a challenge for veterinary practitioners regarding anti-parasitical efficacy, adverse effects and cost. Current leishmanicidal drugs, such as antimonials, miltefosine and allopurinol, can clinically cure dogs or temporarily improve clinical signs, but no evidence of efficacy in terms of a parasitological cure exists for any of them [8,9]. Consequently, clinical recurrences associated with high parasite loads in a number of treated dogs are frequent at diverse time intervals post treatment [10]. In Brazil, where most of the South American cases of L. infantum infection occur, the use of antimonials for canine treatment is prohibited [11].
The success of some antileishmanial protocols in reducing L. infantum load and blocking parasite transmission to phlebotomine vectors have been evidenced by xenodiagnoses [12,13,14]. However, failure of anti-Leishmania therapy depends on drug pharmacodynamic effects, parasite’s inherent virulence or drug resistance development and host’s immune and nutritional status, or co-infections, which contribute decisively to the recurrence [15,16]. Prognosis of response to treatment largely depends on the severity of the visceral disease, as determined by the clinicopathological alterations, particularly those related to renal function [17,18].
Because dogs respond differently to anti-parasite treatments and there are still no predictive tools to discriminate dogs that respond to therapy from those that maintain high parasitic loads despite treatment, we aimed at devoting the present study to this aspect. This study was a retrospective longitudinal analysis of parasitological, biochemical and hematological parameters in a cohort of dogs treated for CanL with second-line antileishmanial drugs, as reported in the literature [8,9,19,20,21,22]. The study’s objective was to identify biomarkers and to develop a prototype mathematical model with predictive value to estimate the success of a leishmanicidal therapy as a tool for improved diagnosis and treatment monitoring.
2. Materials and Methods
2.1. Ethics Approval
This work was approved by the Ethics Committee on Animal Use of the Federal University of Bahia, Brazil (CEUA-UFBA, n. 19/2011), substantiated in the bioethical principles of animal experimentation. All procedures were followed in accordance with the guidelines of the Brazilian Council of Animal Experimentation (CONCEA—Conselho Nacional de Controle de Experimentação Animal) and strictly followed the Brazilian law for “Procedures for the Scientific Use of Animals” (11.794/2008). All manipulation in dogs, whether invasive or non-invasive, was performed or monitored by veterinarians. The group of non-responder dogs was designed based on the parasitic load whose quantitative test was performed at the end of the study. Therefore, throughout the course of the study, dogs that developed other diseases or acquired co-infections were removed to receive proper additional treatment.
2.2. Animals and Sampling
A one-year longitudinal analysis of hematological and biochemical parameters was conducted in 26 dogs, naturally infected by L. infantum in the state of Bahia in Brazil, submitted to leishmanicidal treatment. The inclusion criteria were confirmed infection with Leishmania spp. via parasitological and/or molecular techniques, and consent of the guardians for the treatment with the multi-drug antiparasitic regimen (metronidazole + ketoconazole + allopurinol). The coexistence of other diseases or co-infections and terminal CanL were the exclusion criteria in the beginning and at any other time of examination in the course of the follow-up. Dogs were evaluated at six different time points during the year. The time 0 (T0) consisted of the moment in which the dogs arrived at the Zoonotic Leishmaniasis Outpatient Clinic at the Teaching Hospital of Veterinary Medicine of the Federal University of Bahia (ALZ-UFBA) for diagnosis and first clinical care. Dogs were evaluated monthly during the first three months (T1, T2, T3), and then at the sixth (T6) and twelfth month (T12) post-treatment. At each evaluation, blood was collected from the jugular or cephalic vein and stored in EDTA tubes (BD Vacutainer; Becton, Dickinson) for hemogram and search for hematozoa. Blood was also collected into EDTA-free tubes (BD Vacutainer; Becton, Dickinson) for the determination of serological and biochemical parameters. Urine was collected by cystocentesis (in females) and urethral catheterization (in males) for urinalysis and urinary protein-creatinine ratio (UPC). For the collection of bone marrow and spleen samples by aspiration biopsy (performed at T0, T6 and T12), 20 mL syringes and needles 40 × 12 mm and 40 × 16 mm were used respectively, inserted in an anatomic-topographic region of the spleen [23] and sternum [24], with previous local antisepsis and mild sedation with acepromazine (0.02 mg/kg).
2.3. Clinical Evaluation and Staging
Dogs were clinically evaluated for the presence or absence of CanL-compatible clinical signs and definition of clinical staging, as determined by the LeishVet Group guidelines [8]. Weight loss, appetite alterations, facies, mucosal staining, dermatopathies (ulcers, cutaneous vasculitis, onychogryphosis, desquamation, alopecia, crusts, hyperkeratosis, depigmentation of snout, nodule), lymphadenopathy, ophthalmopathy, presence or absence of fever, diarrhea, epistaxis and enlargement of the spleen by palpation were assessed. Based on clinical and laboratory parameters, dogs were categorized in clinical staging I (mild disease), II (moderate disease), III (severe disease) or IV (very severe to terminal disease).
2.4. Anti-Leishmania Chemotherapy
Antiparasitic treatment consisted in the administration of metronidazole (25 mg/kg/twice daily) for 30 days associated to ketoconazole (10 mg/kg/once a day) for 40 days, followed by a maintenance treatment with allopurinol (10 mg/kg/twice daily) during at least one year. Prednisolone was given (beginning with 0.5 mg/kg/twice daily) for 30 days (including gradual withdrawal) [20,25,26] to minimize systemic immune complex formation, thus indirectly to prevent kidney injury during the four-week initial course of treatment with anti-leishmanial drugs. The multi-drug therapeutic regimen for CanL used in the present study has been previously described [14].
2.5. Hematological and Biochemical Parameters
Hemograms, characterization of blood cell morphology and search for hematozoa infection were performed as described [27]. The following biochemical parameters were measured: enzymes (alanine aminotransferase (ALT), aspartate transaminase (AST), creatine kinase (CK) and its isoenzyme creatine kinase MB (CK-MB), pseudocholinesterase and gamma-glutamyl transferase (GGT) (Cormay, Łomianki, Poland)), protein (albumin and total protein (Cormay, Łomianki, Poland)), substrates (creatinine, urea) and electrolytes (calcium, phosphorus and magnesium (Spinreact, Barcelona, Spain)). The urinary protein-creatinine (UPC) and serum calcium/phosphorus ratio were also quantified. All variables were measured in the automatic (Prestige 24i, Tokyo Japan) and semi-automatic (BioPlus-200, São Paulo Brazil) biochemical analyzer, as described [28].
2.6. Quantification of Total Globulin and Specific Anti-Leishmania Antibodies
For the quantification of total globulins, total protein values were subtracted from albumin values. Specific anti-Leishmania IgG antibodies were quantified in the sera as previously described [29], with minor modifications. Ninety-six-well plates (BioLegend, San Diego USA) were coated with 10 μg/mL of L. infantum soluble promastigote antigens. After washing with 3% PBS-low-fat-milk and 0.05% PBS-Tween (PBS-T), 100 μL/well of canine serum diluted 1:50,000 in PBS-T was added in duplicate and incubated at 30 min at 37 °C. The reaction was completed by adding the anti-dog IgG-HRP diluted at 1:5000 (Bethyl Laboratories, Montgomery, USA) for 30 min at 37 °C, and further incubation with 0.5 mg/mL of o-phenylenediamine dihydrochloride (OPD, Sigma, Darmstadt Germany) for 10 min. The reaction was stopped using 50 μL/well of 3 M HCl. Plates were read at 492 nm (Synergy, Biotek, Tokyo Japan).
2.7. Parasitic Load
DNA of the collected aspirated bone marrow and spleen at T0, T6 and T12 was extracted using the commercial kit PureLink Genomic DNA® (Invitrogen, Carlsbad USA) following the manufacturer’s recommendations. The quality and concentration of the DNA in each eluate were evaluated in the L-QUANT spectrophotometer (Loccus, São Paulo Brazil). For the quantification of L. infantum DNA in the spleen and bone marrow samples, qPCR assays were performed using the protocol described by Rolão et al. [30], with some modifications. The primers 5′-GGTTAGCCGATGGTGGTCTT-3′ (forward), 5′-GCTATATCATATGTCCAAGCACTTACCT3′ (reverse) and the probe TaqMan® (Applied Biosystems Foster City USA) (5′- ACCACCTAAGGTCAACCC-3′) were used. The final reaction volume (25 μL) consisted of 4 μL of DNA, standardized at the concentration of 20 ng/μL, 12.5 mL of Mastermix Universal® (Life Technology Corporation, Carlsbad USA), 5 μM of each primer and 10 μM of the probe TaqMan. qPCR was run at 95 °C for 10 min, 40 cycles at 95° C for 15 s and 60 °C for 1 min. All samples were analyzed in duplicate. Reactions were performed on the Applied Biosystems 7500 Real-Time PCR System (Life Technology Corporation Carlsbad USA).
2.8. Quantitative PCR
Splenic mRNA expression of cytokines IFN-γ, IL-2, TNF-α, IL-17A, IL-22, IL-4, IL-5 and IL-10 was determined using real time PCR. Spleen fragments were macerated for total RNA extraction using Trizol reagent (Invitrogen, Carlsbad USA), as recommended by the manufacturer. RNA concentration was determined by OD260 measurement using a L-quant 1.0 spectrophotometer (Loccus®, São Paulo, Brazil). cDNA synthesis was performed using 1 µg of total RNA plus 10 µL of High-Capacity cDNA Reverse Transcription master mix (Applied Biosystems, Foster City USA), according to the manufacturer instructions. Real-Time quantitative PCR (qRT-PCR) reactions were run for each sample on a Bio-Rad CFX96 Real-Time System C1000 Thermal Cycler (Bio-rad Berkeley USA). Primer sequences were obtained from IDT (Lovaina, Belgium) and thoroughly tested. Specific oligonucleotides are: IL2 (forward) CCCAAGAAGGCCACAGAATTTA, (reverse) TCCTTGGTGTCTGTCAAGTGAA; IL4 (forward) CTAGCACTCACCAGCACCTT, (reverse) CACGAGTCGTTTCTCGCTGT; IL5 (forward) GGCGATGGGAACCTGATGAT, (reverse) CGTGGGCAGTTTGGTTCTTC; IL10 (forward) CAAGCCCTGTCGGAGATGAT, (reverse) AGAAATCGGTGACAGCGTCG; IFNG (forward) TCAAATTCCTGTGAACGATCTGC, (reverse) TTATTTCGATGCTCTGCGGC; TNFA (forward) CTCCAACTAATCAGCCCTCTTG, (reverse) GGGTTTGCTACAACATGAGCTACT; GAPDH (forward) GCTGGTGCTGAGTATGTTGTGGAG, (reverse) CAGCAGAAGGAGCAGAGATGATGA. The RT product was expanded using the NZYSpeedy qPCR Green Master Mix kit (NZYTech, Lisbon Portugal) and results were normalized to the expression of the housekeeping gene Gapdh. After amplification, cycle threshold-values (Ct-values) were calculated for all samples and gene expression changes were analyzed in the CFX Manager Software (Bio-Rad) and represented as arbitrary units (AU).
2.9. Statistical Analysis
The Kruskal–Wallis test followed by the Dunn test were used for data analysis between two groups. A one-way analysis of variance (ANOVA) followed by a Bonferroni’s post-hoc test was employed for multiple group comparisons. Exploratory logistic regression models were made in R using the package Bias Reduction in Binomial-Response Generalized Linear Models [31,32]. The Chi-square test was used for statistical analysis to evaluate the significant association between variables. Data are reported as means ± standard deviation (SD). Statistically significant values are as follows: * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Results
3.1. Responder Dogs Exhibit a Decrease in Splenic and Bone Marrow Parasite Loads and Improvement of Clinical Staging
Leishmania infantum absolute parasite load evolution in spleen and bone marrow of a cohort of dogs under leishmanicidal treatment for 12 months were distinct between two groups. Dogs that presented a significant reduction in parasite loads from T0 to T12 were grouped as chemotherapy Responders (GR, 46%, 12 dogs), while those that failed to have the parasite burden reduced were grouped as Non-Responders (GNR, 54%, 14 dogs; Figure 1A, B). At T0, before treatment, there were no parasitic loads’ differences between groups in both organs (Figure 1A, B), as well as in the clinical staging (Figure 1C). At T6, GR exhibited a noticeable parasitic load reduction in comparison to that of GNR. After one year of treatment (T12), GR’s parasitic loads were significantly lower than those at T0 (p < 0.001) in both tissues (Figure 1A, B), having decreased 97.3% and 98.5% in bone marrow and spleen, respectively. In opposition, no significant difference was observed for GNR (Figure 1A, B). At T0, both groups presented the same clinical stage—II, characterized by moderate disease. Accompanying the parasite load data, at T12, GR exhibited on average a mild disease (stage I), while GNR remained in stage II (Figure 1C).
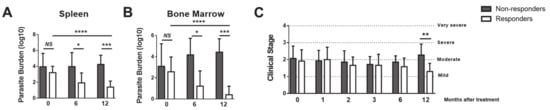
Figure 1.
Responder dogs exhibited decreased parasite loads and improved the clinical staging. The canine cohort was separated into responder and non-responder dogs regarding the effect of anti-Leishmania chemotherapy. The parasite burden was quantified by qPCR in the spleen (A) and bone marrow (B) before and at 6- and 12-months post-treatment. The clinical stage of each dog was recorded at the time of the diagnosis (T0) and upon 1 (T1), 2 (T2), 3 (T3), 6 (T6) and 12 (T12) months of treatment (C). Data are shown as mean ± standard deviation (SD), Responder dogs (n = 12) Non responder dogs (n = 14). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
3.2. Responder Dogs Display a Th1 Signature
We next addressed the immune response developed by GR and GNR. The median monocytes’ counts was similar and within the reference range in both groups (Figure 2A). The lymphocytes’ counts, however, changed at T3 and T6 (Figure 2B), not only increasing from T2 to T3 within GR, but also becoming consistently higher than those of GNR at T3 and T6. To further explore, we performed a qPCR analysis on spleen samples at T0, T6 and T12. The GR exhibited a Th1 signature, with increased levels of IL2, IFNG and TNFA transcripts at T6 (Figure 2C), while no differences were found in IL17 nor IL22 (Supplementary Figure S1A). Moreover, a significant reduction on IL10 transcripts of GR was accompanied by similar levels of Th2-associated IL4 and IL5 cytokines (Figure 2C), indicating an effective Th1 response underlying the protective response. Remarkably, these effects on transcripts could be verified in all individual dogs analyzed (Supplementary Figure S1B, C). Overall, our data showed that increased levels of blood lymphocytes and a splenic Th1 response guide the response to therapy.
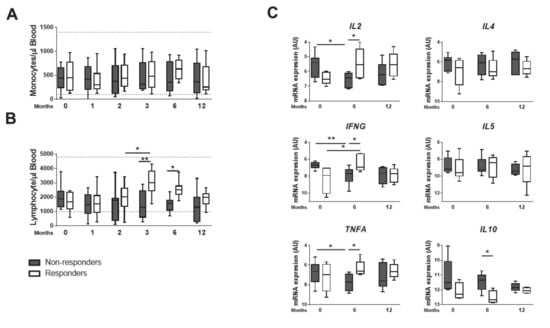
Figure 2.
Responder dogs display Th1 signature. The serum absolute numbers of monocytes (A) and lymphocytes (B) was quantified from T0 until 12 months post treatment. mRNA was isolated from spleen biopsies at T0, T6 and T12. Quantitative PCR was performed for IFNG, TNFA, IL2, IL4, IL5 and IL10 (C). Data are shown as mean ± SD or in a box and whisker plot format, Responder dogs (n = 9) Non responder dogs (n = 8) * p < 0.05; ** p < 0.01.
3.3. Non-Responder Dogs Failed to Restore the Hematological Parameters to Normal Reference Values
At T0, average GR dogs displayed median erythrocyte counts, hemoglobin and hematocrit levels within the normal reference range, while in GNR dogs, those parameters were sub-optimal (Figure 3A–C). Of note, at T0, 8 out of 14 (57.14%) of GNR had anemia; of these, 4 out of 8 (50%) presented a reticulocyte counting below 60 × 103/mm3 (had non-regenerative anemia). Regarding the GR dogs, 3 out of 12 (25%) were anemic, with 2 of these presenting a reticulocyte counting superior to 60 × 103/mm3 (had regenerative anemia). At T12, 9 out of 13 (69.2%) GNR dogs were anemic, compared to none of the GR dogs; among those 9 dogs, only one presented a reticulocyte counting superior to 60 × 103/mm3. This is strongly suggestive that non-regenerative anemia indeed progressed in the GNR group of dogs during the one year of follow up, despite treatment. Total globulin levels were increased in both groups, but the GR displayed a significantly lower level (Figure 3D). Interestingly, the difference in globulins was not equivalent to anti-Leishmania-specific IgG titters, which could not discriminate GR from GNR (Figure 3E).
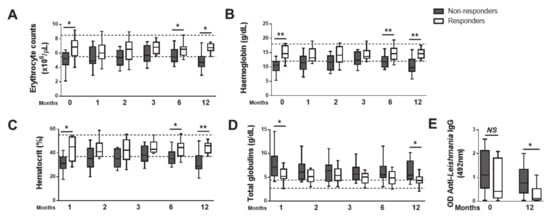
Figure 3.
Success of anti-Leishmania therapy is associated with a restoration of erythrocyte, hemoglobin, hematocrit, as well as total and Leishmania-specific globulin values. Erythrocyte number (A) and the values of hemoglobin (B), hematocrit (C), total globulins (D) and anti-Leishmania IgG (E) were quantified in the serum at diagnosis (0) and upon 1, 2, 3, 6 and 12 months of anti-Leishmania treatment. Data are shown as box and whisker plot format, Responder dogs (n = 12), Non responder dogs (n = 14). * p < 0.05; ** p < 0.01.
The cohort of dogs was monitored during one year through hematological and biochemical analysis performed at one-, two-, three-, six- and twelve-months post-treatment (T1, T2, T3, T6 and T12) (Figure 3A–D). Although a tendency for hematological improvement was observed in all parameters until the third month of treatment, non-responder dogs declined in months 6 and 12 (Figure 3A–D). In opposition, all responder dogs displayed erythrocyte numbers, hemoglobin and hematocrit within the normal reference values upon 12 months of treatment, apart from total globulins, where 3 out of 11 dogs still presented values above the upper limit (Figure 3A–D). Moreover, the observed recovery of responder dogs was accompanied by a significant reduction in the anti-Leishmania-specific IgG (Figure 3E).
Finally, given that our data was suggestive that erythrocyte number, hemoglobin and hematocrit values can be predictive of the therapeutic response in a first moment of evaluation of sick dogs, before treatment, we calculated the relative risk (RR) and the 95% confidence interval (CI) to determine the likelihood of each of these parameters to be associated with the progression of CanL upon treatment. A significant association between decreased hemoglobin (3.14 RR with 1.14–8.7 CI), hematocrit (3.75 RR with 1.05–13.38 CI) and erythrocyte levels (1.82 RR with 0.88–3.73 CI) with the failure of CanL treatment was observed, reinforcing their utility to the prediction of therapeutic response.
3.4. Serum Biochemistry Parameters Are Predictive of Visceral Organ Injury in Non-Responder Dogs
Serum levels of hepatocellular damage markers, alanine and aspartate transaminase (Supplementary Figure S2A, B), and biliary tract disease marker γ-glutamiltransferase (Supplementary Figure S2C), were within the reference range during follow-up in both groups. However, cholinesterase and albumin, proteins synthesized in the liver, were significantly reduced in the GNR at T0, T6 and T12 (Figure 4A and Supplementary Figure S2D). In GNR, while urea and creatinine values were within the reference range (Supplementary Figure S2E, F), the urinary protein/creatinine ratio (UPC), a predictor of renal disease, was elevated (Figure 4B). These peculiar changes are indicative of early hepatic and renal disease in the GNR. The values of hematological, serum biochemistry and parasitic load of the initial and final times of the responder and non-responder dogs to the treatment are shown in Table 1.
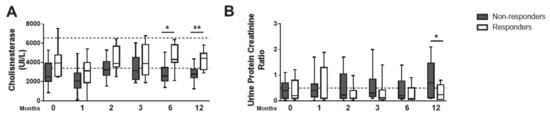
Figure 4.
Non-responder dogs develop sub-clinical hepatic and renal lesions. Serum values of cholinesterase (A) and urine protein/creatinine ratio (B) were quantified at T0, T1, T2, T3, T6 and T12. Data are shown as mean ± SD or in a box and whisker plot format, Responder dogs (n = 12), Non responder dogs (n = 14). * p < 0.05; ** p < 0.01.

Table 1.
Values of hematological, serum biochemistry, oxidative stress parameters and parasitic load of the initial and final times of the responder and non-responder dogs to the treatment
3.5. Development of a Mathematical Model with Predictive Value for the Success of CanL Chemotherapy
Apart from erythrocytes, hemoglobin and hematocrit (Figure 3A–C), phosphorous, and to a lesser extent, potassium and albumin also significantly discriminated GR from GNR at T0 (Figure 5A). High Spearman correlation coefficients were found between erythrocytes, hematocrit and hemoglobin (Figure 5B). Logistic regression models with the lowest Akaike information criterion (AIC) and higher accuracy using a leave-one-out cross-validation were selected for phosphorus plus one of the three highly correlated variables erythrocytes, hematocrit or hemoglobin (Figure 5C). The highest accuracy was obtained considering phosphorus and erythrocytes. For this case, the log(odds) of a successful treatment, using the full dataset, can be given by:
log(odds) = 14.067 − 5.289 × [Phosphorus] + 1.170 × Number of erythrocytes
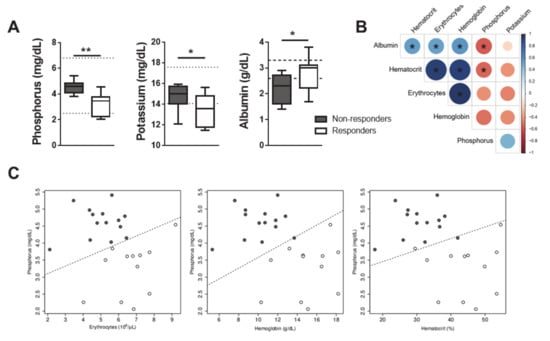
Figure 5.
Development of a mathematical model with predictive value for the success of CanL chemotherapy including the measurement of the erythrocyte and phosphorus parameters. (A) The serum values of phosphorous, potassium and albumin were quantified at T0). (B) The Spearman linear correlation coefficient is shown for serum albumin, hematocrit, erythrocytes, hemoglobin, phosphorous and potassium values. (C) Scatterplots between phosphorous and hematocrit or erythrocytes or hemoglobin serum values. Data are shown as mean ± SD or in a box and whisker plot format. Responder dogs (n = 12), Non responder dogs (n = 14). *p < 0.05; **p < 0.01.
The probability is obtained by applying the logistic function: (exp(x)/(1 + exp(x)), where x is the log(odds). Remarkably, this formula allowed 100% discrimination between GR and GNR, demonstrating the high predictive power of our mathematical model.
4. Discussion
Until recently, most studies of prognostic factors for treatment success or failure in leishmaniasis were restricted to humans [33,34,35,36,37], and the infected dog has been seen only as a protozoan reservoir. This is an innovative longitudinal study of prognostic factors in L. infantum naturally infected dogs under treatment that resulted in a clear distinction of two profiles of CanL dogs that responded differently to the same antiparasitic therapy, based on their tissue parasite load reduction. Dogs with reduced parasite numbers are less likely to develop clinical signs and are less infectious to vectors [5].
Studies indicate that the successful resolution of Leishmania infections depend on a response characterized by IFN-γ, IL-2 and TNF-α predominance, which increase phagocytic efficiency and lymphocyte cytotoxicity, triggering a protective immune response [38,39]. Accordingly, we observed that responder dogs exhibited a consistent Th1 signature that accompanied parasitism reduction upon treatment, in opposition to the non-responder group.
In the present study, the standard parameters for clinical staging of CanL [8] and follow-up of the dogs, such as albumin/globulin ratio, non-regenerative anemia or renal functions, were assessed individually from the beginning to the end of the study. However, only at the end of the one-year study did the treatment outcome allow the determination of which dogs could be classified as responders or non-responders. The evident dichotomy between GR and GNR dogs allowed for identifying two laboratory parameters predictive of therapeutic success: erythrocyte counts and serum phosphorus dosage. Anemia is commonly observed in human and canine leishmaniasis and can originate from reduced production and/or increased destruction of erythrocytes [27,40,41]. During chronic inflammatory diseases, anemia results from low iron circulating levels, while inflammatory cytokines such TFN-α, IL-1 and IFN-γ inhibit erythropoiesis, and erythrocyte membrane damage by oxidizing agents shortens the lifespan of erythrocytes [42]. In chronic infections such as CanL, these damaged erythrocytes are retained and destroyed in the spleen, which becomes enlarged as a clinical sign of the disease. This is accompanied by a dysregulation of iron transporting proteins leading to the accumulation of storage iron [43,44]. This explains the normocytic normochromic type of anemia most dogs had in the beginning of the present study, which also demonstrates the improper iron supplementation during the treatment, as already described in the literature [45,46,47]. In chronic renal disease, which is common in CanL, anemia also results from decreased erythropoietin production in the kidney [8,48]. Kidneys are the main route for phosphorus excretion and hyperphosphatemia promotes progressive renal lesions [49], as described in dogs with late-stage CanL [50]. Serum globulin and acute phase proteins levels, proteinuria and UPC ratio are considered biomarkers for the clinical monitoring of dogs during leishmanicidal treatment and post-treatment [3,17,18,51,52]. However, very few studies have approached the identification of factors, to be used as reliable prognosis predictors, based on the outcome (death or cure) upon therapy [53]. In the present study, moderate-stage CanL dogs exhibited overall serum phosphorus—a marker of renal failure—within the reference range, but the logistic regression models clearly indicated that phosphorus’ in addition to erythrocyte’s changes were sensitive enough to predict chemotherapy success or failure.
Phosphorus interplays with calcium via modulation of several hormones and their serum concentration is approximately inversely related [50]. Overall, non-responder dogs of our study displayed lower magnesium, higher phosphorus and lower Ca/P ratio compared to responder dogs, with renal consequences demonstrated by elevated UPC ratio. Renal impairment-related ion imbalances underlie several clinical manifestations; for instance, magnesium is an enzymatic cofactor that participates in diverse metabolic reactions [40,54], including those related to erythropoiesis [55,56].
Cholinesterase and albumin serum levels normalized in responder dogs after six months of therapy, unlike in non-responders. As the liver is a site of L. infantum activity [57], this discrete interference with hepatic synthetic function could explain our findings in non-responder dogs. Here, serum urea and creatinine were within the reference values in both group of dogs. This corroborates literature data showing that azotemia is an uncommon finding, despite elevated frequencies of renal pathologies in CanL [58]. Creatinine is a by-product of CK, whose levels are reduced in dogs with LCan [59]; therefore, we suggest that this bias of azotemia diagnosis in advanced CanL results from sub-concentration of creatinine and might lead to kidney disease underdiagnoses. It has been stressed in the literature that the active infection by L. infantum and CanL progression relates with immune complex-mediated disease [60], which plays an important role in the pathophysiology of diverse clinical manifestations, including immune-mediated hemolytic anemia, glomerular lesions and renal failure [38,61]. In this sense, prednisolone was chosen as an anti-inflammatory drug in the present study during the short-term in all dogs to minimize the formation and circulation of soluble immune complexes and reduce inflammation through an inhibitory effect on complement activation [62,63]. Glucocorticoid therapy benefits in the treatment of CanL have been described previously [20,25,26]. It is worth mentioning that several studies have shown that the short- or long-term oral glucocorticoid administration in dogs do not induce significant changes in the several hematological and biochemical parameters [64,65,66,67]. The four-week course of prednisolone did not seem to have interfered with the biomarkers evaluated herein, as evaluations at T1 did not behave differently than those seen at subsequent follow-up points, as demonstrated in Figure 2, Figure 3 and Figure 4. Upon corticotherapy at anti-inflammatory doses, a discrete elevation in liver enzymes is expected in dogs, as previously described [66,68].
Altogether, we developed a mathematical formula using erythrocytes and phosphorus as markers for a prognosis of antiparasitic therapy success in CanL at the time of first diagnosis. Nevertheless, the study displays the reduced cohort studied, and the use of second line anti-parasite drugs, given the constraints for therapeutic approaches to CanL in Brazil, as major limitations. Because this was a long-term clinical study, it resulted in a small number of dogs that were followed up until the last evaluation. Nevertheless, the gathered data was so consistent that it allowed the creation of the formula, which can be an initial guide for further studies with larger sample sizes and different methods of cross-validation and in cohorts treated with first-line drugs. Taken together, these results are highly encouraging and may represent a new paradigm for clinical assistance, allowing a precocious therapeutic interference based on an improved diagnosis.
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/2076-2607/8/5/745/s1.
Author Contributions
Conceptualization, F.A.P., R.S. and S.M.B-M.; methodology, F.A.P., R.S. and S.M.B-M.; formal analysis, R.S.G., F.A.P., R.J.D-O., R.A., J.G., E.M.R-S., D.F.L., H.G., R.S. and S.M.B-M.; investigation, R.S.G., F.A.P., R.J.D-O., R.A., J.G., D.F.L., E.M.R-S., H.G., R.S. and S.M.B-M.; resources, R.J.D-O., H.G., R.S. and S.M.B-M.; data curation, R.S.G., F.A.P., R.A., J.G., E.M.R-S., H.G. and S.M.B-M.; writing—original draft preparation, R.S.G., F.A.P., R.S. and S.M.B-M.; writing—review and editing, R.S. and S.M.B-M.; supervision, R.S. and S.M.B-M.; project administration, R.S. and S.M.B-M.; funding acquisition, R.J.D-O., R.S. and S.M.B-M.. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Brazilian agencies Bahia Research Foundation—FAPESB (Grant nº PRONEM 498/2011-PNE 0002/2011 to S.M.B-M), National Council for Scientific and Technological Development —CNPq (PQ scholarship nº 307813/2018-5 to SMBM, and nº 303621/2015-0 to HG) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior —CAPES (PDSE scholarship nº 88881.189587/2018-01 to R.S.G; Finance Code 001 and PV scholarship nº 23066.033859/2018-73 to R.S.). This work was supported by grants from CESPU (TramTap-CESPU-2016, Chronic-TramTap_CESPU_2017 and TraTapMDMA-CESPU-2018), from the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) (NORTE-01-0145-FEDER-000013), funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and the Fundação para a Ciência e Tecnologia (FCT) (contract IF/00021/2014 to R.S.), Infect-Era (project INLEISH to R.S.) and Proyecto SNIP N° 292900 “Creación del Servicio de Laboratorio de Enfermedades Infecciosas y Parasitarias de Animales Domésticos de la Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas.Instituto de Investigación en Ganadería y Biotecnología-IGBI. Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| AIC | Akaike information criterion |
| ALT | Alanine aminotransferase |
| AST | Aspartate transaminase |
| Ca | Calcium |
| CanL | Canine leishmaniasis |
| CI | Confidence interval |
| CK | Creatine kinase |
| CK-MB | Creatine kinase MB |
| DOAJ | Directory of open access journals |
| GGT | Gamma-glutamyl transferase |
| GNR | Group of non-responder dogs |
| GR | Group of responder dogs |
| IFNG/IFN-γ | Interferon-gamma |
| IL1 | Interleukin-1 |
| IL2 | Interleukin-2 |
| IL4 | Interleukin-4 |
| IL5 | Interleukin-5 |
| IL10 | Interleukin-10 |
| IL17 | Interleukin-17 |
| IL22 | Interleukin-22 |
| LD | Linear dichroism |
| MDPI | Multidisciplinary Digital Publishing Institute |
| P | Phosphorus |
| q-PCR | Quantitative polymerase chain reaction |
| RR | Relative risk |
| Th1 | T helper 1 cell |
| Th2 | T helper 2 cell |
| TLA | Three letter acronym |
| TNFA/TNF-α | Tumor necrosis factor alpha |
| UPC | Urinary protein/creatinine ratio |
References
- Sevá, A.P.; Ovallos, F.G.; Amaku, M.; Carrillo, E.; Moreno, J.; Galati, E.A.B.; Lopes, E.G.; Soares, R.M.; Ferreira, F. Canine-Based Strategies for Prevention and Control of Visceral Leishmaniasis in Brazil. PLoS ONE 2016, 11, e0160058. [Google Scholar]
- Lippi, I.; Guidi, G.; Marchetti, V.; Tognetti, R.; Meucci, V. Prognostic role of the product of serum calcium and phosphorus concentrations in dogs with chronic kidney disease: 31 cases (2008–2010). J. Am. Vet. Med. Assoc. 2014, 245, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Gradoni, L.; Roura, X.; Zatelli, A.; Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45, 552–578. [Google Scholar] [CrossRef]
- WHO. Leishmaniasis. Available online: https://www.who.int/leishmaniasis/burden/en/ (accessed on 20 November 2019).
- Magalhães-Junior, J.T.; Feitosa, T.; Porfirio-Passos, G.; Farias, D.; Roberto, C.; Barrouin-Melo, S.M. Xenodiagnosis on dogs with visceral leishmaniasis : Canine and sand fly aspects related to the parasite transmission. Vet. Parasitol. 2016, 223, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Borja, L.S.; Sousa, O.M.F.; Solcà, M.D.S.; Bastos, L.A.; Bordoni, M.; Magalhães, J.T.; Larangeira, D.F.; Barrouin-Melo, S.M.; Fraga, D.B.M.; Veras, P.S.T. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet. Parasitol. 2016, 132, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Torrecilha, R.B.P.; Utsunomiya, Y.T.; Bosco, A.M.; Almeida, B.F.; Pereira, P.P.; Narciso, L.G.; Pereira, D.C.M.; Baptistiolli, L.; Calvo-bado, L.; Courtenay, O. Correlations between peripheral parasite load and common clinical and laboratory alterations in dogs with visceral leishmaniasis. Prev. Vet. Med. 2016, 132, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. The LeishVet Group LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Oliva, G.; Roura, X.; Crotti, A.; Maroli, M.; Castagnaro, M.; Gradoni, L.; Lubas, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Guidelines for treatment of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1192–1198. [Google Scholar] [CrossRef]
- Manna, L.; Corso, R.; Galiero, G.; Cerrone, A.; Muzj, P.; Gravino, A.E. Long-term follow-up of dogs with leishmaniosis treated with meglumine antimoniate plus allopurinol versus miltefosine plus allopurinol. Parasites Vectors 2015, 8, 289. [Google Scholar] [CrossRef]
- Marcondes, M.; Day, M.J. Current status and management of canine leishmaniasis in Latin America. Res. Vet. Sci. 2019, 123, 261–272. [Google Scholar] [CrossRef]
- Nogueira, S.; Avino, V.C.; Galvis-Ovallos, F.; Pereira-Chioccola, V.L.; Antonio, M.; Moreira, B.; Paula, A.; Lopes, P.; Molla, L.M.; Menz, I. Use of miltefosine to treat canine visceral leishmaniasis caused by Leishmania infantum in Brazil. Parasites Vectors 2019, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.; Amorim, I.F.G.; Ribeiro, R.R.; Azevedo, E.G.; Demicheli, C.; Melo, M.N.; Tafuri, W.L.; Gontijo, N.F.; Michalick, M.S.M.; Frézard, F. Efficacy of combined therapy with liposome-encapsulated meglumine antimoniate and allopurinol in treatment of canine visceral leishmaniasis. Antimicrob. Agents Chemother. 2012, 56, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Nery, G.; Becerra, D.R.D.; Borja, L.S.; Magalhães-Junior, J.T.; Souza, B.M.P.S.; Franke, C.R.; Veras, P.S.T.; Larangeira, D.F.; Barrouin-Melo, S.M. Evaluation of parasite infectivity for Lutzomyia longipalpis by xenodiagnosis in dogs treated for natural visceral leishmaniasis. Braz. J. Vet. Res. 2017, 37, 701–707. [Google Scholar]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.; Barrett, M.P.; Garcı, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef] [PubMed]
- Baxarias, M.; Álvarez-Fernández, A.; Martínez-Orellana, P.; Montserrat-Sangrà, S.; Ordeix, L.; Rojas, A.; Nachum-Biala, Y.; Baneth, G.; Solano-Gallego, L. Does co-infection with vector-borne pathogens play a role in clinical canine leishmaniosis? Parasites Vectors 2018, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Roura, X.; Fondati, A.; Lubas, G.; Gradoni, L.; Maroli, M.; Oliva, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Prognosis and monitoring of leishmaniasis in dogs: A working group report. Vet. J. 2013, 198, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Campino, L. Biomarkers Associated with Leishmania infantum Exposure, Infection, and Disease in Dogs. Front. Cell. Infect. Microbiol. 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Sellon, R.K.; Menard, M.M.; Meuten, D.J.; Lengerich, E.J.; Steurer, F.J.; Breitschwerdt, E.B. Endemic Visceral Leishmaniasis in a Dog from Texas. J. Vet. Intern. Med. 1993, 7, 16–19. [Google Scholar] [CrossRef]
- Ciaramella, P. Canine Leishmaniasis: Therapeutic Aspects *. Compend. Contin. Educ. Pract. Vet. 2003, 25, 370–375. [Google Scholar]
- Noli, C.; Auxilia, S.T. Treatment of canine Old World visceral leishmaniasis. Vet. Dermatol. 2005, 16, 213–232. [Google Scholar]
- Pennisi, M.G.; De Majo, M.; Masucci, M.; Britti, D.; Vitale, F.; Del Maso, R. Efficacy of the treatment of dogs with leishmaniosis with a combination of metronidazole and spiramycin. Vet. Rec. 2005, 156, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Barrouin-Melo, S.M.; Larangeira, D.F.; de Andrade Filho, F.A.; Trigo, J.; Julião, F.S.; Franke, C.R.; Palis Aguiar, P.H.; Conrado dos-Santos, W.L.; Pontes-de-Carvalho, L. Can spleen aspirations be safely used for the parasitological diagnosis of canine visceral leishmaniosis? A study on assymptomatic and polysymptomatic animals. Vet. J. 2006, 171, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Paparcone, R.; Fiorentino, E.; Cappiello, S.; Gizzarelli, M.; Gradoni, L.; Oliva, G.; Manzillo, V.F. Sternal aspiration of bone marrow in dogs: A practical approach for canine leishmaniasis diagnosis and monitoring. J. Vet. Med. 2013, 2013, 217314. [Google Scholar] [CrossRef] [PubMed]
- Adamama-Moraitou, K.K.; Saridomichelakis, M.N.; Polizopoulou, Z.; Kritsepi, M.; Tsompanakou, A.; Koutinas, A.F. Short-term exogenous glucocorticosteroidal effect on iron and copper status in canine leismaniasis (Leishmania infantum). Can. J. Vet. Res. 2005, 69, 287–292. [Google Scholar]
- Cortese, L.; Pelagalli, A.; Piantedosi, D.; Mastellone, V.; Di Loria, A.; Lombardi, P.; Ciaramella, P.; Avallone, L. The effects of prednisone on haemostasis in leishmaniotic dogs treated with meglumine antimoniate and allopurinol. Vet. J. 2008, 177, 405–410. [Google Scholar] [CrossRef]
- Harvey, J.W.; Stevens, A.; Lowe, J.S.; Scott, I. Veterinary Hematology; Elsevier: St. Louis, MO, USA, 2012; ISBN 9781437701739. [Google Scholar]
- Costa, I.; Carvalho, F.; Magalhães, T.; Guedes De Pinho, P.; Silvestre, R.; Dinis-Oliveira, R.J. Promising blood-derived biomarkers for estimation of the postmortem interval. Toxicol. Res. (Camb) 2015, 4, 1443–1452. [Google Scholar] [CrossRef]
- Silvestre, R.; Santarém, N.; Cunha, J.; Cardoso, L.; Nieto, J.; Carrillo, E.; Moreno, J.; Cordeiro-da-Silva, A. Serological evaluation of experimentally infected dogs by LicTXNPx-ELISA and amastigote-flow cytometry. Vet. Parasitol. 2008, 158, 23–30. [Google Scholar] [CrossRef]
- Rolão, N.; Cortes, S.; Rodrigues, O.R.; Campino, L. Quantification of Leishmania infantum parasites in tissue biopsies by Real-Time polymerase chain reaction and polymerase chain reaction—Enzyme-linked immunosorbent assay real-time polymerase chain reaction and polymerase chain reaction—Enzyme-linked i. J. Parasitol. 2004, 90, 1150–1154. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 20 November 2019).
- Kosmidis I Bias Reduction in Binary-Response Generalized Linear Models_. R Package Version 0.6.2. Available online: https://cran.r-project.org/package=brglm%3E (accessed on 20 November 2019).
- Tourinho, B.D.; Amâncio, F.F.; Ferraz, M.L.; Carneiro, M. Prognostic factors for death from visceral leishmaniasis in patients treated with liposomal amphotericin B in an endemic state in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 163–171. [Google Scholar] [CrossRef]
- Da Silva, T.A.M.; Morais, M.H.F.; Lopes, H.M.O.R.; Gonçalves, S.A.; Magalhães, F.D.C.; Amâncio, F.F.; Antunes, C.M.F.; Carneiro, M. Prognostic factors associated with death from visceral leishmaniasis: A case-control study in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2020, 7, 346–354. [Google Scholar] [CrossRef]
- Belo, V.S.; Struchiner, C.J.; Barbosa, D.S.; Nascimento, B.W.L.; Horta, M.A.P.; da Silva, E.S.; Werneck, G.L. Risk Factors for Adverse Prognosis and Death in American Visceral Leishmaniasis: A Meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e2982. [Google Scholar] [CrossRef] [PubMed]
- Coura-Vital, W.; de Araújo, V.E.M.; Reis, I.A.; Amancio, F.F.; Reis, A.B.; Carneiro, M. Prognostic Factors and Scoring System for Death from Visceral Leishmaniasis: An Historical Cohort Study in Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3374. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.L.; Rocha, R.L.; de Brito Ferreira Chaves, E.; de Vasconcelos Batista, V.G.; Costa, H.L.; Nery Costa, C.H. Predicting death from kala-azar: Construction, development, and validation of a score set and accompanying software. Rev. Soc. Bras. Med. Trop. 2016, 49, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Moreno, J. Cytokine profiles in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Alvar, J. Canine leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002, 18, 399–405. [Google Scholar] [CrossRef]
- Varma, N.; Naseem, S. Hematologic changes in visceral Leishmaniasis/Kala Azar. Indian J. Hematol. Blood Transfus. 2010, 26, 78–82. [Google Scholar] [CrossRef]
- De Pinho, F.A.; Vendrame, C.M.V.; MacIel, B.L.L.; Dos Santos Silva, L.; Miyashiro, S.I.; Jerônimo, S.M.B.; Goto, H. Association between insulin-like growth factor-i levels and the disease progression and anemia in visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2019, 4, 808–815. [Google Scholar] [CrossRef]
- Madu, A.J.; Ughasoro, M.D. Anaemia of Chronic Disease: An In-Depth Review. Med. Princ. Pract. 2017, 26, 1–9. [Google Scholar] [CrossRef]
- Zaidi, A.; Singh, K.P.; Ali, V. Leishmania and its quest for iron: An update and overview. Mol. Biochem. Parasitol. 2017, 211, 15–25. [Google Scholar] [CrossRef]
- Laranjeira-Silva, M.F.; Hamza, I.; Pérez-Victoria, J.M. Iron and Heme Metabolism at the Leishmania–Host Interface. Trends Parasitol. 2020, 36, 279–289. [Google Scholar] [CrossRef]
- Poggiali, E.; Migone De Amicis, M.; Motta, I. Anemia of chronic disease: A unique defect of iron recycling for many different chronic diseases. Eur. J. Intern. Med. 2014, 25, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Goheen, M.M.; Fulford, A.; Prentice, A.M.; Elnagheeb, M.A.; Patel, J.; Fisher, N.; Taylor, S.M.; Kasthuri, R.S.; Cerami, C. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat. Commun. 2014, 5, 4446. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, F.M.; Barie, P.S. Iron and the risk of infection. Surg. Infect. (Larchmt) 2005, 6, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. J. Am. Med. Assoc. 2019, 13, 1294–1304. [Google Scholar] [CrossRef]
- Martorelli, C.R.; Kogika, M.M.; Chacar, F.C.; Caragelasco, D.S.; de Campos Fonseca Pinto, A.C.B.; Lorigados, C.A.B.; Andrade, L.C. Urinary fractional excretion of phosphorus in dogs with spontaneous chronic kidney disease. Vet. Sci. 2017, 4, 67. [Google Scholar] [CrossRef]
- Cortadellas, O.; Fernández-del Palacio, M.J.; Talavera, J.; Bayón, A. Serum phosphorus concentrations in dogs with leishmaniosis at different stages of chronic kidney disease. Vet. Rec. 2009, 164, 487–490. [Google Scholar] [CrossRef]
- Rougier, S.; Hasseine, L.; Delaunay, P.; Michel, G.; Marty, P. One-year clinical and parasitological follow-up of dogs treated with marbofloxacin for canine leishmaniosis. Vet. Parasitol. 2012, 186, 245–253. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Di Filippo, L.; Ordeix, L.; Planellas, M.; Roura, X.; Altet, L.; Martínez-orellana, P. Early reduction of Leishmania infantum—Specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasites Vectors 2016, 9, 235. [Google Scholar] [CrossRef]
- Zanette, M.F.; de Lima, V.M.F.; Laurenti, M.D.; Rossi, C.N.; Vides, J.P.; da Costa Vieira, R.F.; Biondo, A.W.; Marcondes, M. Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Rev. Soc. Bras. Med. Trop. 2014, 47, 105–107. [Google Scholar] [CrossRef]
- Kaneko, J.; Harvey, J.; Bruss, M. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123704917. [Google Scholar]
- Elin, R.J.; Utter, A.; Tan, H.K.; Corash, L. Effect of magnesium deficiency on erythrocyte aging in rats. Am. J. Pathol. 1980, 100, 765–778. [Google Scholar]
- Nicolato, R.D.C.; De Abreu, R.T.; Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Reis, L.E.S.; Carvalho, M.D.G.; Carneiro, C.M.; Giunchetti, R.C.; Bouillet, L.E.M.; Lemos, D.S.; et al. Clinical forms of canine visceral leishmaniasis in naturally Leishmania infantum-infected dogs and related myelogram and hemogram changes. PLoS ONE 2013, 8, e82947. [Google Scholar] [CrossRef] [PubMed]
- Tonin, A.A.; Calado, A.M.C.; Bottari, N.B.; Dalenogare, D.; Thomé, G.R.; Duarte, T.; Duarte, M.M.M.F.; Morsch, V.M.; Schetinger, M.R.C.; Alves, L.C.; et al. Novel markers of inflammatory response and hepatic dysfunction in canine leishmaniasis. Comp. Immunol. Microbiol. Infect. Dis. 2016, 44, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Lazo, A.; Ordeix, L.; Planellas, M.; Pastor, J.; Solano-Gallego, L. Clinicopathological findings in sick dogs naturally infected with Leishmania infantum: Comparison of five different clinical classification systems. Res. Vet. Sci. 2018, 117, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Pintore, L.; Balducci, F.; Giordano, A.; Costabile, A.; Bernardini, M. Serum creatine kinase isoenzymes and macroenzymes in dogs with different neurologic diseases. Vet. Clin. Pathol. 2017, 46, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology 2016, 144, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Koutinas, A.; Miró, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef]
- Whitley, N.T.; Day, M.J. Immunomodulatory drugs and their application to the management of canine immune-mediated disease. J. Small Anim. Pract. 2011, 52, 70–85. [Google Scholar] [CrossRef]
- Miller, E. Immunosuppressive Therapy in the Treatment of Immune-mediated Disease. J. Vet. Intern. Med. 1992, 6, 206–213. [Google Scholar] [CrossRef]
- Moore, G.E.; Mahaffey, E.A.; Hoenig, M. Hematologic and serum biochemical effects of long-term administration of anti-inflammatory doses of prednisone in dogs. Am. J. Vet. Res. 1992, 53, 1033–1037. [Google Scholar]
- Muñoz, J.; Soblechero, P.; Duque, F.J.; Macías-García, B.; Ruiz, P.; Zaragoza, C.; Barrera, R. Effects of Oral Prednisone Administration on Serum Cystatin C in Dogs. J. Vet. Intern. Med. 2017, 31, 1765–1770. [Google Scholar] [CrossRef]
- Masters, A.K.; Berger, D.J.; Ware, W.A.; Langenfeld, N.R.; Coetzee, J.P.M.; Ward, J.L. Effects of short-term anti-inflammatory glucocorticoid treatment on clinicopathologic, echocardiographic, and hemodynamic variables in systemically healthy dogs. Am. J. Vet. Res. 2017, 79, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tinklenberg, R.L.; Murphy, S.D.; Mochel, J.P.; Seo, Y.-J.; Mahaffey, A.L.; Yan, Y.; Ward, J.L. Evaluation of dose-response effects of short-term oral prednisone administration on clinicopathologic and hemodynamic variables in healthy dogs. Am. J. Vet. Res. 2020, 81, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, J.C.; Mooney, A.P.; Price, J.M.; Thomason, J. Clinical, clinicopathologic, and gastrointestinal changes from aspirin, prednisone, or combination treatment in healthy research dogs: A double-blind randomized trial. J. Vet. Intern. Med. 2019, 33, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).