Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Strategy of Bibliographic Search
2.2. Inclusion–Exclusion Criteria
2.3. Data Extraction and Assessment for Study Quality
2.4. Quality Assessment
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Literature Search and Characteristics of the Studies
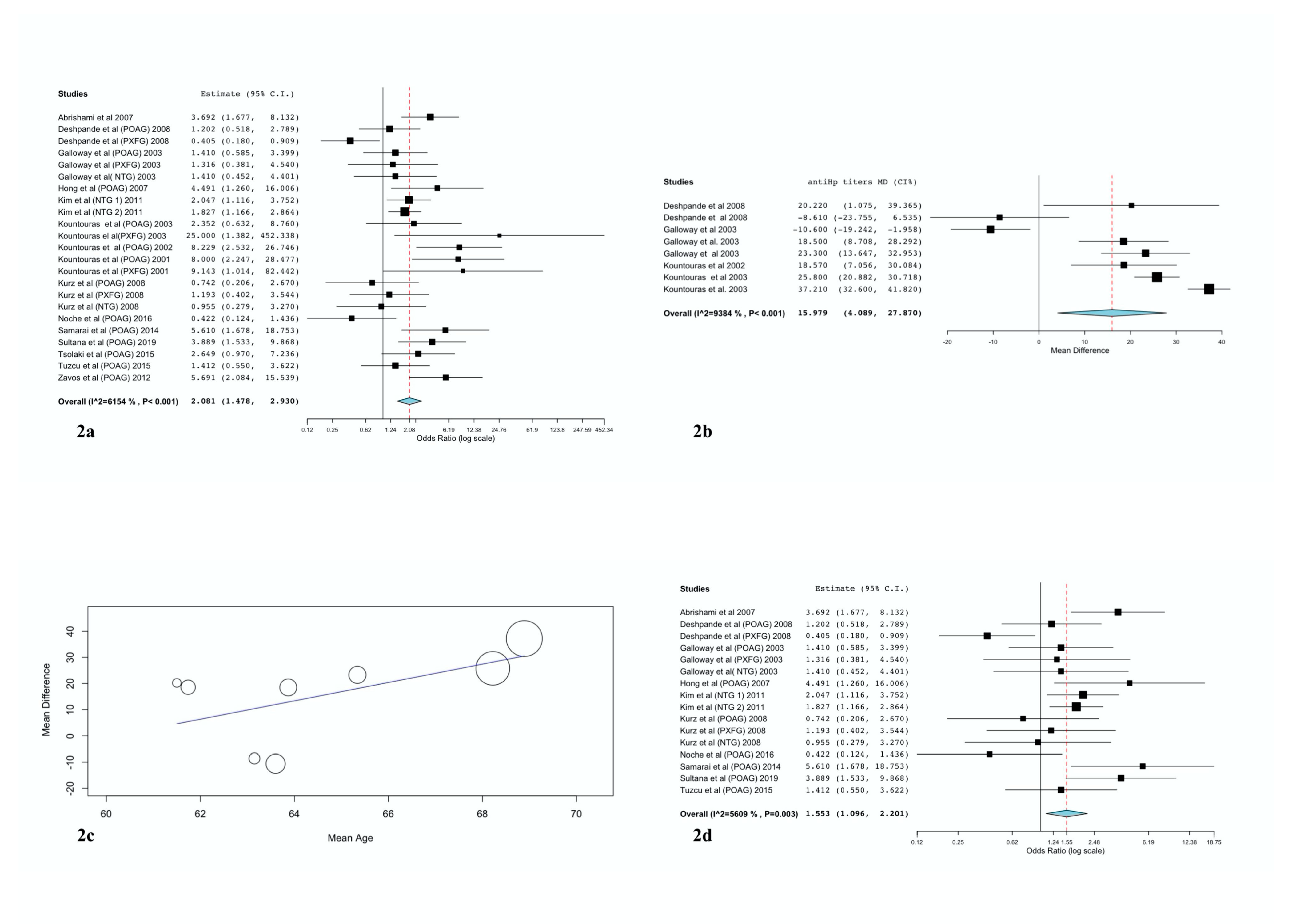
3.2. Overall Results and Subgroup Analyses
3.3. Sensitivity Analysis
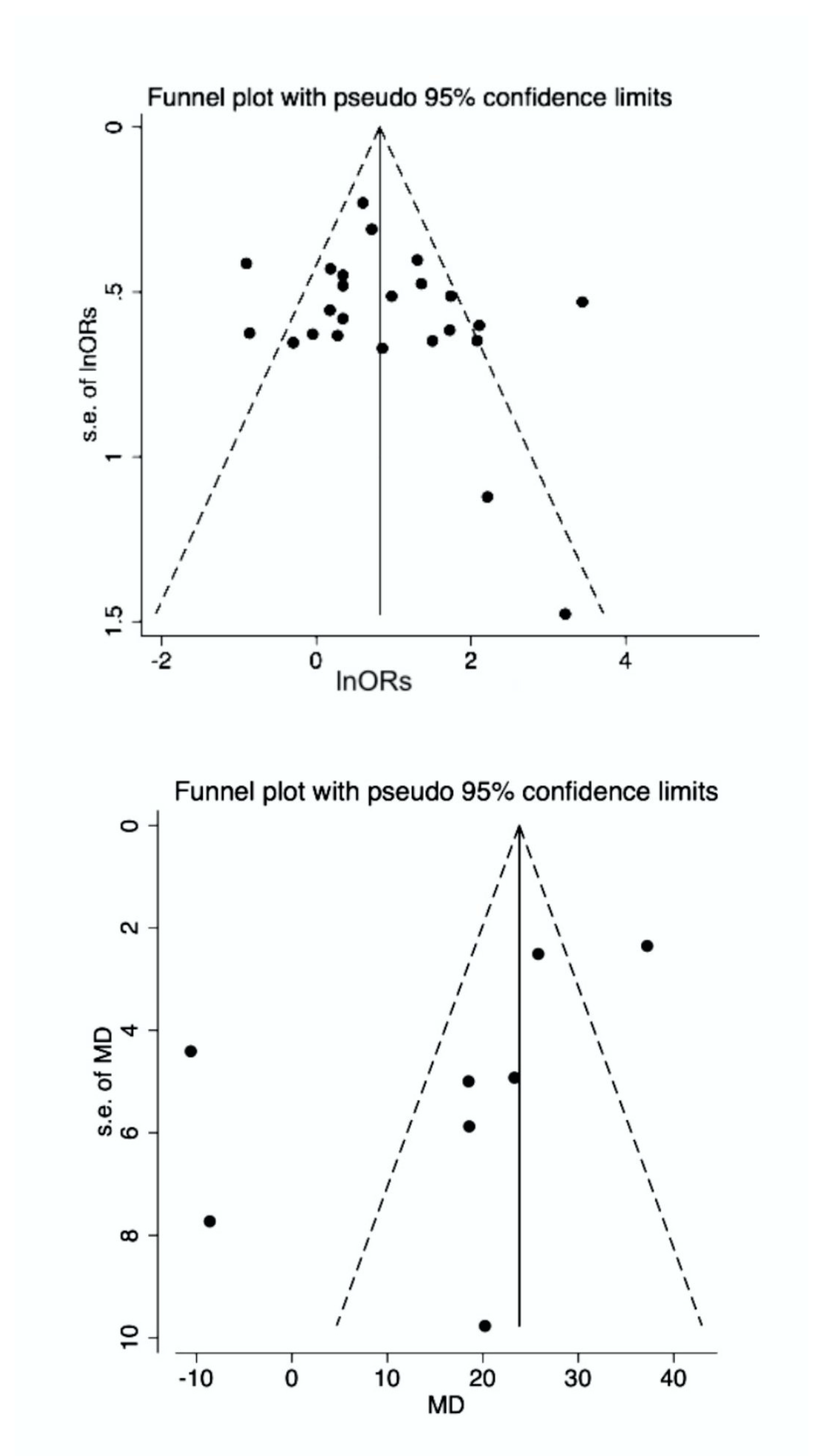
3.4. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| BOB | Blood-Ocular-Barrier |
| CI | Confidence Interval |
| Hsps | heat shock proteins |
| H. pylori | Helicobacter pylori |
| HLA | Human leukocyte antigens |
| IG | Immunoglobulin |
| NOD | Nucleotide-Binding Oligomerisation Domain |
| NTG | Normal Tension Glaucoma |
| POAG | Primary Open-Angle Glaucoma |
| PEG | Pseudo-Exfoliation Glaucoma |
| RUT | Rapid Urease Test |
| SD | Standard Deviation |
| WMD | Weighted Mean Differences |
References
- Chmiela, M.; Karwowska, Z.; Gonciarz, W.; Allushi, B.; Staczek, P. Host pathogen interactions in Helicobacter pylori related gastric cancer. World J. Gastroenterol. 2017, 23, 1521–1540. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Gerges, S.E.; Alosh, T.K.; Khalil, S.H.; El Din, M.M.W. Relevance of Helicobacter pylori infection in Egyptian multiple sclerosis patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2018, 54, 41. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Vardaka, E.; Tzivras, D.; Dardiotis, E.; Deretzi, G.; Giartza-Taxidou, E.; Grigoriadis, S.; et al. A perspective on risk factors for esophageal adenocarcinoma: Emphasis on Helicobacter pylori infection. Ann. N. Y. Acad. Sci. 2019, 1452, 12–17. [Google Scholar] [CrossRef]
- Mentis, A.-F.A.; Boziki, M.; Grigoriadis, N.; Papavassiliou, A.G. Helicobacter pylori infection and gastric cancer biology: Tempering a double-edged sword. Cell. Mol. Life Sci. 2019, 76, 2477–2486. [Google Scholar] [CrossRef]
- Crowe, S.E. Helicobacter pylori Infection. N. Engl. J. Med. 2019, 380, 1158–1165. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Cheng, A.-L. Helicobacter pylori and mucosa-associated lymphoid tissue: What’s new. Hematol. Am. Soc. Hematol. Educ. Progr. 2013, 2013, 109–117. [Google Scholar] [CrossRef]
- Romano, M.; Ricci, V.; Zarrilli, R. Mechanisms of Disease: Helicobacter pylori-related gastric carcinogenesis—Implications for chemoprevention. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 622–632. [Google Scholar] [CrossRef]
- Stewart, O.A.; Wu, F.; Chen, Y. The role of gastric microbiota in gastric cancer. Gut Microbes 2020, 1–11. [Google Scholar] [CrossRef]
- Ricci, V.; Giannouli, M.; Romano, M.; Zarrilli, R. Helicobacter pylori gamma-glutamyl transpeptidase and its pathogenic role. World J. Gastroenterol. 2014, 20, 630–638. [Google Scholar] [CrossRef]
- Roubaud Baudron, C.; Franceschi, F.; Salles, N.; Gasbarrini, A. Extragastric Diseases and Helicobacter pylori. Helicobacter 2013, 18, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Gasbarrini, A.; Polyzos, S.A.; Kountouras, J. Extragastric Diseases and Helicobacter pylori. Helicobacter 2015, 20, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Zagari, R.M.; De Musis, C.; Romano, L.; Loguercio, C.; Romano, M. Helicobacter pylori and extragastric diseases: A review. World J. Gastroenterol. 2018, 24, 3204–3221. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, F.; Tortora, A.; Gasbarrini, G.; Gasbarrini, A. Helicobacter pylori and Extragastric Diseases. Helicobacter 2014, 19, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet (Lond. Engl.) 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Chou, T.-H.; Musada, G.R.; Romano, G.L.; Bolton, E.; Porciatti, V. Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma. Int. J. Mol. Sci. 2018, 19, 237. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, J.; Zhou, X.; Li, X. Modified phacoemulsification plus goniosynechialysis compared with conventional surgery for cataract and glaucoma. Exp. Ther. Med. 2019, 1, 131–136. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, H.; Liu, X.; Ding, C.; Liuz, X.; Ding, C. The relationship between Helicobacter pylori infection and open-angle glaucoma: A meta-analysis. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5238–5245. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.H.; Park, K.H.; Han, S.Y.; Shim, H.S. Investigation of the association between Helicobacter pylori infection and normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2011, 52, 665–668. [Google Scholar] [CrossRef]
- Kountouras, J.; Zavos, C.; Deretzi, G. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 2679–2680. [Google Scholar]
- Zavos, C. Immuno-Gastroenterology: A New Multi-Disciplinary Journal. ImmunoGastroenterology 2013, 1, 1. [Google Scholar] [CrossRef]
- Deshpande, N.; Lalitha, P.; Krishna das, S.R.; Jethani, J.; Pillai, R.M.; Robin, A. Karthik Helicobacter pylori IgG Antibodies in Aqueous Humor and Serum of Subjects With Primary Open Angle and Pseudo-exfoliation Glaucoma in a South Indian Population. J. Glaucoma 2008, 17, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Regenbogen, M.; Goldiner, I.; Horowitz, N.; Moshkowitz, M. No Association Between Helicobacter pylori Infection or CagA-bearing Strains and Glaucoma. J. Glaucoma 2008, 17, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Galloway, P.H.; Warner, S.J.; Morshed, M.G.; Mikelberg, F.S. Helicobacter pylori infection and the risk for open-angle glaucoma. Ophthalmology 2003, 110, 922–925. [Google Scholar] [CrossRef]
- Moran, A.P.; Prendergast, M.M. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: Contribution of gastrointestinal infections to autoimmunity. J. Autoimmun. 2001, 16, 241–256. [Google Scholar] [CrossRef]
- Kountouras, J.; Deretzi, G.; Zavos, C.; Karatzoglou, P.; Touloumis, L.; Nicolaides, T.; Chatzopoulos, D.; Venizelos, I. Association between Helicobacter pylori infection and acute inflammatory demyelinating polyradiculoneuropathy. Eur. J. Neurol. 2005, 12, 139–143. [Google Scholar] [CrossRef]
- Kountouras, J.; Deretzi, G.; Grigoriadis, N.; Zavos, C.; Boziki, M.; Gavalas, E.; Katsinelos, P.; Tzilves, D.; Giouleme, O.; Lazaraki, G. Guillain-Barré syndrome. Lancet Neurol. 2008, 7, 1080–1081; author reply 1083–1085. [Google Scholar] [CrossRef]
- Kountouras, J.; Zavos, C.; Chatzopoulos, D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J. Cell. Mol. Med. 2005, 9, 196–207. [Google Scholar] [CrossRef]
- Frulloni, L.; Lunardi, C.; Simone, R.; Dolcino, M.; Scattolini, C.; Falconi, M.; Benini, L.; Vantini, I.; Corrocher, R.; Puccetti, A. Identification of a Novel Antibody Associated with Autoimmune Pancreatitis. N. Engl. J. Med. 2009, 361, 2135–2142. [Google Scholar] [CrossRef]
- Guarneri, F.; Guarneri, C.; Benvenga, S. Helicobacter pylori and autoimmune pancreatitis: Role of carbonic anhydrase via molecular mimicry? J. Cell. Mol. Med. 2005, 9, 741–744. [Google Scholar] [CrossRef]
- Romano, C.; Barrett, D.A.; Li, Z.; Pestronk, A.; Wax, M.B. Anti-rhodopsin antibodies in sera from patients with normal-pressure glaucoma. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1968–1975. [Google Scholar]
- Doulberis, M.; Kotronis, G.; Thomann, R.; Polyzos, S.A.; Boziki, M.; Gialamprinou, D.; Deretzi, G.; Katsinelos, P.; Kountouras, J. Impact of helicobacter pylori on alzheimer’s disease: What do we know so far? Helicobacter 2017. [Google Scholar] [CrossRef]
- Kountouras, J. Helicobacter pylori: An intruder involved in conspiring glaucomatous neuropathy. Br. J. Ophthalmol. 2009, 93, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Mylopoulos, N.; Konstas, A.G.P.A.G.P.; Zavos, C.; Chatzopoulos, D.; Boukla, A. Increased levels of Helicobacter pylori IgG antibodies in aqueous humor of patients with primary open-angle and exfoliation glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2003, 241, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Tsolaki, M.; Gavalas, E.; Boziki, M.; Zavos, C.; Karatzoglou, P.; Chatzopoulos, D.; Venizelos, I. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 2006, 66, 938–940. [Google Scholar] [CrossRef]
- Tu, H.; Sun, L.; Dong, X.; Gong, Y.; Xu, Q.; Jing, J.; Yuan, Y. Serum anti-Helicobacter pylori immunoglobulin G titer correlates with grade of histological gastritis, mucosal bacterial density, and levels of serum biomarkers. Scand. J. Gastroenterol. 2014, 49, 259–266. [Google Scholar] [CrossRef]
- Chung, H.A.; Lee, S.-Y.; Moon, H.W.; Kim, J.H.; Sung, I.-K.; Park, H.S.; Shim, C.S.; Han, H.S. Does the antibody production ability affect the serum anti-Helicobacter pylori IgG titer? World J. Gastrointest. Pathophysiol. 2016, 7, 288–295. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.R.D. MOOSE Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies. Jama 2000, 283, 2008–2012. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.-J.; Sheen, S.-S.; Hahn, S.; Jang, B.-H.; Son, H.-J. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, C.; Duan, L.; Wang, W. Relationship between Helicobacter pylori infection and open angle glaucoma in China. Asian J. Ophthalmol. 2017, 9, 205–208. [Google Scholar]
- Kountouras, J.; Mylopoulos, N.; Chatzopoulos, D.; Zavos, C.; Boura, P.; Konstas, A.G.P.; Venizelos, J. Eradication of Helicobacter pylori may be beneficial in the management of chronic open-angle glaucoma. Arch. Intern. Med. 2002, 162, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Ubani, U.A.; Timothy, C.O.; Ihesiulor, G.C. Assessment of Helicobacter Pylori in Glaucoma Disease in Abia State of Nigeria. Int. Educ. Appl. Sci. Res. J. 2017, 2, 7–12. [Google Scholar]
- Ozturk, F.; Kurt, E.; Ubeyt Inan, U.; Samet Ermis, S.; Cetinkaya, Z.; Altindis, M. Is there a relationship between glaucoma and Helicobacter pylori? Afr. J. Microbiol. Res. 2009, 3, 560–564. [Google Scholar]
- Samarai, V.; Sharifi, N.; Nateghi, S. Association between helicobacter pylori infection and primary open angle glaucoma. Glob. J. Health Sci. 2014, 6, 13–17. [Google Scholar] [CrossRef][Green Version]
- Sultana, S.; Khan, N.; Ghosh, C.K.; Saleh, A.A.; Islam, M.S. Association between Helicobacter pylori infection and primary open-angle glaucoma. Bangabandhu Sheikh Mujib Med. Univ. J. 2019, 12, 25–28. [Google Scholar] [CrossRef]
- Tsolaki, F.; Kountouras, J.; Topouzis, F.; Tsolaki, M. Helicobacter pylori infection, dementia and primary open-angle glaucoma: Are they connected? BMC Ophthalmol. 2015, 15, 24. [Google Scholar] [CrossRef]
- Tuzcu, E.A.; Aydogan, F.; Motor, V.K.; Ilhan, O.; Daglioglu, M.C.; Coskun, M.; Parlakfikirer, N.; Keskin, U. Investigation of the association between glaucoma and Helicobacter pylori infection using the 14C-urea breath test. Arq. Bras. Oftalmol. 2015, 78, 229–231. [Google Scholar] [CrossRef][Green Version]
- Zavos, C.; Kountouras, J.; Sakkias, G.; Venizelos, I.; Deretzi, G.; Arapoglou, S. Histological presence of Helicobacter pylori bacteria in the trabeculum and iris of patients with primary open-angle glaucoma. Ophthalmic Res. 2012, 47, 150–156. [Google Scholar] [CrossRef]
- Abrishami, M.; Kargozar, A.; Rashed, T.; Shoeibi, N.; Attaranzadeh, A. Association of Helicobacter Pylori Infection with Primary Open Angle Glaucoma. Bina J. Ophthalmol. 2007, 12, 289–293. [Google Scholar]
- Kountouras, J.; Mylopoulos, N.; Boura, P.; Bessas, C.; Chatzopoulos, D.; Venizelos, J.; Zavos, C. Relationship between Helicobacter pylori infection and glaucoma. Ophthalmology 2001, 108, 599–604. [Google Scholar]
- Noche, C.D.; Njajou, O.; Etoa, F.X. No Association between CagA- and VacA-Positive Strains of Helicobacter pylori and Primary Open-Angle Glaucoma: A Case-Control Study. Ophthalmol. Eye Dis. 2016, 8, 1–4. [Google Scholar] [CrossRef]
- Pezzullo, L.; Streatfeild, J.; Simkiss, P.; Shickle, D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv. Res. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Al-Mugheiry, T.S.; Normando, E.M.; Cordeiro, M.F. Real-Time Imaging of Retinal Cell Apoptosis by Confocal Scanning Laser Ophthalmoscopy and Its Role in Glaucoma. Front. Neurol. 2018, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, P.; Javanian, M.; Koppolu, V.; Vasigala, V.R.; Ebrahimpour, S. Helicobacter pylori infection in children: An overview of diagnostic methods. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1035–1045. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Zhu, H.; Zhao, J.; She, Q.; Zhang, G.-X. Diagnostic Performance of Urea Breath Test, Rapid Urea Test, and Histology for Helicobacter pylori Infection in Patients with Partial Gastrectomy. J. Clin. Gastroenterol. 2012, 46, 285–292. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Chmiela, M.; Gonciarz, W. Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 2017, 23, 3964. [Google Scholar] [CrossRef]
- Saccà, S.C.; Vagge, A.; Pulliero, A.; Izzotti, A. Helicobacter pylori Infection and Eye Diseases. Medicine (Baltim.) 2014, 93, e216. [Google Scholar] [CrossRef]
- Unterlauft, J.D.; Böhm, M.R.R. Rolle des alternden visuellen Systems bei Glaukomen. Der Ophthalmol. 2017, 114, 108–113. [Google Scholar] [CrossRef]
- Day, A.L.; Singh, J.A. Cardiovascular Disease Risk in Older Adults and Elderly Patients with Rheumatoid Arthritis: What Role Can Disease-Modifying Antirheumatic Drugs Play in Cardiovascular Risk Reduction? Drugs Aging 2019, 36, 493–510. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2014, 30, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Edward, D.P.; Wax, M.B. Serum Autoantibodies to Optic Nerve Head Glycosaminoglycans in Patients with Glaucoma. Arch. Ophthalmol. 1999, 117, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Wax, M.B.; Tezel, G.; Saito, I.; Gupta, R.S.; Harley, J.B.; Li, Z.; Romano, C. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am. J. Ophthalmol. 1998, 125, 145–157. [Google Scholar] [CrossRef]
- Kornberg, A.J.; Pestronk, A. Immune-mediated neuropathies. Curr. Opin. Neurol. 1993, 6, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.J. Immune-Related Disease and Normal-Tension Glaucoma. Arch. Ophthalmol. 1992, 110, 500. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S. The important role played by chemokines influence the clinical outcome of Helicobacter pylori infection. Life Sci. 2019, 231, 116688. [Google Scholar] [CrossRef]
- Lang, B.J.; Gorrell, R.J.; Tafreshi, M.; Hatakeyama, M.; Kwok, T.; Price, J.T. The Helicobacter pylori cytotoxin CagA is essential for suppressing host heat shock protein expression. Cell Stress Chaperones 2016, 21, 523–533. [Google Scholar] [CrossRef]
- Duarte, H.; Freitas, D.; Gomes, C.; Gomes, J.; Magalhães, A.; Reis, C. Mucin-Type O-Glycosylation in Gastric Carcinogenesis. Biomolecules 2016, 6, 33. [Google Scholar] [CrossRef]
- Gönen, S.; Sari, S.; Kandur, Y.; Dalgiç, B.; Söylemezoğlu, O. Evaluation of human leukocyte antigen class I and II antigens in Helicobacter pylori-positive pediatric patients with active gastritis and duodenal ulcer. Arq. Gastroenterol. 2017, 54, 297–299. [Google Scholar] [CrossRef]
- Claeys, D.; Faller, G.; Appelmelk, B.J.; Negrini, R.; Kirchner, T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 1998, 115, 340–347. [Google Scholar] [CrossRef]
- Terraciano, A.J.; Wang, N.; Schuman, J.; Haffner, G.; Panjwani, N.; Zhao, Z.; Yang, Z. Sialyl Lewis X, Lewis X, and N-acetyllactosamine expression on normal and glaucomatous eyes. Curr. Eye Res. 1999, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Baudouin, C.; Hamard, P.; Creuzot-Garcher, C.; Warnet, J.-M.; Brignole-Baudouin, F. [Activation of TH1/TH2 pathways detected through the expression of CCR4 and CCR5 on the ocular surface of glaucomatous patients treated over the long term]. J. Fr. Ophtalmol. 2006, 29, 121–126. [Google Scholar] [CrossRef]
- Tsai, T.; Reinehr, S.; Maliha, A.; Joachim, S. Degeneration and Possible Protection in Glaucoma. Front. Neurosci. 2019, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Seigel, G.M.; Wax, M.B. Autoantibodies to small heat shock proteins in glaucoma. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2277–2287. [Google Scholar]
- Cvenkel, B.; Kopitar, A.N.; Ihan, A. Correlation Between Filtering Bleb Morphology, Expression of Inflammatory Marker HLA-DR by Ocular Surface, and Outcome of Trabeculectomy. J. Glaucoma 2013, 22, 15–20. [Google Scholar] [CrossRef]
- David, R.; Maier, G.; Baumgarten, I.; Abrahams, C. HLA antigens in glaucoma and ocular hypertension. Br. J. Ophthalmol. 1979, 63, 293–296. [Google Scholar] [CrossRef]
- Kountouras, J.; Boziki, M.; Polyzos, S.A.; Katsinelos, P.; Gavalas, E.; Zeglinas, C.; Tzivras, D.; Romiopoulos, I.; Giorgakis, N.; Anastasiadou, K.; et al. The emerging role of helicobacter pylori-induced metabolic gastrointestinal dysmotility and neurodegeneration. Curr. Mol. Med. 2017, 17, 389–404. [Google Scholar] [CrossRef]
- Sakimoto, S.; Okazaki, T.; Usui, S.; Ishibashi, T.; Oura, Y.; Nishida, K.; Miki, A.; Kawasaki, R.; Matsushita, K.; Sakaguchi, H.; et al. Cross-Sectional Imaging Analysis of Epiretinal Membrane Involvement in Unilateral Open-Angle Glaucoma Severity. Investig. Opthalmol. Vis. Sci. 2018, 59, 5745. [Google Scholar] [CrossRef]
- Cui, Q.N.; Ramakrishnan, M.S.; Gudiseva, H.V.; Collins, D.W.; Pistilli, M.; Lee, R.; Chavali, V.M.; Lehman, A.; Addis, V.M.; O’Brien, J.M. Mitochondrial haplogroup L1c2 is associated with increased disease severity in African American patients with primary open-angle glaucoma. J. Clin. Exp. Ophthalmol. 2019, 10, 799. [Google Scholar]
- Clyne, M.; Rowland, M. The Role of Host Genetic Polymorphisms in Helicobacter Pylori Mediated Disease Outcome; Springer Nature Switzerland AG: Basel, Switzerland, 2019; pp. 151–172. [Google Scholar]
- Suzuki, H.; Mori, H. Different Pathophysiology of Gastritis between East and West? An Asian Perspective. Inflamm. Intest. Dis. 2016, 1, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Tserentogtokh, T.; Gantuya, B.; Subsomwong, P.; Oyuntsetseg, K.; Bolor, D.; Erdene-Ochir, Y.; Azzaya, D.; Davaadorj, D.; Uchida, T.; Matsuhisa, T.; et al. Western-Type Helicobacter pylori CagA are the Most Frequent Type in Mongolian Patients. Cancers 2019, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Papaefthymiou, A.; Doulberis, M.; Katsinelos, P.; Liatsos, C.; Polyzos, S.A.; Kotronis, G.; Papanikolaou, K.; Kountouras, J. Impact of nitric oxide’s bidirectional role on glaucoma: Focus on Helicobacter pylori—Related nitrosative stress. Ann. N. Y. Acad. Sci. 2019, 1465, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Ala, S.; Maleki, I.; Sanjari Araghi, A.; Sahebnasagh, A.; Shahraki, A. Helicobacter pylori eradication in the management of glaucoma. Casp. J. Intern. Med. 2020, 11, 143–149. [Google Scholar]
- Chen, H.-Y.; Lin, C.-L.; Chen, W.-C.; Kao, C.-H. Does Helicobacter pylori Eradication Reduce the Risk of Open Angle Glaucoma in Patients with Peptic Ulcer Disease? Medicine (Baltim.) 2015, 94, e1578. [Google Scholar] [CrossRef]
- Jin, S.; Trope, G.E.; Buys, Y.M.; Badley, E.M.; Thavorn, K.; Yan, P.; Nithianandan, H.; Jin, Y.-P. Reduced social participation among seniors with self-reported visual impairment and glaucoma. PLoS ONE 2019, 14, e0218540. [Google Scholar] [CrossRef]

| No | First Author | Year of Publication | Type of Study | Country (Region) | Host Journal | Glaucoma Cases | Controls | Mean Age (years) * | Controls Deposit | Glaucoma Subtype | H. pylori Infection Diagnosis | MD of Anti-H. pylori Titers | NOS Score | Relationship between H. pylori and Glaucoma (OR, CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abrishami et al. | 2007 | Cohort | Iran (East) | Bina J Ophthalmol | 44 | 79 | 60.8 | Cataract | POAG | ELISA | N/A | N/A | 3.69 (1.68–8.13) |
| 2 | Deshpande et al. | 2008 | Case-control | India (East) | J Glaucoma | 50 | 50 | 63.7 | Cataract | POAG | ELISA | 20′220 | 8 | 1.20 (0.52–2.79) |
| 3 | Deshpande et al. | 2008 | Case-control | India (East) | J Glaucoma | 50 | 50 | 67 | Cataract | PEG | ELISA | −8′610 | 0.41 (0.18–0.91) | |
| 4 | Galloway et al. | 2003 | Cohort | Canada (West) | Ophthalmology | 38 | 94 | 63.2 | Healthy | POAG | ELISA | −10′600 | 8 | 1.41 (0.59–3.4) |
| 5 | Galloway et al. | 2003 | Cohort | Canada (West) | Ophthalmology | 16 | 94 | 73.2 | Healthy | PEG | ELISA | 18′500 | 1.32 (0.38–4.54) | |
| 6 | Galloway et al. | 2003 | Cohort | Canada (West) | Ophthalmology | 19 | 94 | 67.7 | Healthy | NTG | ELISA | 23′300 | 1.41 (0.45–4.4) | |
| 7 | Hong et al. | 2007 | Case-control | China (East) | Asian J Ophtholmol | 24 | 24 | 63.9 | Healthy | POAG | UBT | N/A | 8 | 4.49 (1.26–16) |
| 8 | Kim et al. | 2011 | Retrospective Cohort | South Korea (East) | IOVS | 100 | 88 | 55.6 | Healthy | NTG | ELISA | N/A | 8 | 2.05 (1.12–3.75) |
| 9 | Kim et al. | 2011 | Retrospective Cohort | South Korea (East) | IOVS | 104 | 1116 | 53.4 | Healthy | NTG | ELISA | N/A | 1.83 (1.17–2.86) | |
| 10 | Kountouras et al. | 2003 | Cohort | Greece (West) | Graefe’s Arch Clin Exp Ophthalmol | 27 | 31 | 70.6 | Cataract | POAG | ELISA | 37′210 | 8 | 2.35 (0.63–8.76) |
| 11 | Kountouras et al. | 2003 | Cohort | Greece (West) | Graefe’s Arch Clin Exp Ophthalmol | 19 | 31 | 69.2 | Cataract | PEG | ELISA | 25′800 | 25 (1.38–452.34) | |
| 12 | Kountouras et al. | 2002 | Cohort | Greece (West) | Arch Intern Med | 41 | 30 | 61.4 | Anemia | POAG | HISTOLOGY | 18′570 | 8 | 8.23 (2.53–26.75) |
| 13 | Kountouras et al. | 2001 | Case-control | Greece (West) | Ophthalmology | 32 | 30 | 64 | Anemia | POAG | HISTOLOGY | N/A | 8 (2.25–28.48) | |
| 14 | Kountouras et al. | 2001 | Case-control | Greece (West) | Ophthalmology | 9 | 30 | 62 | Anemia | PEG | HISTOLOGY | N/A | 7 | 9.14 (1.01–82.44) |
| 15 | Kurtz et al. | 2008 | Cohort | Israel (West) | J Glaucoma | 13 | 36 | 67.7 | Cataract | POAG | ELISA | N/A | 8 | 0.74 (0.21–2.67) |
| 16 | Kurtz et al. | 2008 | Cohort | Israel (West) | J Glaucoma | 23 | 36 | Cataract | PEG | ELISA | N/A | 1.19 (0.4–3.54) | ||
| 17 | Kurtz et al. | 2008 | Cohort | Israel (West) | J Glaucoma | 15 | 36 | Cataract | NTG | ELISA | N/A | 0.96 (0.28–3.27) | ||
| 18 | Noche et al. | 2016 | Case-control | Cameroon (Africa) | Ophthalmology and Eye Diseases | 50 | 31 | 58.52 | Healthy | POAG | ELISA | N/A | 8 | 0.42 (0.12–1.43) |
| 19 | Samarai et al. | 2014 | Case-control | Iran (East) | Global Journal of Health Science | 37 | 42 | 73.05 | Anemia | POAG | ELISA | N/A | 8 | 5.61 (1.68–18.75) |
| 20 | Sultana et al. | 2019 | Case-control | Bangladesh (East) | BSMMU J | 40 | 40 | 51.4 | Healthy | POAG | ELISA | N/A | 8 | 3.89 (1.53–9.87) |
| 21 | Tsolaki et al. | 2015 | Cohort | Greece (West) | BMC Ophthalmology | 35 | 31 | 62.18 | Healthy | POAG | HISTOLOGY | N/A | 7 | 2.65 (0.97–7.24) |
| 22 | Tuzcu et al. | 2015 | Case-control | Turkey (East) | Arq Bras Oftalmol | 35 | 35 | 59.08 | Healthy | POAG | UBT | N/A | 8 | 1.41 (0.55–3.62) |
| 23 | Zavos et al. | 2012 | Case-control | Greece (West) | Ophthalmic Res | 51 | 35 | 71.4 | Anemia | POAG | HISTOLOGY | N/A | 8 | 5.69 (2.08–15.54) |
| Random Effects Model | Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | Datasets | OR | CI 95% | p | Q | I2 (%) | t2 | p |
| Total | 23 | 2.08 | 1.48–2.93 | <0.001 | 57.21 | 61.54 | 0.39 | <0.001 |
| Region | ||||||||
| EAST | 8 | 2.1 | 1.23–3.56 | 0.006 | 25.1 | 72.11 | <0.001 | |
| WEST | 14 | 2.32 | 1.47–3.64 | <0.001 | 25.51 | 49.04 | 0.029 | |
| AFRICA | 1 | 0.42 | 0.12–1.44 | N/A | N/A | N/A | N/A | |
| Glaucoma Subtype | ||||||||
| POAG | 14 | 2.57 | 1.66–3.99 | <0.001 | 31.59 | 58.85 | 0.003 | |
| PEG | 5 | 1.69 | 0.53–5.34 | 0.373 | 13.68 | 70.76 | 0.008 | |
| NTG | 4 | 1.76 | 1.27–2.46 | <0.001 | 1.36 | 0 | 0.715 | |
| Study Design | ||||||||
| Cohort | 13 | 1.98 | 1.44–2.74 | 0.141 | 17.24 | 30.4 | 0.141 | |
| Case-control | 10 | 2.33 | 1.13–4.81 | <0.001 | 39.95 | 77.47 | <0.001 | |
| Control groups | ||||||||
| Cataract | 8 | 1.37 | 0.7–2.67 | 0.362 | 20.52 | 65.89 | 0.005 | |
| Anemia | 5 | 6.78 | 3.88–11.83 | <0.001 | 0.45 | 0 | 0.98 | |
| Healthy | 10 | 1.82 | 1.33–2.5 | <0.001 | 11.7 | 23.07 | 0.231 | |
| H. pylori infection diagnosis | ||||||||
| ELISA | 16 | 1.57 | 1.09–2.28 | 0.017 | 35.35 | 57.57 | 0.002 | |
| UBT | 2 | 2.32 | 0.76–7.13 | 0.142 | 2.06 | 51.35 | 0.152 | |
| HISTOLOGY | 5 | 5.4 | 3.17–9.2 | <0.001 | 3.02 | 0 | 0.555 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doulberis, M.; Papaefthymiou, A.; Polyzos, S.A.; Bargiotas, P.; Liatsos, C.; Srivastava, D.S.; Zavos, C.; Katsinelos, P.; Kountouras, J. Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 894. https://doi.org/10.3390/microorganisms8060894
Doulberis M, Papaefthymiou A, Polyzos SA, Bargiotas P, Liatsos C, Srivastava DS, Zavos C, Katsinelos P, Kountouras J. Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis. Microorganisms. 2020; 8(6):894. https://doi.org/10.3390/microorganisms8060894
Chicago/Turabian StyleDoulberis, Michael, Apostolis Papaefthymiou, Stergios A. Polyzos, Panagiotis Bargiotas, Christos Liatsos, David Shiva Srivastava, Christos Zavos, Panagiotis Katsinelos, and Jannis Kountouras. 2020. "Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis" Microorganisms 8, no. 6: 894. https://doi.org/10.3390/microorganisms8060894
APA StyleDoulberis, M., Papaefthymiou, A., Polyzos, S. A., Bargiotas, P., Liatsos, C., Srivastava, D. S., Zavos, C., Katsinelos, P., & Kountouras, J. (2020). Association between Active Helicobacter pylori Infection and Glaucoma: A Systematic Review and Meta-Analysis. Microorganisms, 8(6), 894. https://doi.org/10.3390/microorganisms8060894








