Crossing the Vacuolar Rubicon: Structural Insights into Effector Protein Trafficking in Apicomplexan Parasites
Abstract
1. Introduction
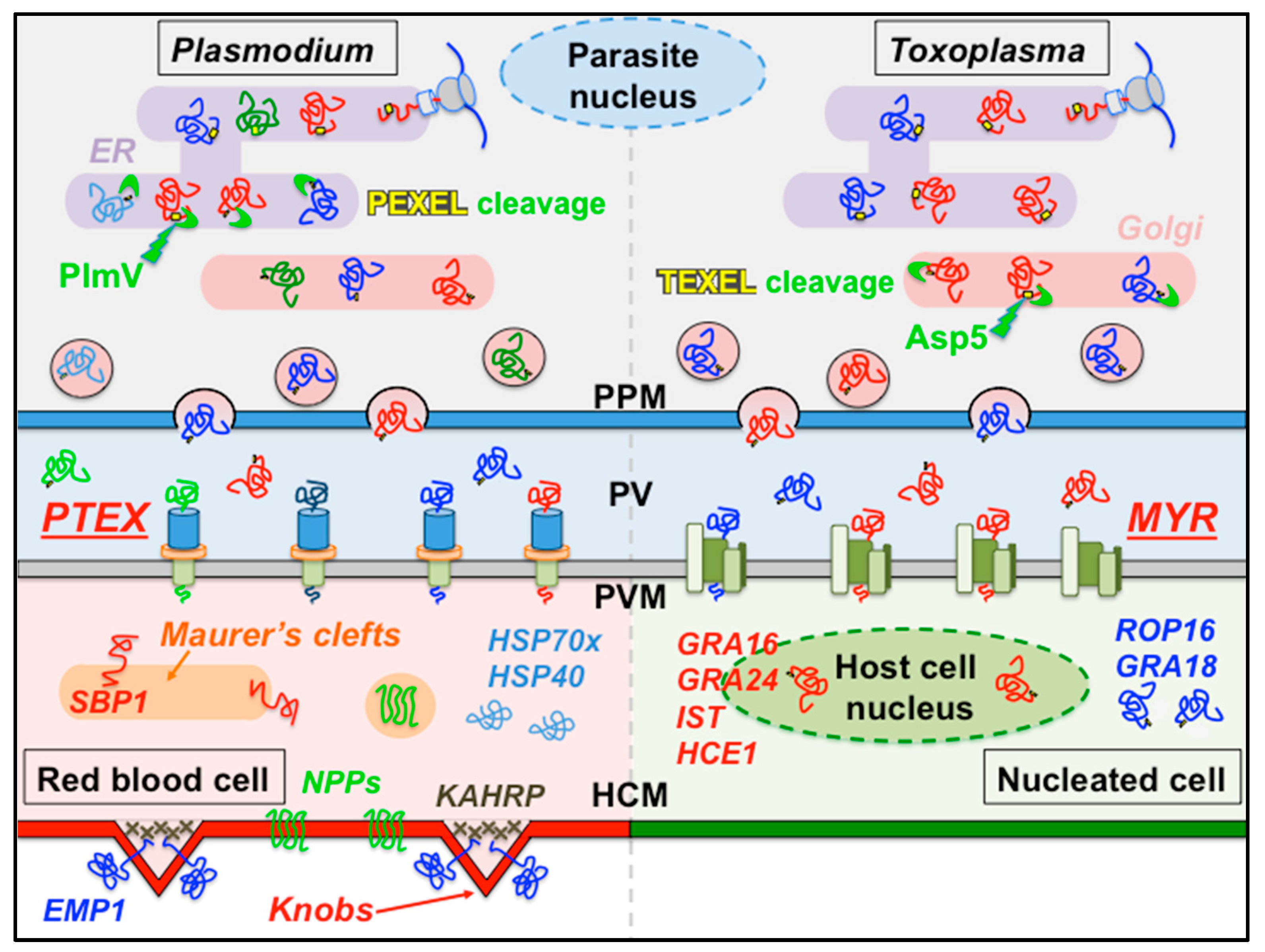
2. Effector and Virulence Factor Export across the Parasitophorous Vacuole Requires Specialized Vacuolar Translocons
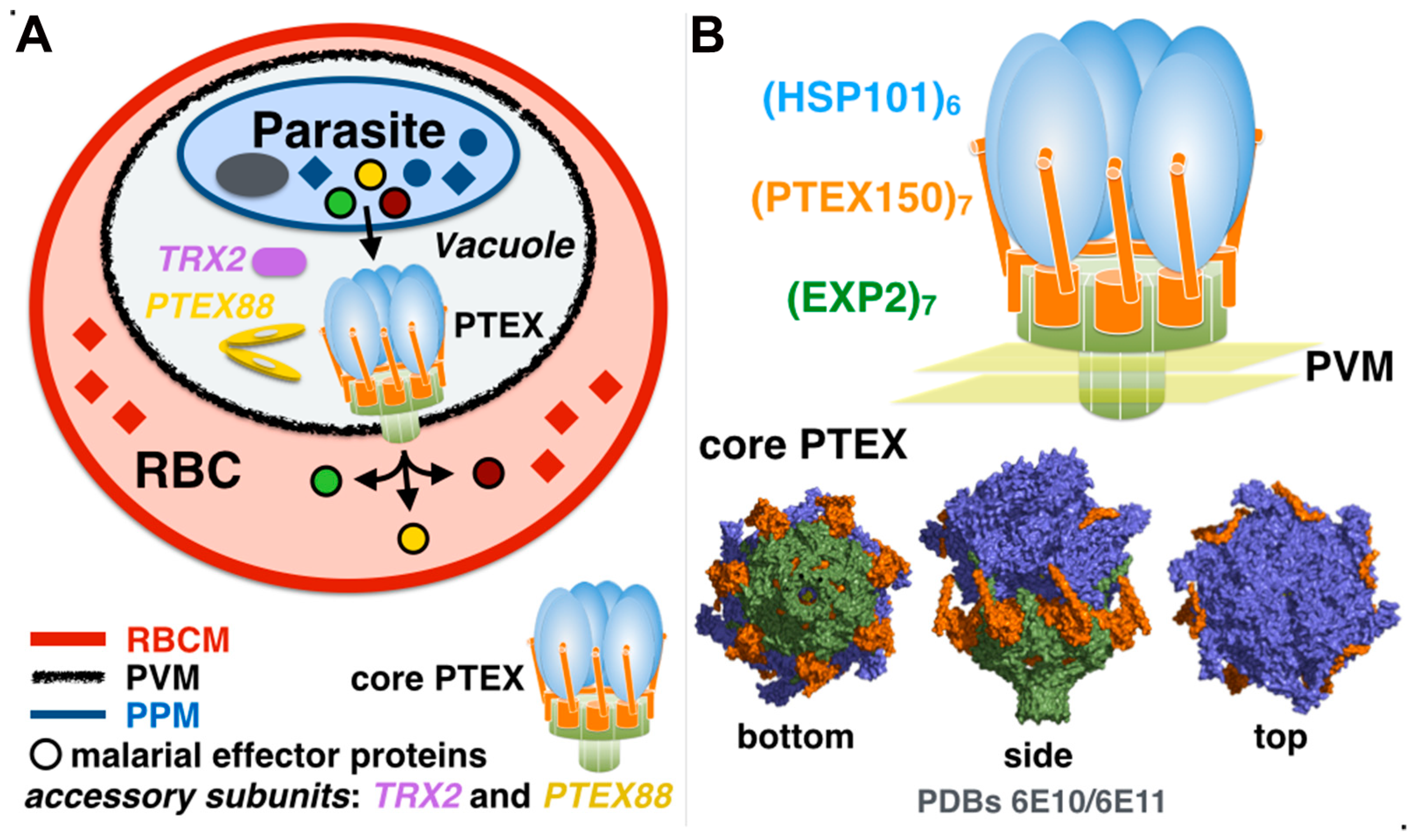
3. The Plasmodium Translocon of Exported Proteins
3.1. Exported Protein-2: An Unusual Pore-Forming Protein with Multiple Functions?
3.2. Disorderly Functional: The Adaptor Protein PTEX150 and the Roles of Low-Complexity Regions in the Plasmodium Proteome
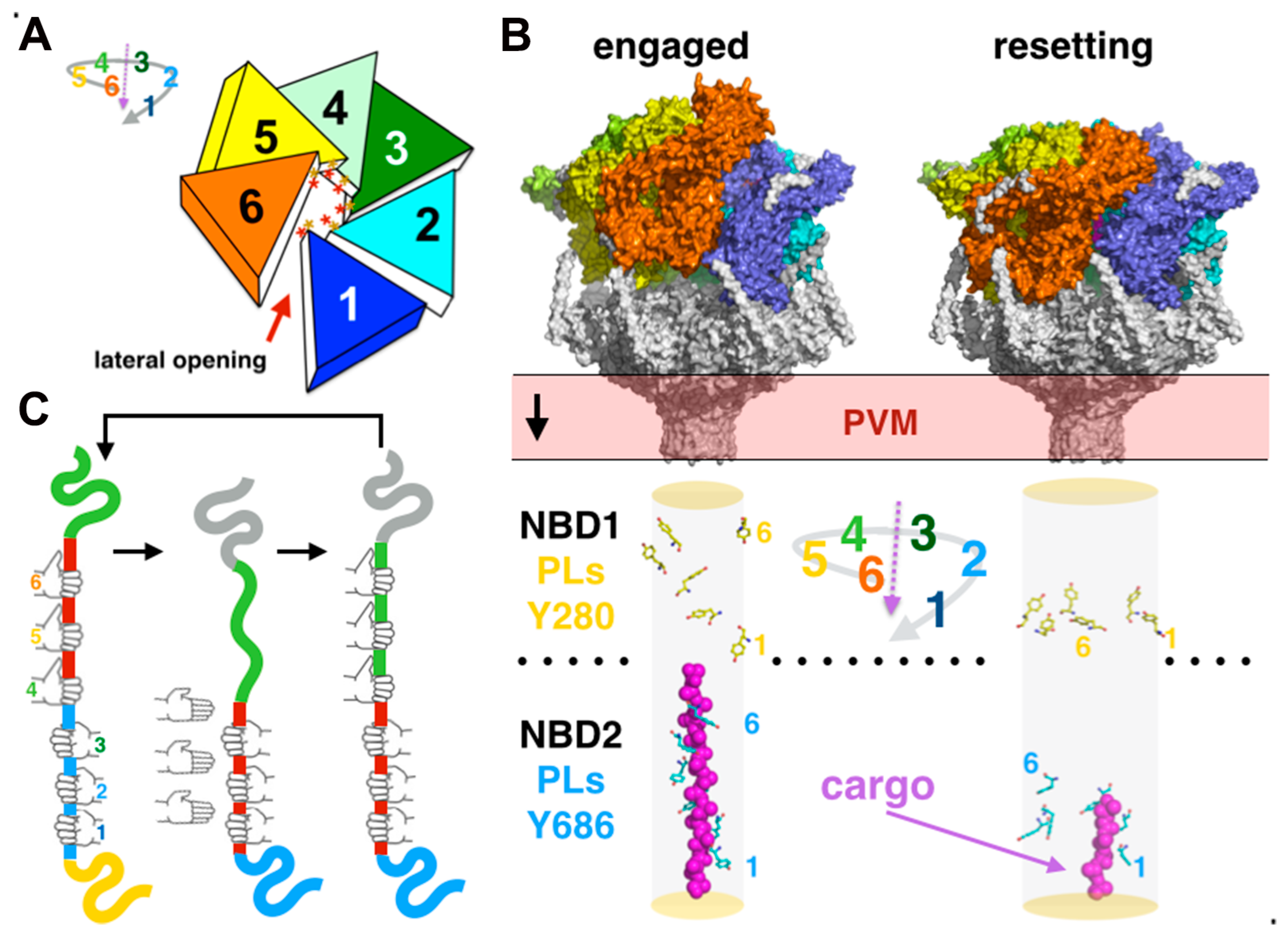
3.3. Transmembrane Pore Rigidity, Geometry, and Physicochemical Properties
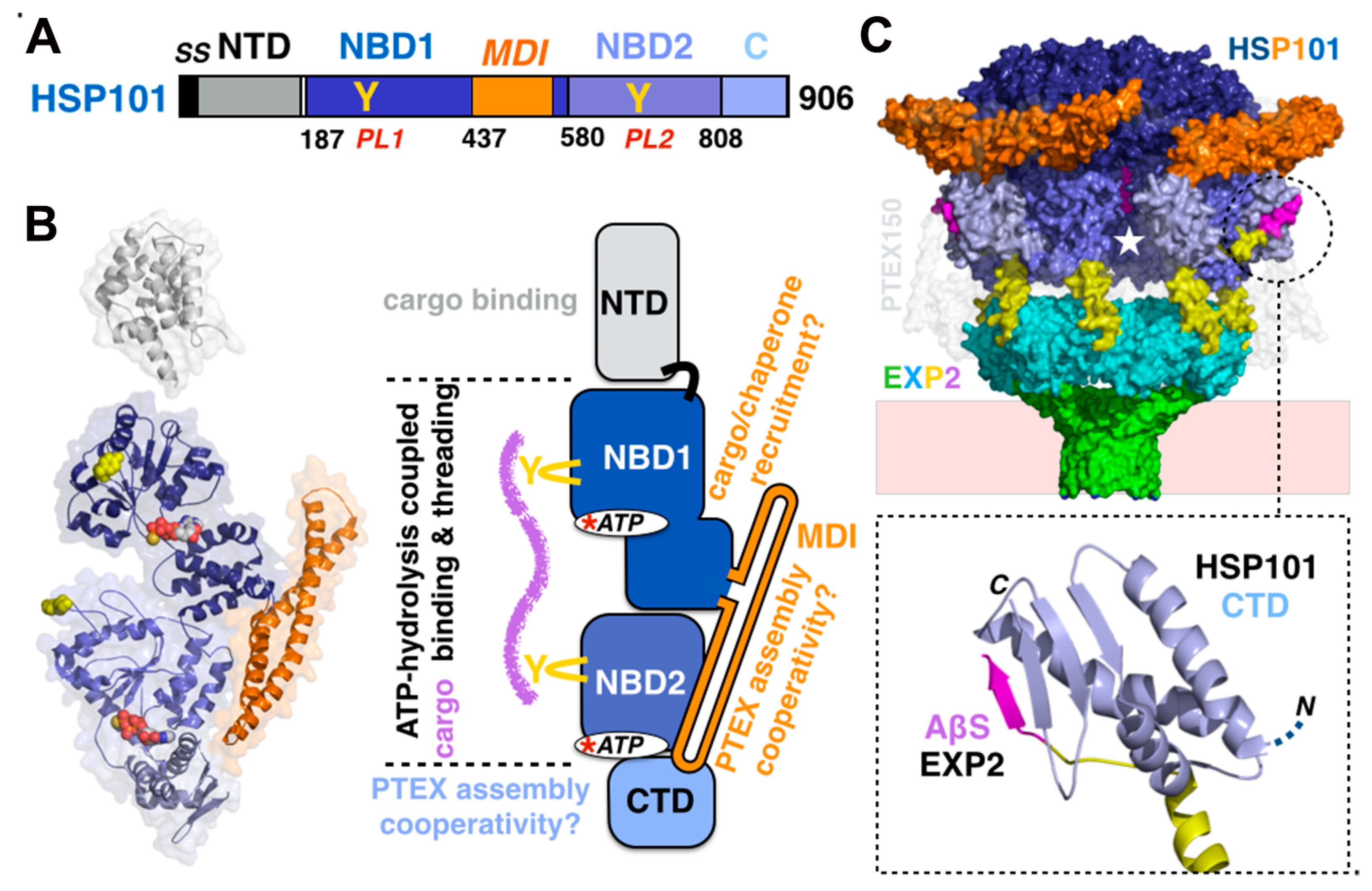
3.4. Energizing Effector Translocation in Plasmodium: The AAA+ Protein Unfoldase HSP101
3.4.1. HSP101/ClpB2: The AAA+ Protein Unfoldase that Drives Protein Export
3.4.2. Hexameric Spiral Staircase Assembly and Cargo Translocation Coupled to ATP-Binding and Hydrolysis
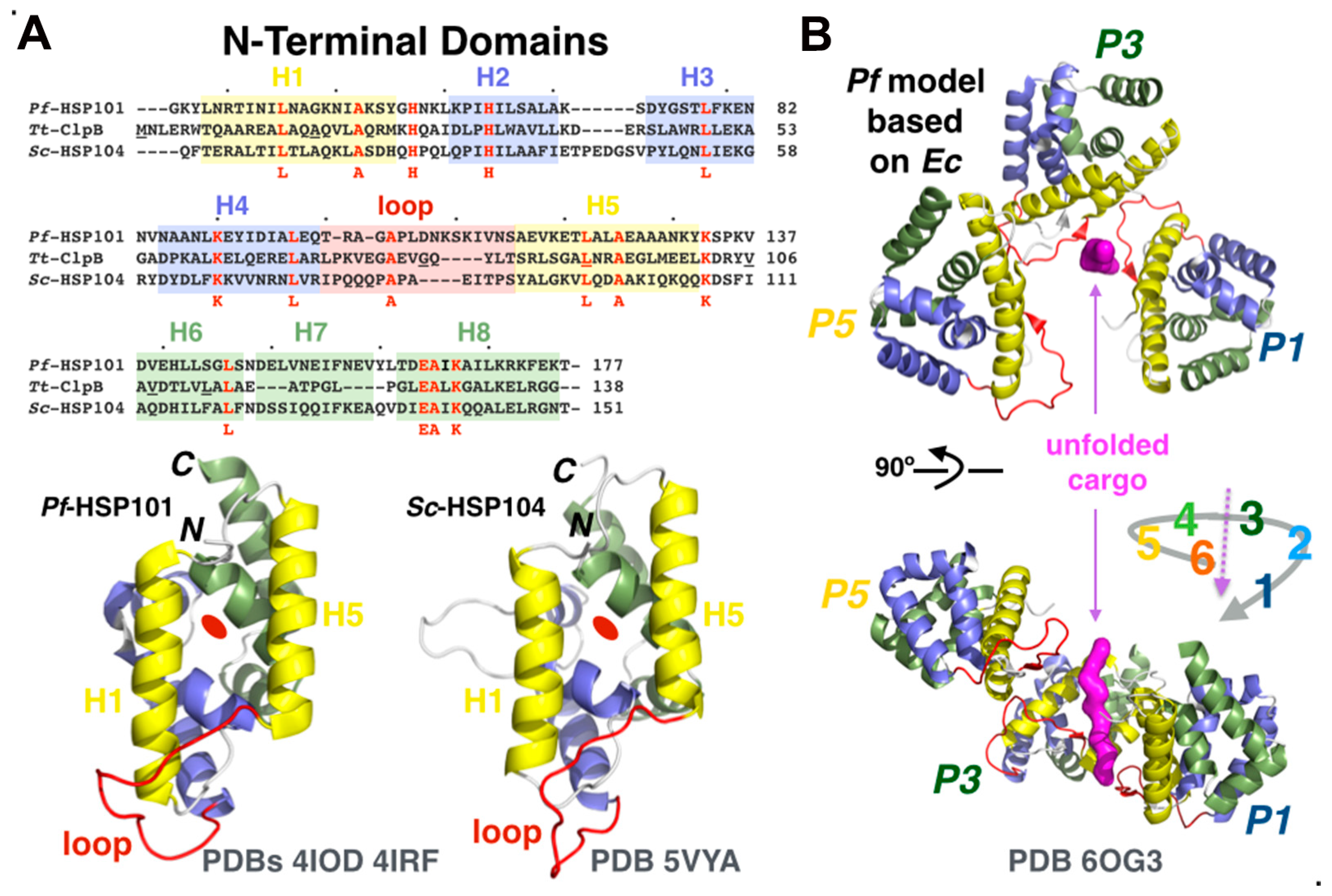
3.4.3. The Role of N-Terminal Domains in Cargo Unfolding, Binding, and Recognition
3.5. Accessory Proteins TRX2 and PTEX88
4. The Perplexing Roles of Vacuolar Targeting Signals (PEXELs and TEXELs) and Their Licensing Proteases in Apicomplexan Effector Protein Export
5. Secretion Across the Parasitophorous Vacuole in Other Apicomplexans: Toxoplasma and Other Coccidia
6. Parasitic Vacuolar Secretion Pathways as Drug Targets
7. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Van Dooren, G.G.; Striepen, B. The algal past and parasite present of the apicoplast. Annu. Rev. Microbiol. 2013, 67, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Arisue, N.; Hashimoto, T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015, 64, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.E.; Zimmerberg, J. Hardly Vacuous: The Parasitophorous Vacuolar Membrane of Malaria Parasites. Trends Parasitol. 2020, 36, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.M.; Beck, J.R.; Blackman, M.J. The parasitophorous vacuole of the blood-stage malaria parasite. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef]
- Spillman, N.J.; Beck, J.R.; Goldberg, D.E. Protein export into malaria parasite-infected erythrocytes: Mechanisms and functional consequences. Annu. Rev. Biochem. 2015, 84, 813–841. [Google Scholar] [CrossRef]
- de Koning-Ward, T.F.; Dixon, M.W.; Tilley, L.; Gilson, P.R. Plasmodium species: Master renovators of their host cells. Nat. Rev. Microbiol. 2016, 14, 494–507. [Google Scholar] [CrossRef]
- Hakimi, M.A.; Olias, P.; Sibley, L.D. Toxoplasma Effectors Targeting Host Signaling and Transcription. Clin. Microbiol. Rev. 2017, 30, 615–645. [Google Scholar] [CrossRef]
- Rastogi, S.; Cygan, A.M.; Boothroyd, J.C. Translocation of effector proteins into host cells by Toxoplasma gondii. Curr. Opin. Microbiol. 2019, 52, 130–138. [Google Scholar] [CrossRef]
- de Koning-Ward, T.F.; Gilson, P.R.; Boddey, J.A.; Rug, M.; Smith, B.J.; Papenfuss, A.T.; Sanders, P.R.; Lundie, R.J.; Maier, A.G.; Cowman, A.F.; et al. A newly discovered protein export machine in malaria parasites. Nature 2009, 459, 945–949. [Google Scholar] [CrossRef]
- Marino, N.D.; Panas, M.W.; Franco, M.; Theisen, T.C.; Naor, A.; Rastogi, S.; Buchholz, K.R.; Lorenzi, H.A.; Boothroyd, J.C. Identification of a novel protein complex essential for effector translocation across the parasitophorous vacuole membrane of Toxoplasma gondii. PLoS Pathog. 2018, 14, e1006828. [Google Scholar] [CrossRef]
- Marti, M.; Good, R.T.; Rug, M.; Knuepfer, E.; Cowman, A.F. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 2004, 306, 1930–1933. [Google Scholar] [CrossRef] [PubMed]
- Hiller, N.L.; Bhattacharjee, S.; van Ooij, C.; Liolios, K.; Harrison, T.; Lopez-Estrano, C.; Haldar, K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 2004, 306, 1934–1937. [Google Scholar] [CrossRef] [PubMed]
- Boddey, J.A.; Cowman, A.F. Plasmodium nesting: Remaking the erythrocyte from the inside out. Annu. Rev. Microbiol. 2013, 67, 243–269. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Babbitt, S.; Muralidharan, V.; Butler, T.; Oksman, A.; Goldberg, D.E. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 2010, 463, 632–636. [Google Scholar] [CrossRef]
- Boddey, J.A.; Hodder, A.N.; Gunther, S.; Gilson, P.R.; Patsiouras, H.; Kapp, E.A.; Pearce, J.A.; de Koning-Ward, T.F.; Simpson, R.J.; Crabb, B.S.; et al. An aspartyl protease directs malaria effector proteins to the host cell. Nature 2010, 463, 627–631. [Google Scholar] [CrossRef]
- Coffey, M.J.; Sleebs, B.E.; Uboldi, A.D.; Garnham, A.; Franco, M.; Marino, N.D.; Panas, M.W.; Ferguson, D.J.; Enciso, M.; O’Neill, M.T.; et al. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. eLife 2015, 4. [Google Scholar] [CrossRef]
- Hammoudi, P.M.; Jacot, D.; Mueller, C.; Di Cristina, M.; Dogga, S.K.; Marq, J.B.; Romano, J.; Tosetti, N.; Dubrot, J.; Emre, Y.; et al. Fundamental Roles of the Golgi-Associated Toxoplasma Aspartyl Protease, ASP5, at the Host-Parasite Interface. PLoS Pathog. 2015, 11, e1005211. [Google Scholar] [CrossRef]
- Egea, P.F.; Stroud, R.M.; Walter, P. Targeting proteins to membranes: Structure of the signal recognition particle. Curr. Opin. Struct. Biol. 2005, 15, 213–220. [Google Scholar] [CrossRef]
- Marapana, D.S.; Dagley, L.F.; Sandow, J.J.; Nebl, T.; Triglia, T.; Pasternak, M.; Dickerman, B.K.; Crabb, B.S.; Gilson, P.R.; Webb, A.I.; et al. Plasmepsin V cleaves malaria effector proteins in a distinct endoplasmic reticulum translocation interactome for export to the erythrocyte. Nat. Microbiol. 2018, 3, 1010–1022. [Google Scholar] [CrossRef]
- Mundwiler-Pachlatko, E.; Beck, H.P. Maurer’s clefts, the enigma of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2013, 110, 19987–19994. [Google Scholar] [CrossRef]
- Martin, R.E. The transportome of the malaria parasite. Biol. Rev. 2019. [Google Scholar] [CrossRef]
- Lim, D.C.; Cooke, B.M.; Doerig, C.; Saeij, J.P. Toxoplasma and Plasmodium protein kinases: Roles in invasion and host cell remodelling. Int. J. Parasitol. 2012, 42, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Naor, A.; Panas, M.W.; Marino, N.; Coffey, M.J.; Tonkin, C.J.; Boothroyd, J.C. MYR1-Dependent Effectors Are the Major Drivers of a Host Cell’s Early Response to Toxoplasma, Including Counteracting MYR1-Independent Effects. mBio 2018, 9. [Google Scholar] [CrossRef]
- Panas, M.W.; Naor, A.; Cygan, A.M.; Boothroyd, J.C. Toxoplasma Controls Host Cyclin E Expression through the Use of a Novel MYR1-Dependent Effector Protein, HCE1. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Bullen, H.E.; Charnaud, S.C.; Kalanon, M.; Riglar, D.T.; Dekiwadia, C.; Kangwanrangsan, N.; Torii, M.; Tsuboi, T.; Baum, J.; Ralph, S.A.; et al. Biosynthesis, localisation and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins; PTEX. J. Biol. Chem. 2012, 287, 7871–7884. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Beck, J.R.; Lai, M.; Cui, Y.; Goldberg, D.E.; Egea, P.F.; Zhou, Z.H. Malaria parasite translocon structure and mechanism of effector export. Nature 2018, 561, 70–75. [Google Scholar] [CrossRef]
- de Koning-Ward, T.F. Spotlight on proteins that aid malaria. Nature 2018, 561, 41–43. [Google Scholar] [CrossRef]
- Matthews, K.M.; Pitman, E.L.; de Koning-Ward, T.F. Illuminating how malaria parasites export proteins into host erythrocytes. Cell Microbiol. 2019, 21, e13009. [Google Scholar] [CrossRef]
- Beck, J.R.; Muralidharan, V.; Oksman, A.; Goldberg, D.E. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 2014, 511, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Elsworth, B.; Matthews, K.; Nie, C.Q.; Kalanon, M.; Charnaud, S.C.; Sanders, P.R.; Chisholm, S.A.; Counihan, N.A.; Shaw, P.J.; Pino, P.; et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 2014, 511, 587–591. [Google Scholar] [CrossRef]
- Garten, M.; Nasamu, A.S.; Niles, J.C.; Zimmerberg, J.; Goldberg, D.E.; Beck, J.R. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat. Microbiol. 2018, 3, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Charnaud, S.C.; Kumarasingha, R.; Bullen, H.E.; Crabb, B.S.; Gilson, P.R. Knockdown of the translocon protein EXP2 in Plasmodium falciparum reduces growth and protein export. PLoS ONE 2018, 13, e0204785. [Google Scholar] [CrossRef] [PubMed]
- Sanders, P.R.; Dickerman, B.K.; Charnaud, S.C.; Ramsland, P.A.; Crabb, B.S.; Gilson, P.R. The N-terminus of EXP2 forms the membrane-associated pore of the protein exporting translocon PTEX in Plasmodium falciparum. J. Biochem. 2019, 165, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gold, D.A.; Kaplan, A.D.; Lis, A.; Bett, G.C.; Rosowski, E.E.; Cirelli, K.M.; Bougdour, A.; Sidik, S.M.; Beck, J.R.; Lourido, S.; et al. The Toxoplasma Dense Granule Proteins GRA17 and GRA23 Mediate the Movement of Small Molecules between the Host and the Parasitophorous Vacuole. Cell Host Microbe 2015, 17, 642–652. [Google Scholar] [CrossRef]
- Paredes-Santos, T.; Wang, Y.; Waldman, B.; Lourido, S.; Saeij, J.P. The GRA17 Parasitophorous Vacuole Membrane Permeability Pore Contributes to Bradyzoite Viability. Front. Cell. Infect. Microbiol. 2019, 9, 321. [Google Scholar] [CrossRef]
- Mesen-Ramirez, P.; Bergmann, B.; Tran, T.T.; Garten, M.; Stacker, J.; Naranjo-Prado, I.; Hohn, K.; Zimmerberg, J.; Spielmann, T. EXP1 is critical for nutrient uptake across the parasitophorous vacuole membrane of malaria parasites. PLoS Biol. 2019, 17, e3000473. [Google Scholar] [CrossRef]
- Nessel, T.; Beck, J.M.; Rayatpisheh, S.; Jami-Alahmadi, Y.; Wohlschlegel, J.A.; Goldberg, D.E.; Beck, J.R. EXP1 is required for organisation of EXP2 in the intraerythrocytic malaria parasite vacuole. Cell Microbiol. 2020, 22, e13168. [Google Scholar] [CrossRef]
- Speed, M.A.; Wang, D.I.; King, J. Multimeric intermediates in the pathway to the aggregated inclusion body state for P22 tailspike polypeptide chains. Protein Sci. 1995, 4, 900–908. [Google Scholar] [CrossRef]
- Speed, M.A.; Wang, D.I.; King, J. Specific aggregation of partially folded polypeptide chains: The molecular basis of inclusion body composition. Nat. Biotechnol. 1996, 14, 1283–1287. [Google Scholar] [CrossRef]
- Santner, A.A.; Croy, C.H.; Vasanwala, F.H.; Uversky, V.N.; Van, Y.Y.; Dunker, A.K. Sweeping away protein aggregation with entropic bristles: Intrinsically disordered protein fusions enhance soluble expression. Biochemistry 2012, 51, 7250–7262. [Google Scholar] [CrossRef]
- Jamecna, D.; Polidori, J.; Mesmin, B.; Dezi, M.; Levy, D.; Bigay, J.; Antonny, B. An Intrinsically Disordered Region in OSBP Acts as an Entropic Barrier to Control Protein Dynamics and Orientation at Membrane Contact Sites. Dev. Cell 2019, 49, 220–234.e8. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Oksman, A.; Pal, P.; Lindquist, S.; Goldberg, D.E. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat. Commun. 2012, 3, 1310. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Goldberg, D.E. Asparagine repeats in Plasmodium falciparum proteins: Good for nothing? PLoS Pathog. 2013, 9, e1003488. [Google Scholar] [CrossRef] [PubMed]
- Malinovska, L.; Palm, S.; Gibson, K.; Verbavatz, J.M.; Alberti, S. Dictyostelium discoideum has a highly Q/N-rich proteome and shows an unusual resilience to protein aggregation. Proc. Natl. Acad. Sci. USA 2015, 112, E2620–E2629. [Google Scholar] [CrossRef]
- Dal Peraro, M.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef]
- Jiang, J.; Pentelute, B.L.; Collier, R.J.; Zhou, Z.H. Atomic structure of anthrax protective antigen pore elucidates toxin translocation. Nature 2015, 521, 545–549. [Google Scholar] [CrossRef]
- Tribensky, A.; Graf, A.W.; Diehl, M.; Fleck, W.; Przyborski, J.M. Trafficking of PfExp1 to the parasitophorous vacuolar membrane of Plasmodium falciparum is independent of protein folding and the PTEX translocon. Cell Microbiol. 2017, 19. [Google Scholar] [CrossRef][Green Version]
- Van den Berg, B.; Clemons, W.M., Jr.; Collinson, I.; Modis, Y.; Hartmann, E.; Harrison, S.C.; Rapoport, T.A. X-ray structure of a protein-conducting channel. Nature 2004, 427, 36–44. [Google Scholar] [CrossRef]
- Egea, P.F.; Stroud, R.M. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc. Natl. Acad. Sci. USA 2010, 107, 17182–17187. [Google Scholar] [CrossRef]
- Li, W.; Schulman, S.; Boyd, D.; Erlandson, K.; Beckwith, J.; Rapoport, T.A. The plug domain of the SecY protein stabilizes the closed state of the translocation channel and maintains a membrane seal. Mol. Cell 2007, 26, 511–521. [Google Scholar] [CrossRef]
- Park, E.; Rapoport, T.A. Preserving the membrane barrier for small molecules during bacterial protein translocation. Nature 2011, 473, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, T.A.; Li, L.; Park, E. Structural and Mechanistic Insights into Protein Translocation. Annu. Rev. Cell Dev. Biol. 2017, 33, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Krantz, B.A.; Finkelstein, A.; Collier, R.J. Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J. Mol. Biol. 2006, 355, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Tsukazaki, T. Structure-based working model of SecDF, a proton-driven bacterial protein translocation factor. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Tsukazaki, T. Structural Basis of the Sec Translocon and YidC Revealed Through X-ray Crystallography. Protein J. 2019, 38, 249–261. [Google Scholar] [CrossRef]
- Duran, E.C.; Weaver, C.L.; Lucius, A.L. Comparative Analysis of the Structure and Function of AAA+ Motors ClpA, ClpB, and Hsp104: Common Threads and Disparate Functions. Front. Mol. Biosci. 2017, 4, 54. [Google Scholar] [CrossRef]
- Schirmer, E.C.; Glover, J.R.; Singer, M.A.; Lindquist, S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996, 21, 289–296. [Google Scholar] [CrossRef]
- El Bakkouri, M.; Pow, A.; Mulichak, A.; Cheung, K.L.; Artz, J.D.; Amani, M.; Fell, S.; de Koning-Ward, T.F.; Goodman, C.D.; McFadden, G.I.; et al. The Clp chaperones and proteases of the human malaria parasite Plasmodium falciparum. J. Mol. Biol. 2010, 404, 456–477. [Google Scholar] [CrossRef]
- AhYoung, A.P.; Koehl, A.; Cascio, D.; Egea, P.F. Structural mapping of the ClpB ATPases of Plasmodium falciparum: Targeting protein folding and secretion for antimalarial drug design. Protein Sci. 2015, 24, 1508–1520. [Google Scholar] [CrossRef]
- Remaut, H.; Waksman, G. Protein-protein interaction through beta-strand addition. Trends Biochem. Sci. 2006, 31, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.M.; Potts, J.R. The tandem beta-zipper: Modular binding of tandem domains and linear motifs. FEBS Lett. 2013, 587, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Olson, A.J. Structural symmetry and protein function. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 105–153. [Google Scholar] [CrossRef]
- Beuron, F.; Maurizi, M.R.; Belnap, D.M.; Kocsis, E.; Booy, F.P.; Kessel, M.; Steven, A.C. At sixes and sevens: Characterization of the symmetry mismatch of the ClpAP chaperone-assisted protease. J. Struct. Biol. 1998, 123, 248–259. [Google Scholar] [CrossRef]
- Gates, S.N.; Martin, A. Stairway to Translocation: AAA+ motor structures reveal the mechanisms of ATP-dependent substrate translocation. Protein Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Puchades, C.; Sandate, C.R.; Lander, G.C. The molecular principles governing the activity and functional diversity of AAA+ proteins. Nat. Rev. Mol. Cell Biol. 2020, 21, 43–58. [Google Scholar] [CrossRef]
- Gehde, N.; Hinrichs, C.; Montilla, I.; Charpian, S.; Lingelbach, K.; Przyborski, J.M. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol. Microbiol. 2009, 71, 613–628. [Google Scholar] [CrossRef]
- Mesen-Ramirez, P.; Reinsch, F.; Blancke Soares, A.; Bergmann, B.; Ullrich, A.K.; Tenzer, S.; Spielmann, T. Stable Translocation Intermediates Jam Global Protein Export in Plasmodium falciparum Parasites and Link the PTEX Component EXP2 with Translocation Activity. PLoS Pathog. 2016, 12, e1005618. [Google Scholar] [CrossRef]
- Matthews, K.M.; Kalanon, M.; de Koning-Ward, T.F. Uncoupling the Threading and Unfoldase Actions of Plasmodium HSP101 Reveals Differences in Export between Soluble and Insoluble Proteins. mBio 2019, 10. [Google Scholar] [CrossRef]
- Zeth, K.; Ravelli, R.B.; Paal, K.; Cusack, S.; Bukau, B.; Dougan, D.A. Structural analysis of the adaptor protein ClpS in complex with the N-terminal domain of ClpA. Nat. Struct. Biol. 2002, 9, 906–911. [Google Scholar] [CrossRef]
- AhYoung, A.P.; Koehl, A.; Vizcarra, C.L.; Cascio, D.; Egea, P.F. Structure of a putative ClpS N-end rule adaptor protein from the malaria pathogen Plasmodium falciparum. Protein Sci. 2015. [Google Scholar] [CrossRef]
- Wang, F.; Mei, Z.; Qi, Y.; Yan, C.; Hu, Q.; Wang, J.; Shi, Y. Structure and mechanism of the hexameric MecA-ClpC molecular machine. Nature 2011, 471, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.Y.; Houry, W.A. ClpP: A distinctive family of cylindrical energy-dependent serine proteases. FEBS Lett. 2007, 581, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Trentini, D.B.; Suskiewicz, M.J.; Heuck, A.; Kurzbauer, R.; Deszcz, L.; Mechtler, K.; Clausen, T. Arginine phosphorylation marks proteins for degradation by a Clp protease. Nature 2016, 539, 48–53. [Google Scholar] [CrossRef]
- Rosenzweig, R.; Farber, P.; Velyvis, A.; Rennella, E.; Latham, M.P.; Kay, L.E. ClpB N-terminal domain plays a regulatory role in protein disaggregation. Proc. Natl. Acad. Sci. USA 2015, 112, E6872–E6881. [Google Scholar] [CrossRef]
- Rizo, A.N.; Lin, J.; Gates, S.N.; Tse, E.; Bart, S.M.; Castellano, L.M.; DiMaio, F.; Shorter, J.; Southworth, D.R. Structural basis for substrate gripping and translocation by the ClpB AAA+ disaggregase. Nat. Commun. 2019, 10, 2393. [Google Scholar] [CrossRef]
- Yokom, A.L.; Gates, S.N.; Jackrel, M.E.; Mack, K.L.; Su, M.; Shorter, J.; Southworth, D.R. Spiral architecture of the Hsp104 disaggregase reveals the basis for polypeptide translocation. Nat. Struct. Mol. Biol. 2016, 23, 830–837. [Google Scholar] [CrossRef]
- Gates, S.N.; Yokom, A.L.; Lin, J.; Jackrel, M.E.; Rizo, A.N.; Kendsersky, N.M.; Buell, C.E.; Sweeny, E.A.; Mack, K.L.; Chuang, E.; et al. Ratchet-like polypeptide translocation mechanism of the AAA+ disaggregase Hsp104. Science 2017, 357, 273–279. [Google Scholar] [CrossRef]
- Yu, H.; Lupoli, T.J.; Kovach, A.; Meng, X.; Zhao, G.; Nathan, C.F.; Li, H. ATP hydrolysis-coupled peptide translocation mechanism of Mycobacterium tuberculosis ClpB. Proc. Natl. Acad. Sci. USA 2018, 115, E9560–E9569. [Google Scholar] [CrossRef]
- Elsworth, B.; Sanders, P.R.; Nebl, T.; Batinovic, S.; Kalanon, M.; Nie, C.Q.; Charnaud, S.C.; Bullen, H.E.; de Koning Ward, T.F.; Tilley, L.; et al. Proteomic analysis reveals novel proteins associated with the Plasmodium protein exporter PTEX and a loss of complex stability upon truncation of the core PTEX component, PTEX150. Cell Microbiol. 2016, 18, 1551–1569. [Google Scholar] [CrossRef]
- Chisholm, S.A.; Kalanon, M.; Nebl, T.; Sanders, P.R.; Matthews, K.M.; Dickerman, B.K.; Gilson, P.R.; de Koning-Ward, T.F. The malaria PTEX component PTEX88 interacts most closely with HSP101 at the host-parasite interface. FEBS J. 2018, 285, 2037–2055. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Kalanon, M.; Chisholm, S.A.; Sturm, A.; Goodman, C.D.; Dixon, M.W.; Sanders, P.R.; Nebl, T.; Fraser, F.; Haase, S.; et al. The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol. Microbiol. 2013, 89, 1167–1186. [Google Scholar] [CrossRef]
- Matz, J.M.; Ingmundson, A.; Costa Nunes, J.; Stenzel, W.; Matuschewski, K.; Kooij, T.W. In Vivo Function of PTEX88 in Malaria Parasite Sequestration and Virulence. Eukaryot. Cell 2015, 14, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.A.; McHugh, E.; Lundie, R.; Dixon, M.W.; Ghosh, S.; O’Keefe, M.; Tilley, L.; Kalanon, M.; de Koning-Ward, T.F. Contrasting Inducible Knockdown of the Auxiliary PTEX Component PTEX88 in P. falciparum and P. berghei Unmasks a Role in Parasite Virulence. PLoS ONE 2016, 11, e0149296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dixit, S. Structural insights into thioredoxin-2: A component of malaria parasite protein secretion machinery. Sci. Rep. 2011, 1, 179. [Google Scholar] [CrossRef]
- Peng, M.; Cascio, D.; Egea, P.F. Crystal structure and solution characterization of the thioredoxin-2 from Plasmodium falciparum, a constituent of an essential parasitic protein export complex. Biochem. Biophys. Res. Commun. 2015, 456, 403–409. [Google Scholar] [CrossRef]
- Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 1985, 54, 237–271. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Fernandes, P.A.; Ramos, M.J. Similarities and differences in the thioredoxin superfamily. Prog. Biophys. Mol. Biol. 2006, 91, 229–248. [Google Scholar] [CrossRef]
- Batinovic, S.; McHugh, E.; Chisholm, S.A.; Matthews, K.; Liu, B.; Dumont, L.; Charnaud, S.C.; Schneider, M.P.; Gilson, P.R.; de Koning-Ward, T.F.; et al. An exported protein-interacting complex involved in the trafficking of virulence determinants in Plasmodium-infected erythrocytes. Nat. Commun. 2017, 8, 16044. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. Protein Structure and Function Prediction Using I-TASSER. Curr. Protoc. Bioinform. 2015, 52, 1–15. [Google Scholar] [CrossRef]
- Chen, C.K.; Chan, N.L.; Wang, A.H. The many blades of the beta-propeller proteins: Conserved but versatile. Trends Biochem. Sci. 2011, 36, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Haldar, K. Protein trafficking in apicomplexan parasites: Crossing the vacuolar Rubicon. Curr. Opin. Microbiol. 2016, 32, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nasamu, A.S.; Polino, A.J.; Istvan, E.S.; Goldberg, D.E. Malaria parasite plasmepsins: More than just plain old degradative pepsins. J. Biol. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hodder, A.N.; Sleebs, B.E.; Czabotar, P.E.; Gazdik, M.; Xu, Y.; O’Neill, M.T.; Lopaticki, S.; Nebl, T.; Triglia, T.; Smith, B.J.; et al. Structural basis for plasmepsin V inhibition that blocks export of malaria proteins to human erythrocytes. Nat. Struct. Mol. Biol. 2015, 22, 590–596. [Google Scholar] [CrossRef]
- Nguyen, W.; Hodder, A.N.; de Lezongard, R.B.; Czabotar, P.E.; Jarman, K.E.; O’Neill, M.T.; Thompson, J.K.; Jousset Sabroux, H.; Cowman, A.F.; Boddey, J.A.; et al. Enhanced antimalarial activity of plasmepsin V inhibitors by modification of the P2 position of PEXEL peptidomimetics. Eur. J. Med. Chem. 2018, 154, 182–198. [Google Scholar] [CrossRef]
- Heiber, A.; Kruse, F.; Pick, C.; Gruring, C.; Flemming, S.; Oberli, A.; Schoeler, H.; Retzlaff, S.; Mesen-Ramirez, P.; Hiss, J.A.; et al. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog. 2013, 9, e1003546. [Google Scholar] [CrossRef]
- Gruring, C.; Heiber, A.; Kruse, F.; Flemming, S.; Franci, G.; Colombo, S.F.; Fasana, E.; Schoeler, H.; Borgese, N.; Stunnenberg, H.G.; et al. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe 2012, 12, 717–729. [Google Scholar] [CrossRef]
- Haase, S.; Herrmann, S.; Gruring, C.; Heiber, A.; Jansen, P.W.; Langer, C.; Treeck, M.; Cabrera, A.; Bruns, C.; Struck, N.S.; et al. Sequence requirements for the export of the Plasmodium falciparum Maurer’s clefts protein REX2. Mol. Microbiol. 2009, 71, 1003–1017. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Luisa Hiller, N.; Haldar, K.; Knoll, L.J. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic 2013, 14, 519–531. [Google Scholar] [CrossRef]
- Coffey, M.J.; Jennison, C.; Tonkin, C.J.; Boddey, J.A. Role of the ER and Golgi in protein export by Apicomplexa. Curr. Opin. Cell Biol. 2016, 41, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Curt-Varesano, A.; Braun, L.; Ranquet, C.; Hakimi, M.A.; Bougdour, A. The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell Microbiol. 2016, 18, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.M.; Wickner, S. Hsp104 and ClpB: Protein disaggregating machines. Trends Biochem. Sci. 2009, 34, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Przyborski, J.M.; Diehl, M.; Blatch, G.L. Plasmodial HSP70s are functionally adapted to the malaria parasite life cycle. Front. Mol. Biosci. 2015, 2, 34. [Google Scholar] [CrossRef]
- Mabate, B.; Zininga, T.; Ramatsui, L.; Makumire, S.; Achilonu, I.; Dirr, H.W.; Shonhai, A. Structural and biochemical characterization of Plasmodium falciparum Hsp70-x reveals functional versatility of its C-terminal EEVN motif. Proteins 2018, 86, 1189–1201. [Google Scholar] [CrossRef]
- Franco, M.; Panas, M.W.; Marino, N.D.; Lee, M.C.; Buchholz, K.R.; Kelly, F.D.; Bednarski, J.J.; Sleckman, B.P.; Pourmand, N.; Boothroyd, J.C. A Novel Secreted Protein, MYR1, Is Central to Toxoplasma’s Manipulation of Host Cells. mBio 2016, 7, e02231-15. [Google Scholar] [CrossRef]
- Cao, S.; Du, N.; Chen, H.; Pang, Y.; Zhang, Z.; Zheng, J.; Jia, H. Toxoplasma gondii Clp family protein: TgClpB1 plays a crucial role in thermotolerance. Oncotarget 2017, 8, 86117–86129. [Google Scholar] [CrossRef]
- Panas, M.W.; Ferrel, A.; Naor, A.; Tenborg, E.; Lorenzi, H.A.; Boothroyd, J.C. Translocation of Dense Granule Effectors across the Parasitophorous Vacuole Membrane in Toxoplasma-Infected Cells Requires the Activity of ROP17, a Rhoptry Protein Kinase. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Cygan, A.M.; Theisen, T.C.; Mendoza, A.G.; Marino, N.D.; Panas, M.W.; Boothroyd, J.C. Coimmunoprecipitation with MYR1 Identifies Three Additional Proteins within the Toxoplasma gondii Parasitophorous Vacuole Required for Translocation of Dense Granule Effectors into Host Cells. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Coffey, M.J.; Dagley, L.F.; Seizova, S.; Kapp, E.A.; Infusini, G.; Roos, D.S.; Boddey, J.A.; Webb, A.I.; Tonkin, C.J. Aspartyl Protease 5 Matures Dense Granule Proteins That Reside at the Host-Parasite Interface in Toxoplasma gondii. mBio 2018, 9. [Google Scholar] [CrossRef]
- Laurens, M.B. The Promise of a Malaria Vaccine-Are We Closer? Annu. Rev. Microbiol. 2018, 72, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Hammond, A.M.; Galizi, R. Gene drives to fight malaria: Current state and future directions. Pathog. Glob. Health 2017, 111, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.R.; Chisholm, S.A.; Crabb, B.S.; de Koning-Ward, T.F. Host cell remodelling in malaria parasites: A new pool of potential drug targets. Int. J. Parasitol. 2017, 47, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bedi, R.K.; Patel, C.; Mishra, V.; Xiao, H.; Yada, R.Y.; Bhaumik, P. Understanding the structural basis of substrate recognition by Plasmodium falciparum plasmepsin V to aid in the design of potent inhibitors. Sci. Rep. 2016, 6, 31420. [Google Scholar] [CrossRef]
- Firestone, A.J.; Weinger, J.S.; Maldonado, M.; Barlan, K.; Langston, L.D.; O’Donnell, M.; Gelfand, V.I.; Kapoor, T.M.; Chen, J.K. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 2012, 484, 125–129. [Google Scholar] [CrossRef]
- Chou, T.F.; Brown, S.J.; Minond, D.; Nordin, B.E.; Li, K.; Jones, A.C.; Chase, P.; Porubsky, P.R.; Stoltz, B.M.; Schoenen, F.J.; et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 4834–4839. [Google Scholar] [CrossRef]
- Kehr, S.; Sturm, N.; Rahlfs, S.; Przyborski, J.M.; Becker, K. Compartmentation of redox metabolism in malaria parasites. PLoS Pathog. 2010, 6, e1001242. [Google Scholar] [CrossRef]
- Garrison, J.L.; Kunkel, E.J.; Hegde, R.S.; Taunton, J. A substrate-specific inhibitor of protein translocation into the endoplasmic reticulum. Nature 2005, 436, 285–289. [Google Scholar] [CrossRef]
- Mackinnon, A.L.; Paavilainen, V.O.; Sharma, A.; Hegde, R.S.; Taunton, J. An allosteric Sec61 inhibitor traps nascent transmembrane helices at the lateral gate. eLife 2014, 3, e01483. [Google Scholar] [CrossRef]
- Junne, T.; Wong, J.; Studer, C.; Aust, T.; Bauer, B.W.; Beibel, M.; Bhullar, B.; Bruccoleri, R.; Eichenberger, J.; Estoppey, D.; et al. Decatransin, a new natural product inhibiting protein translocation at the Sec61/SecYEG translocon. J. Cell Sci. 2015, 128, 1217–1229. [Google Scholar] [CrossRef]
- Paatero, A.O.; Kellosalo, J.; Dunyak, B.M.; Almaliti, J.; Gestwicki, J.E.; Gerwick, W.H.; Taunton, J.; Paavilainen, V.O. Apratoxin Kills Cells by Direct Blockade of the Sec61 Protein Translocation Channel. Cell Chem. Biol. 2016, 23, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]





| PTEX subunit | HSP101 | PTEX150 | EXP2 | TRX2 | PTEX88 |
|---|---|---|---|---|---|
| PDB ID | 6E10 | 6E10 | 6E10 | NA | |
| 6E11 | 6E11 | 6E11 | |||
| 4IOD | 3UL3 | ||||
| 4IRF | 4O32 |
| PTEX subunit | HSP101 | PTEX150 | EXP2 | TRX2 | PTEX88 |
|---|---|---|---|---|---|
| Number of Residues | 906 | 993 | 287 | 157 | 777 |
| % composition a | |||||
| % Asn | 6 | 17 | 5 | 7 | 13 |
| % Asp | 5 | 13 | 12 | 6 | 6 |
| % Glu | 8 | 10 | 8 | 2 | 5 |
| % Lys | 12 | 10 | 11 | 13 | 10 |
| structural coverage b | 100% | 20% | 78% | 65–75% | NA |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egea, P.F. Crossing the Vacuolar Rubicon: Structural Insights into Effector Protein Trafficking in Apicomplexan Parasites. Microorganisms 2020, 8, 865. https://doi.org/10.3390/microorganisms8060865
Egea PF. Crossing the Vacuolar Rubicon: Structural Insights into Effector Protein Trafficking in Apicomplexan Parasites. Microorganisms. 2020; 8(6):865. https://doi.org/10.3390/microorganisms8060865
Chicago/Turabian StyleEgea, Pascal F. 2020. "Crossing the Vacuolar Rubicon: Structural Insights into Effector Protein Trafficking in Apicomplexan Parasites" Microorganisms 8, no. 6: 865. https://doi.org/10.3390/microorganisms8060865
APA StyleEgea, P. F. (2020). Crossing the Vacuolar Rubicon: Structural Insights into Effector Protein Trafficking in Apicomplexan Parasites. Microorganisms, 8(6), 865. https://doi.org/10.3390/microorganisms8060865





