Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases
Abstract
1. Introduction
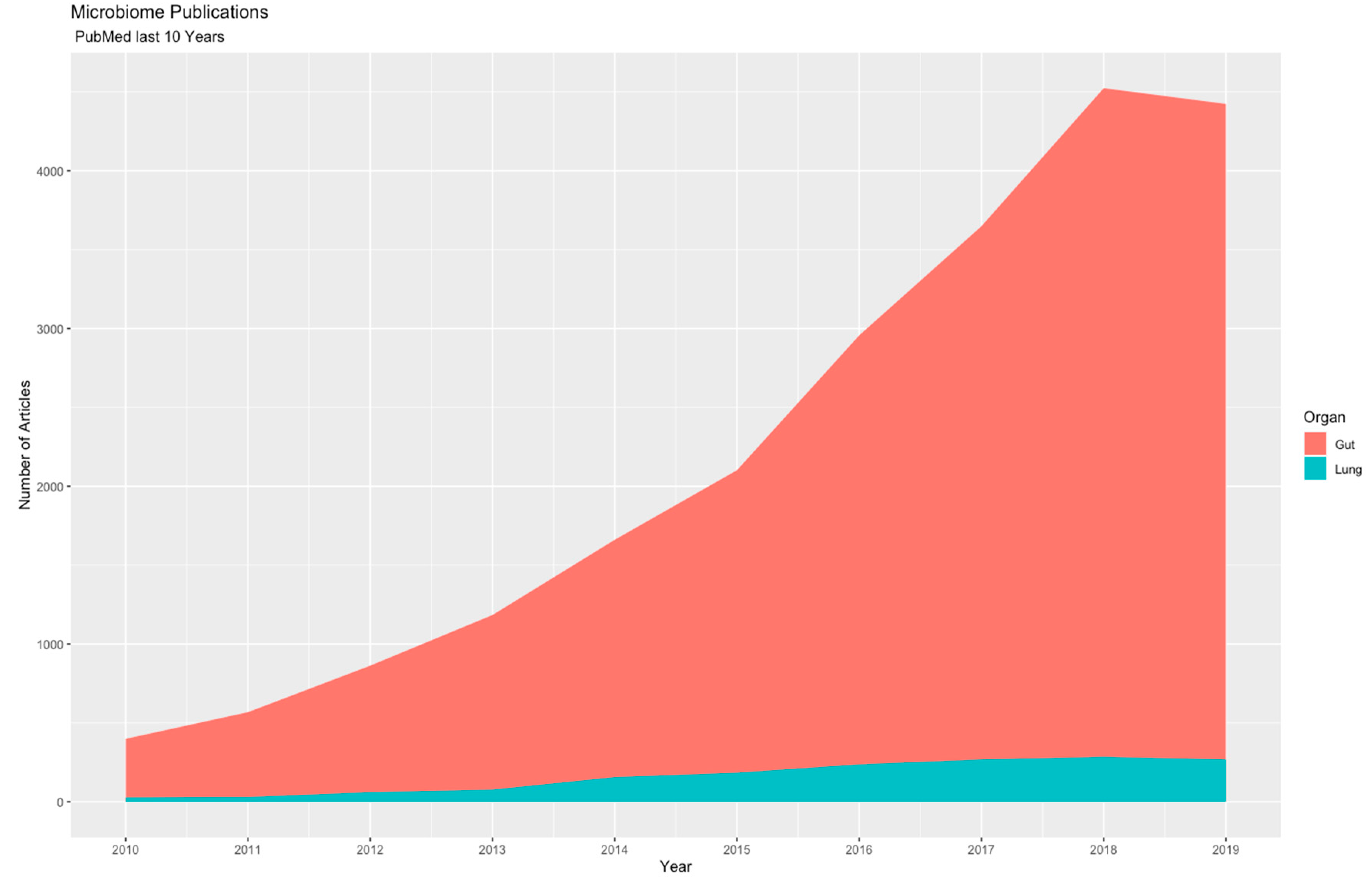
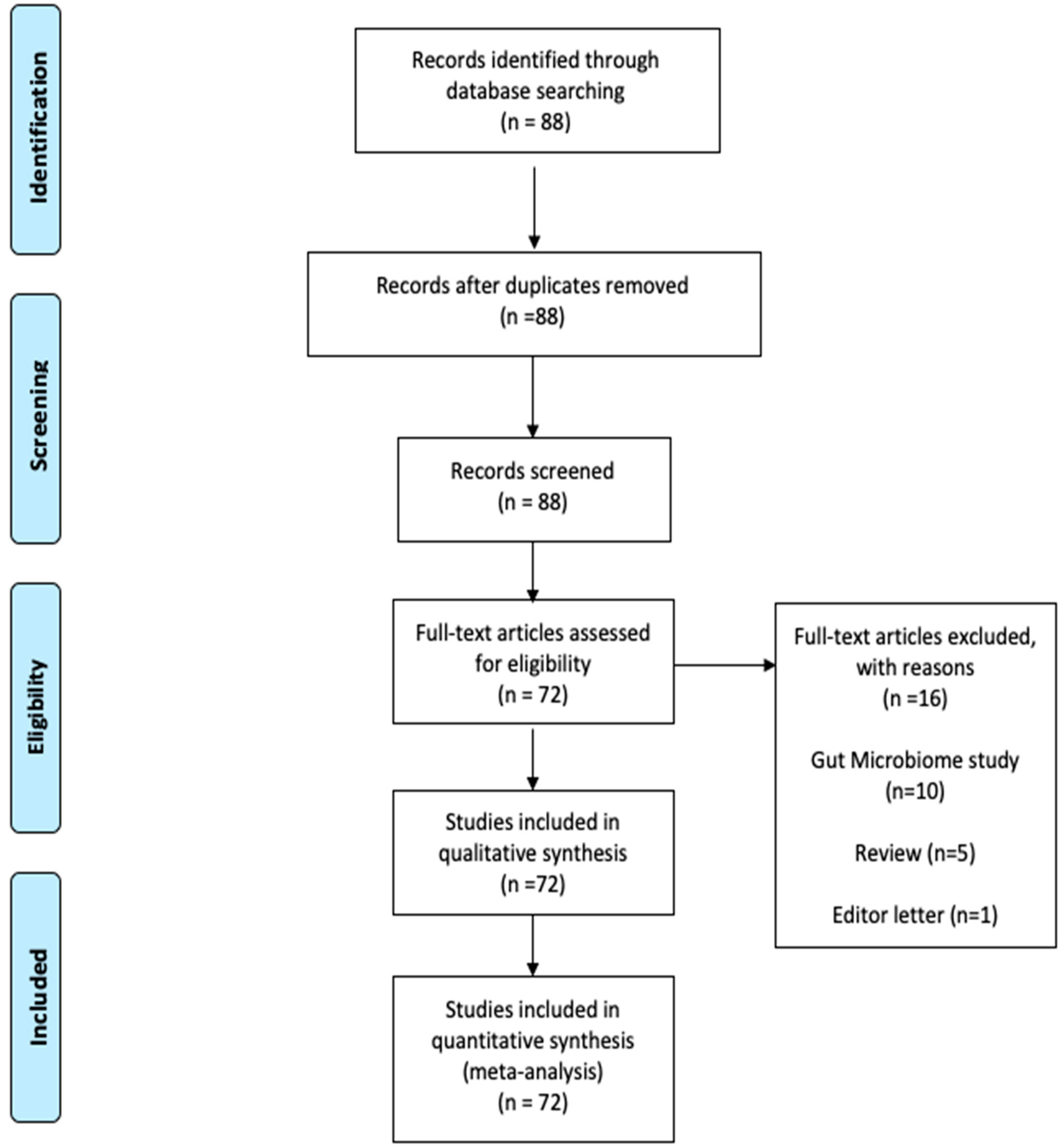
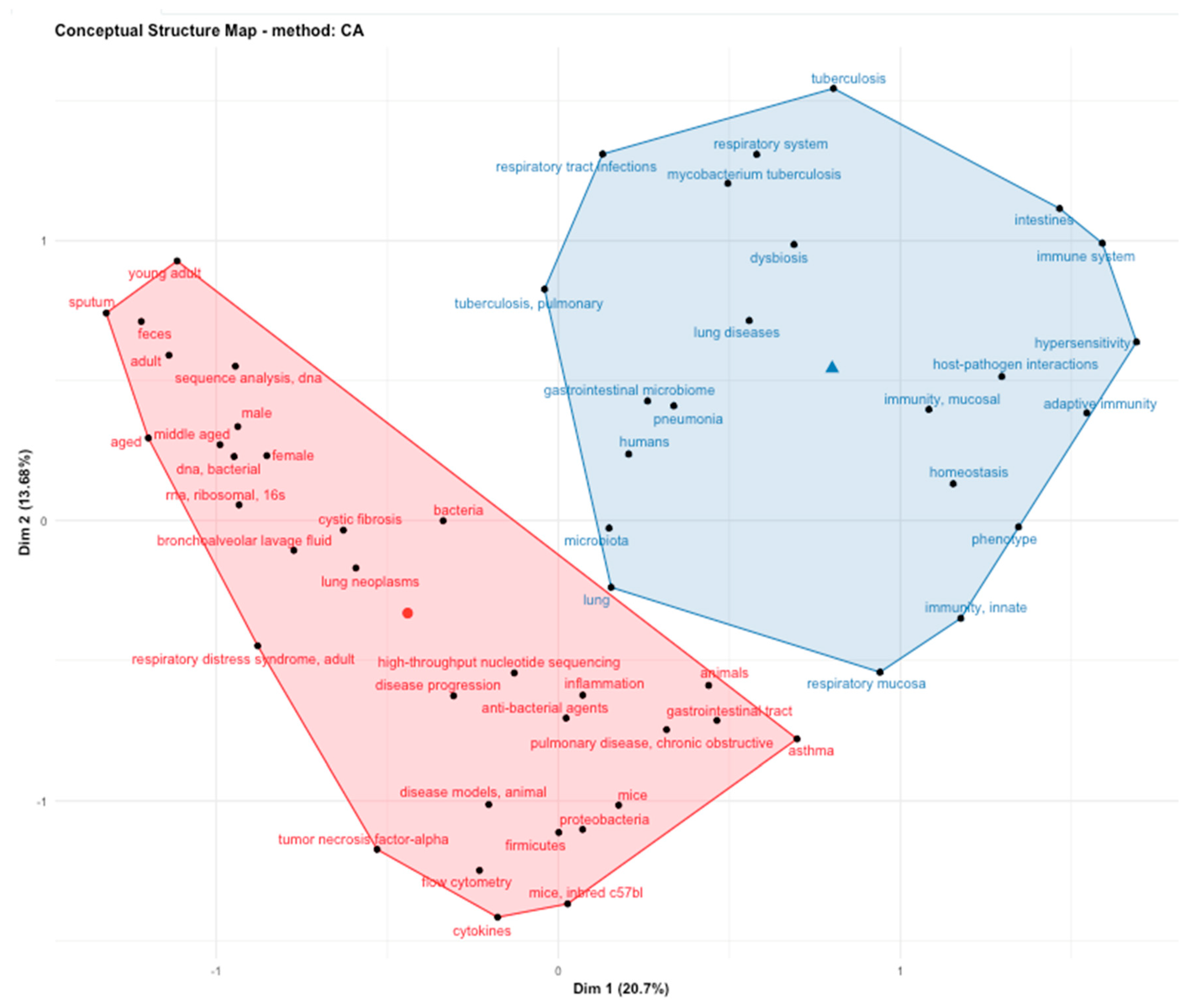
2. Materials and Methods
3. Results
3.1. Lung Microbiome
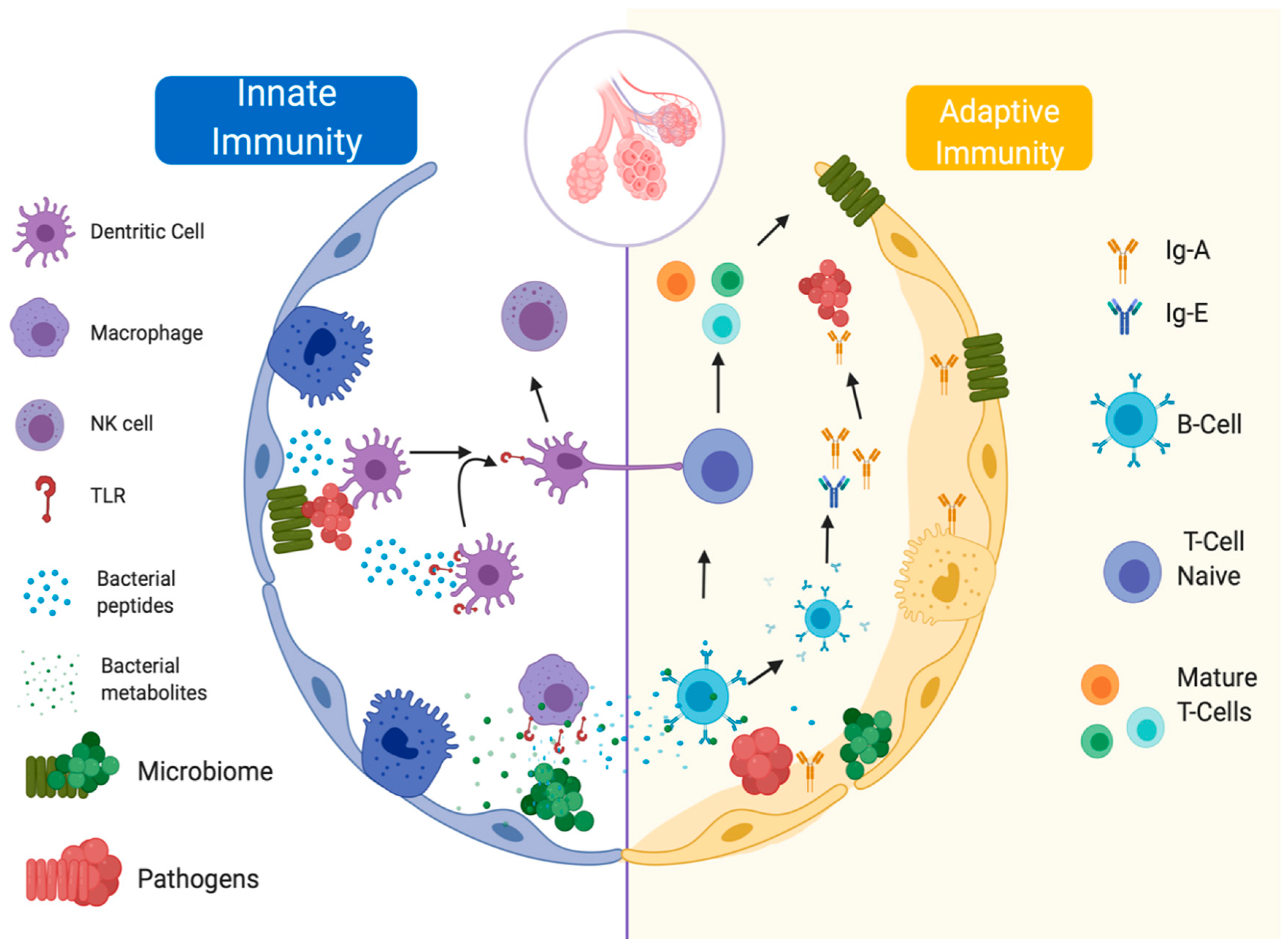
3.2. Lung Microbiome and Immune Response
3.3. Lung Microbiome in the Most Studied Respiratory Pathologies
3.3.1. Cystic Fibrosis (CF)
3.3.2. Asthma
3.3.3. Chronic Obstructive Pulmonary Disease (COPD)
3.3.4. Pulmonary Microbiome in Interstitial Lung Diseases
3.3.5. Pulmonary Microbiome in Lung Infections
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Duan, K.; Dammel, C.; Stein, J.; Rabin, H.; Surette, M.G. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 2003, 50, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Burke, C.; Pedro, H.; Cardenas, P.; Bush, A.; Bossley, C.; Davies, J.; Ervine, A.; Poulter, L.; Pachter, L.; et al. Disordered microbial communities in asthmatic airways. PLoS ONE 2010, 5, e8578. [Google Scholar] [CrossRef]
- Huang, Y.J.; Kim, E.; Cox, M.J.; Brodie, E.L.; Brown, R.; Wiener-Kronish, J.P.; Lynch, S.V. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS A J. Integr. Biol. 2010, 14, 9–59. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Dickson, R.P.; Moore, B.B. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J. Immunol. 2016, 196, 4839–4847. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef]
- Jankauskaitė, L.; Misevičienė, V.; Vaidelienė, L.; Kėvalas, R. Lower Airway Virology in Health and Disease-From Invaders to Symbionts. Medicina (Kaunas) 2018, 54, 72. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Lynch, J.P.; Werder, R.B.; Loh, Z.; Sikder, M.A.A.; Curren, B.; Zhang, V.; Rogers, M.J.; Lane, K.; Simpson, J.; Mazzone, S.B.; et al. Plasmacytoid dendritic cells protect from viral bronchiolitis and asthma through semaphorin 4a-mediated T reg expansion. J. Exp. Med. 2018, 215, 537–557. [Google Scholar] [CrossRef]
- Minota, S. Gut Microbiota and Internal Diseases: Update Information. Topics: V. Gut Microbiota: Topics in Various Medical Fields; 2. Possible causal relationship of microbiota to rheumatoid arthritis and bronchial asthma. Nihon Naika Gakkai Zasshi 2015, 104, 71–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruane, D.; Chorny, A.; Lee, H.; Faith, J.; Pandey, G.; Shan, M.; Simchoni, N.; Rahman, A.; Garg, A.; Weinstein, E.G.; et al. Microbiota regulate the ability of lung dendritic cells to induce IgA class-switch recombination and generate protective gastrointestinal immune responses. J. Exp. Med. 2016, 213, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.G.; Segal, L.N. Lung Microbiota and Its Impact on the Mucosal Immune Phenotype. Microbiology. Spectr. 2017, 5, 161–186. [Google Scholar]
- Kovalchik, S. RISmed: Download Content from NCBI Databases. R Packag. Version 2.1.7. 2017. Available online: https://cran.r-project.org/package=RISmed (accessed on 6 June 2017).
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.A.D.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Mccloskey, L.; Falkowski, N.R.; Huffnagle, G.B. Bacterial Topography of the Healthy Human Lower Respiratory Tract. mBio 2017, 8, e0228-16. [Google Scholar]
- Simpson, J.L.; Daly, J.; Baines, K.J.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Hugenholtz, P.; Willner, D.; et al. Airway dysbiosis: Haemophilus influenza and Tropheryma in poorly controlled asthma. Eur. Respir. J. 2016, 47, 792–800. [Google Scholar] [CrossRef]
- Scher, J.U.; Joshua, V.; Artacho, A.; Abdollahi-Roodsaz, S.; Öckinger, J.; Kullberg, S.; Sköld, M.; Eklund, A.; Grunewald, J.; Clemente, J.C.; et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome 2016, 4, 60. [Google Scholar] [CrossRef]
- Chotirmall, S.H.; Burke, C.M. Aging and the microbiome: Implications for asthma in the elderly? Expert Rev. Respir. Med. 2015, 9, 125–128. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Cao, L.; Li, K.J.; Huang, Y.; Luan, X.Q.; Li, G. Effects of smoking on the lower respiratory tract microbiome in mice. Respir. Res. 2018, 19, 253. [Google Scholar] [CrossRef]
- Erb-Downward, J.R.; Thompson, D.L.; Han, M.K.; Freeman, C.M.; McCloskey, L.; Schmidt, L.A.; Young, V.B.; Toews, G.B.; Curtis, J.L.; Sundaram, B.; et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 2011, 6, e16384. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.P.; Erb-Downward, J.R.; Falkowski, N.R.; Hunter, E.M.; Ashley, S.L.; Huffnagle, G.B. The Lung Microbiota of Healthy Mice Are Highly Variable, Cluster by Environment, and Reflect Variation in Baseline Lung Innate Immunity. Am. J. Respir. Crit. Care Med. 2018, 198, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, R.; Lloyd, C.M.; Molyneaux, P.L. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology 2020, 160, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Tirouvanziam, R.; Laval, J.; Greene, C.M.; Habiel, D.; Sharma, L.; Yildirim, A.Ö.; Dela Cruz, C.S.; Hogaboam, C.M. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. J. Innate Immun. 2018, 10, 487–501. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Marsland, B.J. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunology 2017, 46, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.E.; Xu, Y.; Tuvim, M.J.; Dickey, B.F. Inducible Innate Resistance of Lung Epithelium to Infection. Annu. Rev. Physiol. 2010, 72, 413–435. [Google Scholar] [CrossRef]
- Ferreira, J.A.G.; Penner, J.C.; Moss, R.B.; Haagensen, J.A.J.; Clemons, K.V.; Spormann, A.M.; Nazik, H.; Cohen, K.; Banaei, N.; Carolino, E.; et al. Inhibition of Aspergillus fumigatus and its biofilm by Pseudomonas aeruginosa is dependent on the source, phenotype and growth conditions of the bacterium. PLoS ONE 2015, 10, 1–27. [Google Scholar] [CrossRef]
- Kowalski, M.P.; Dubouix-Bourandy, A.; Bajmoczi, M.; Golan, D.E.; Zaidi, T.; Coutinho-sledge, Y.S.; Gygi, M.P.; Gygi, S.P.; Erik, A.C.; Pier, G.B. Host Resistance to Lung Infection Mediated by Major Vault Protein in Epithelial Cells. Science 2013, 317, 130–132. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Clarke, T.B. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via Nod-like receptor ligands. Infect. Immun. 2014, 82, 4596–4606. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.L.; Sequeira, R.P.; Clarke, T.B. The microbiota protects against respiratory infection via GM-CSF signaling. Nat. Commun. 2017, 8, 1512. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, Y.; Wang, C. Does IL-17 Respond to the Disordered Lung Microbiome and Contribute to the Neutrophilic Phenotype in Asthma? Mediators Inflamm. 2016, 2016, 6470364. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, S.F.; Espindola, M.S.; Vij, R.; Oldham, J.M.; Huffnagle, G.B.; Erb-Downward, J.R.; Flaherty, K.R.; Moore, B.B.; White, E.S.; et al. Microbes are associated with host innate immune response in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 208–219. [Google Scholar] [CrossRef] [PubMed]
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef]
- Han, D.; Walsh, M.C.; Kim, K.S.; Hong, S.W.; Lee, J.; Yi, J.; Rivas, G.; Choi, Y.; Surh, C.D. Dendritic cell expression of the signaling molecule TRAF6 is required for immune tolerance in the lung. Int. Immunol. 2017, 29, 71–78. [Google Scholar] [CrossRef]
- Whiteson, K.; Agrawal, S.; Agrawal, A. Differential responses of human dendritic cells to metabolites from the oral/airway microbiome. Clin. Exp. Immunol. 2017, 188, 371–379. [Google Scholar] [CrossRef]
- Brewington, J.; Goss, C.H.; Benscoter, D.; Clancy, J.P.; Pradeep, K. Dominate Early Infections in Children with Cystic Fibrosis. Cell. Rep. 2019, 27, 1190–1204. [Google Scholar]
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Harris, J.K.; Groote, M.A.; De Sagel, S.D.; Zemanick, E.T.; Kapsner, R.; Penvari, C.; Kaess, H.; Deterding, R.R.; Accurso, F.J.; Pace, N.R. Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc. Natl. Acad. Sci. USA 2007, 104, 20529–20533. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.E.; Wylie, K.M.; Akers, K.; Storch, G.A.; Hatch, J.; Quante, J.; Frayman, K.B.; Clarke, N.; Davis, M.; Stick, S.M.; et al. Association of antibiotics, airway microbiome, and inflammation in infants with cystic fibrosis. Ann. Am. Thorac. Soc. 2017, 14, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.A.; Adem, S.; Mills, R.H.; Comstock, W.; Goldasich, L.D.; Humphrey, G.; Aksenov, A.A.; Melnik, A.V.; Silva, R.; Ackermann, G.; et al. Neutrophilic proteolysis in the cystic fibrosis lung correlates with a pathogenic microbiome. Microbiome 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Fodor, A.A.; Klem, E.R.; Gilpin, D.F.; Elborn, J.S.; Boucher, R.C.; Tunney, M.M.; Wolfgang, M.C. The Adult Cystic Fibrosis Airway Microbiota Is Stable over Time and Infection Type, and Highly Resilient to Antibiotic Treatment of Exacerbations. PLoS ONE 2012, 7, e45001. [Google Scholar] [CrossRef] [PubMed]
- Vandeplassche, E.; Tavernier, S.; Coenye, T.; Crabbé, A. Influence of the lung microbiome on antibiotic susceptibility of cystic fibrosis pathogens. Eur. Respir Rev. 2019, 28, 190041. [Google Scholar] [CrossRef] [PubMed]
- Feigelman, R.; Kahlert, C.R.; Baty, F.; Rassouli, F.; Kleiner, R.L.; Kohler, P.; Brutsche, M.H.; Mering, C.; Mering, C.V. Sputum DNA sequencing in cystic fibrosis: Non-invasive access to the lung microbiome and to pathogen details. Microbiome 2017, 5, 20. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chan, Y.L.; Tsai, Y.S.; Chen, S.A.; Wang, C.J.; Chen, K.F.; Chung, I.F. Airway Microbial Diversity is Inversely Associated with Mite-Sensitized Rhinitis and Asthma in Early Childhood. Sci. Rep. 2017, 7, 1820. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat. Immunol. 2017, 18, 1076–1083. [Google Scholar] [CrossRef]
- Sverrild, A.; Kiilerich, P.; Brejnrod, A.; Pedersen, R.; Porsbjerg, C.; Bergqvist, A.; Erjefält, J.S.; Kristiansen, K.; Backer, V. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J. Allergy Clin. Immunol. 2017, 140, 407–417. [Google Scholar] [CrossRef]
- Smits, H.H.; van der Vlugt, L.E.P.M.; von Mutius, E.; Hiemstra, P.S. Childhood allergies and asthma: New insights on environmental exposures and local immunity at the lung barrier. Curr. Opin. Immunol. 2016, 42, 41–47. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Ma, Q.; Zhang, Q.; Wang, C. Neutrophilic Asthma Is Associated with Increased Airway Bacterial Burden and Disordered Community Composition. Biomed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiu, R.; Yang, Z.; Li, J.; Chung, K.F.; Zhong, N.; Zhang, Q. Sputum microbiota in severe asthma patients: Relationship to eosinophilic inflammation. Respir. Med. 2017, 131, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Durack, J.; Huang, Y.J.; Nariya, S.; Christian, L.S.; Mark Ansel, K.; Beigelman, A.; Castro, M.; Dyer, A.M.; Israel, E.; Kraft, M.; et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome 2018, 6, 104. [Google Scholar] [CrossRef]
- Richmond, B.W.; Brucker, R.M.; Han, W.; Du, R.-H.; Zhang, Y.; Cheng, D.-S.; Gleaves, L.; Abdolrasulnia, R.; Polosukhina, D.; Clark, P.E.; et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat. Commun. 2016, 7, 11240. [Google Scholar] [CrossRef]
- Segal, L.N.; Clemente, J.C.; Tsay, J.C.J.; Koralov, S.B.; Keller, B.C.; Wu, B.G.; Li, Y.; Shen, N.; Ghedin, E.; Morris, A.; et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat. Microbiol. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Sze, M.A.; Dimitriu, P.A.; Suzuki, M.; McDonough, J.E.; Campbell, J.D.; Brothers, J.F.; Erb-Downward, J.R.; Huffnagle, G.B.; Hayashi, S.; Elliott, W.M.; et al. Host response to the lung microbiome in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 192, 438–445. [Google Scholar] [CrossRef]
- Millares, L.; Pascual, S.; Montón, C.; García-Núñez, M.; Lalmolda, C.; Faner, R.; Casadevall, C.; Setó, L.; Capilla, S.; Moreno, A.; et al. Relationship between the respiratory microbiome and the severity of airflow limitation, history of exacerbations and circulating eosinophils in COPD patients. BMC. Pulm. Med. 2019, 19, 112. [Google Scholar] [CrossRef]
- Price, L.C.; Lowe, D.; Hosker, H.S.R.; Anstey, K.; Pearson, M.G.; Roberts, C.M. UK National COPD Audit 2003: Impact of hospital resources and organisation of care on patient outcome following admission for acute COPD exacerbation. Thorax 2006, 61, 837–842. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Tangedal, S.; Nielsen, R.; Aanerud, M.; Persson, L.J.; Wiker, H.G.; Bakke, P.S.; Hiemstra, P.S.; Eagan, T.M. Sputum microbiota and inflammation at stable state and during exacerbations in a cohort of chronic obstructive pulmonary disease (COPD) patients. PLoS ONE 2019, 14, e0222449. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.N.; Clemente, J.C.; Wu, B.G.; Wikoff, W.R.; Gao, Z.; Li, Y.; Ko, J.P.; Rom, W.N.; Blaser, M.J.; Weiden, M.D. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax 2017, 72, 13–22. [Google Scholar] [CrossRef]
- Migliaccio, C.T.; Mauderly, J.L. Biomass smoke exposures: Toxicology and animal study design. Inhal. Toxicol. 2010, 22, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Silvio, D.; Garnier, V.; Garnier, V.; Micro-, M.; Garzoni, C.; Brugger, S.D.; Qi, W.; Wasmer, S.; Cusini, A.; Dumont, P.; et al. Microbial communities in the respiratory tract of patients with interstitial lung disease. Thorax 2013, 68, 1150–1156. [Google Scholar]
- Yang, D.; Chen, X.; Wang, J.; Lou, Q.; Lou, Y.; Li, L.; Wang, H.; Chen, J.; Wu, M.; Song, X.; et al. Dysregulated Lung Commensal Bacteria Drive Interleukin-17B Production to Promote Pulmonary Fibrosis through Their Outer Membrane Vesicles. Immunity 2019, 50, 692–706. [Google Scholar] [CrossRef]
- Han, M.L.K.; Zhou, Y.; Murray, S.; Tayob, N.; Noth, I.; Lama, V.N.; Moore, B.B.; White, E.S.; Flaherty, K.R.; Huffnagle, G.B.; et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: An analysis of the COMET study. Lancet Respir. Med. 2014, 2, 548–556. [Google Scholar] [CrossRef]
- Molyneaux, P.L.; Cox, M.J.; Wells, A.U.; Kim, H.C.; Ji, W.; Cookson, W.O.C.; Moffatt, M.F.; Kim, D.S.; Maher, T.M. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir. Res. 2017, 18, 29. [Google Scholar] [CrossRef]
- Salisbury, M.L.; Han, M.K.; Dickson, R.P.; Molyneaux, P.L. Microbiome in interstitial lung disease: From pathogenesis to treatment target. Curr. Opin. Pulm. Med. 2017, 23, 404–410. [Google Scholar] [CrossRef]
- O’Dwyer, D.N.; Ashley, S.L.; Gurczynski, S.J.; Xia, M.; Wilke, C.; Falkowski, N.R.; Norman, K.C.; Arnold, K.B.; Huffnagle, G.B.; Salisbury, M.L.; et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am. J. Respir. Crit. Care. Med. 2019, 199, 1127–1138. [Google Scholar] [CrossRef]
- Takahashi, Y.; Saito, A.; Chiba, H.; Kuronuma, K.; Ikeda, K.; Kobayashi, T.; Ariki, S.; Takahashi, M.; Sasaki, Y.; Takahashi, H. Impaired diversity of the lung microbiome predicts progression of idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 34. [Google Scholar] [CrossRef]
- Invernizzi, R.; Hewitt, R.; Ghai, P.; Swann, J.; Wu, B.; Segal, L.; Byrne, A.; Maher, T.; Lloyd, C.; Molyneaux, P. The respiratory microbiome and metabolome in chronic hypersensitivity pneumonitis. ERJ Open Res. 2020, 6, 35. [Google Scholar]
- Olson, A.L.; Swigris, J.J.; Sprunger, D.B.; Fischer, A.; Fernandez-Perez, E.R.; Solomon, J.; Murphy, J.; Cohen, M.; Raghu, G.; Brown, K.K. Rheumatoid Arthritis - Interstitial Lung Disease - associated Mortality. Am. J. Respir. Crit. Care. Med. 2011, 183, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.L.; Sreih, A.G.; Najem, C.E.; Grayson, P.C.; Zhao, C.; Bittinger, K.; Collman, R.G.; Merkel, P.A. Characterization of the nasal microbiota in granulomatosis with polyangiitis. Ann. Rheum. Dis. 2018, 77, 1448–1453. [Google Scholar] [CrossRef]
- Vissing, N.H.; Chawes, B.L.K.; Bisgaard, H. Increased risk of pneumonia and bronchiolitis after bacterial colonization of the airways as neonates. Am. J. Respir. Crit. Care Med. 2013, 188, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- De Steenhuijsen Piters, W.A.; Huijskens, E.G.W.; Wyllie, A.L.; Biesbroek, G.; Van Den Bergh, M.R.; Veenhoven, R.H.; Wang, X.; Trzcinski, K.; Bonten, M.J.; Rossen, J.W.A.; et al. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J. 2016, 10, 97–108. [Google Scholar] [CrossRef]
- Lamarche, D.; Johnstone, J.; Zytaruk, N.; Clarke, F.; Hand, L.; Loukov, D.; Szamosi, J.C.; Rossi, L.; Schenck, L.P.; Verschoor, C.P.; et al. Microbial dysbiosis and mortality during mechanical ventilation: A prospective observational study. Respir. Res. 2018, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Asghari, R.; Abri, R.; Shadvar, K.; Sanaie, S. Effect of a Probiotic Preparation on Ventilator-Associated Pneumonia in Critically Ill Patients Admitted to the Intensive Care Unit: A Prospective Double-Blind Randomized Controlled Trial. Nutr. Clin. Pract. 2019, 34, 156–162. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza a virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef]
- Youn, H.; Lee, D.; Lee, Y.; Park, J.; Yuk, S.; Yang, S.; Lee, H.; Woo, S.; Kim, H.; Lee, J.; et al. Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral. Res. 2012, 93, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef]
- Toivonen, L.; Hasegawa, K.; Waris, M.; Ajami, N.J.; Petrosino, J.F.; Camargo, C.A.; Peltola, V. Early nasal microbiota and acute respiratory infections during the first years of life. Thorax 2019, 74, 592–599. [Google Scholar] [CrossRef]
- Antunes, K.H.; Fachi, J.L.; de Paula, R.; da Silva, E.F.; Pral, L.P.; dos Santos, A.Á.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Santos, J.R.; Ribeiro, M.J.; Freitas, G.J.; Bastos, R.W.; Ferreira, G.F.; Miranda, A.S.; Arifa, R.D.; Santos, P.C.; Martins, F.; et al. The absence of microbiota delays the inflammatory response to Cryptococcus gattii. Int. J. Med. Microbiol. 2016, 306, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, M.K.; Iwai, S.; Lin, D.L.; Worodria, W.; Ayakaka, I.; Byanyima, P.; Kaswabuli, S.; Fong, S.; Stone, S.; Chang, E.; et al. Immune response and mortality risk relate to distinct lung microbiomes in patients with HIV and pneumonia. Am. J. Respir. Crit. Care. Med. 2017, 195, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Halwachs, B.; Thallinger, G.G.; Klymiuk, I.; Gorkiewicz, G.; Hoenigl, M.; Prattes, J.; Valentin, T.; Heidrich, K.; Buzina, W.; et al. Characterisation of Candida within the mycobiome/microbiome of the lower respiratory tract of ICU patients. PLoS ONE 2016, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Corral, D.; Colom, A.; Levillain, F.; Peixoto, A.; Hudrisier, D.; Poquet, Y.; Neyrolles, O. The Host Microbiota Contributes to Early Protection Against Lung Colonization by Mycobacterium tuberculosis. Front. Immunol. 2018, 9, 2656. [Google Scholar] [CrossRef]
- Cheung, M.K.; Lam, W.Y.; Fung, W.Y.W.; Law, P.T.W.; Au, C.H.; Nong, W.; Kam, K.M.; Kwan, H.S.; Tsui, S.K.W. Sputum Microbiota in Tuberculosis as Revealed by 16S rRNA Pyrosequencing. PLoS ONE 2013, 8, e54574. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; He, L.; Huang, F.; Chen, J.; Cui, P.; Shen, Y.; Zhao, J.; Wang, W.; Zhang, Y.; et al. Sputum microbiota associated with new, recurrent and treatment failure tuberculosis. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut Microbiome Interactions with Drug Metabolism, Efficacy and Toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Chavira, A.; Belda-Ferre, P.; Kosciolek, T.; Ali, F.; Dorrestein, P.C.; Knight, R. The Microbiome and Its Potential for Pharmacology. Handb. Exp. Pharmacol. 2019, 260, 301–326. [Google Scholar]
- Le Noci, V.; Guglielmetti, S.; Arioli, S.; Camisaschi, C.; Bianchi, F.; Sommariva, M.; Storti, C.; Triulzi, T.; Castelli, C.; Balsari, A.; et al. Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote Immunosurveillance against Lung Metastases. Cell. Rep. 2018, 24, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Marchisio, P.; Santagati, M.; Scillato, M.; Baggi, E. Streptococcus salivarius 24SMB administered by nasal spray for the prevention of acute otitis media in otitis-prone children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira-Lucio, J.A.; Falfán-Valencia, R.; Ramírez-Venegas, A.; Buendía-Roldán, I.; Rojas-Serrano, J.; Mejía, M.; Pérez-Rubio, G. Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases. Microorganisms 2020, 8, 1059. https://doi.org/10.3390/microorganisms8071059
Lira-Lucio JA, Falfán-Valencia R, Ramírez-Venegas A, Buendía-Roldán I, Rojas-Serrano J, Mejía M, Pérez-Rubio G. Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases. Microorganisms. 2020; 8(7):1059. https://doi.org/10.3390/microorganisms8071059
Chicago/Turabian StyleLira-Lucio, Juan Alberto, Ramcés Falfán-Valencia, Alejandra Ramírez-Venegas, Ivette Buendía-Roldán, Jorge Rojas-Serrano, Mayra Mejía, and Gloria Pérez-Rubio. 2020. "Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases" Microorganisms 8, no. 7: 1059. https://doi.org/10.3390/microorganisms8071059
APA StyleLira-Lucio, J. A., Falfán-Valencia, R., Ramírez-Venegas, A., Buendía-Roldán, I., Rojas-Serrano, J., Mejía, M., & Pérez-Rubio, G. (2020). Lung Microbiome Participation in Local Immune Response Regulation in Respiratory Diseases. Microorganisms, 8(7), 1059. https://doi.org/10.3390/microorganisms8071059








