Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Collection and Data Adjustment
3. Results
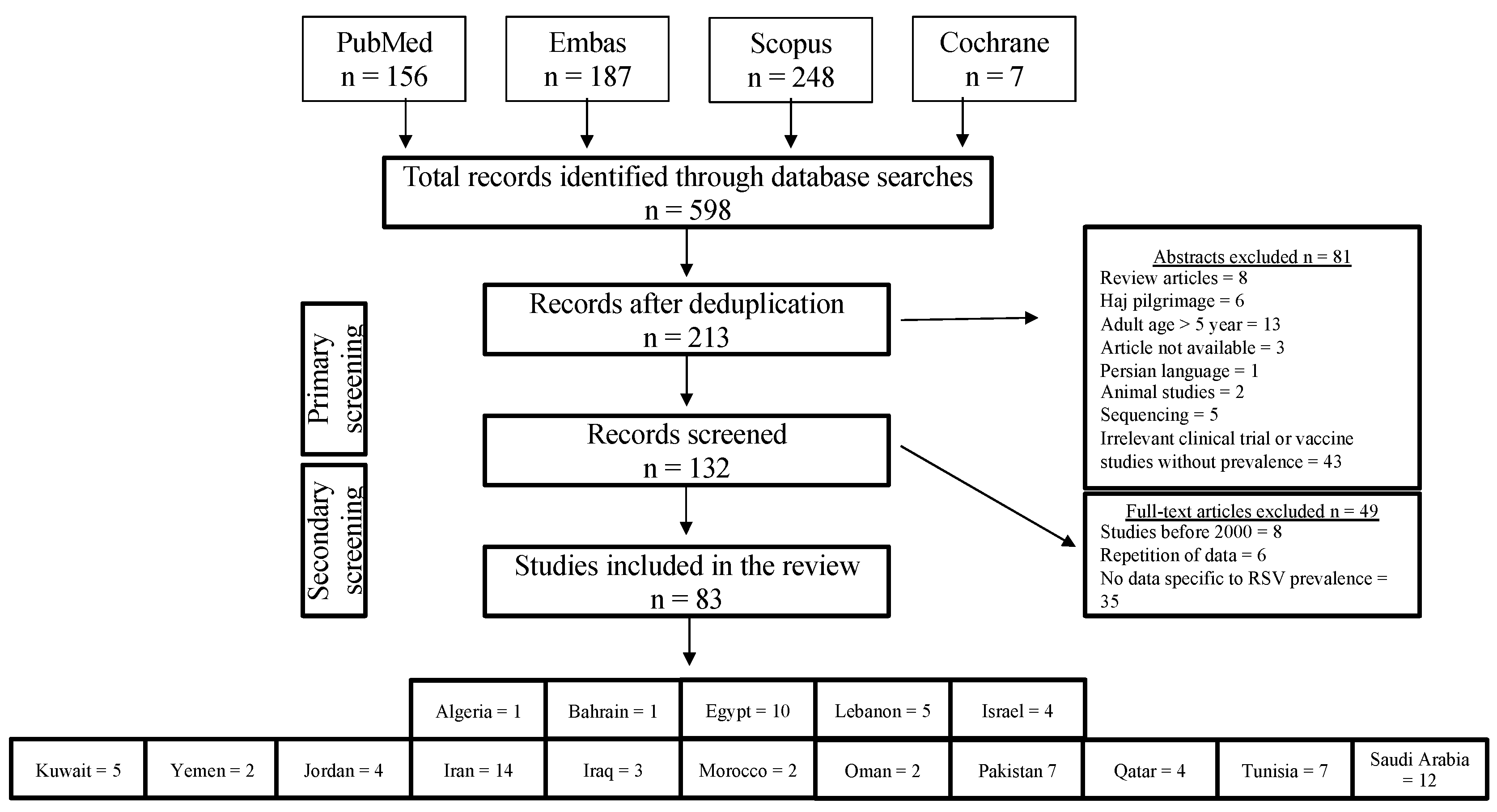
3.1. Literature Search and Selection Process
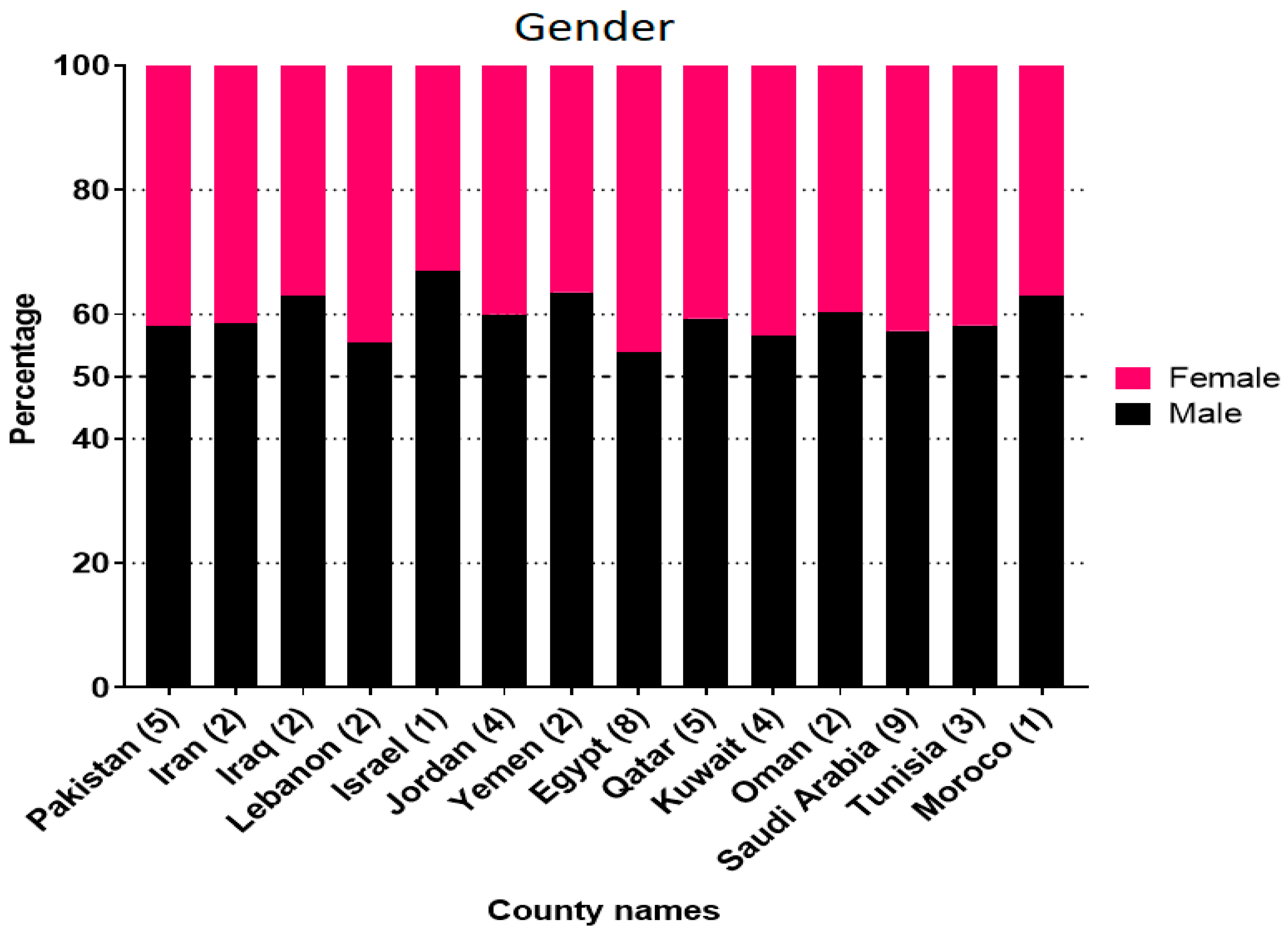
3.2. RSV Prevalence and Population Demography
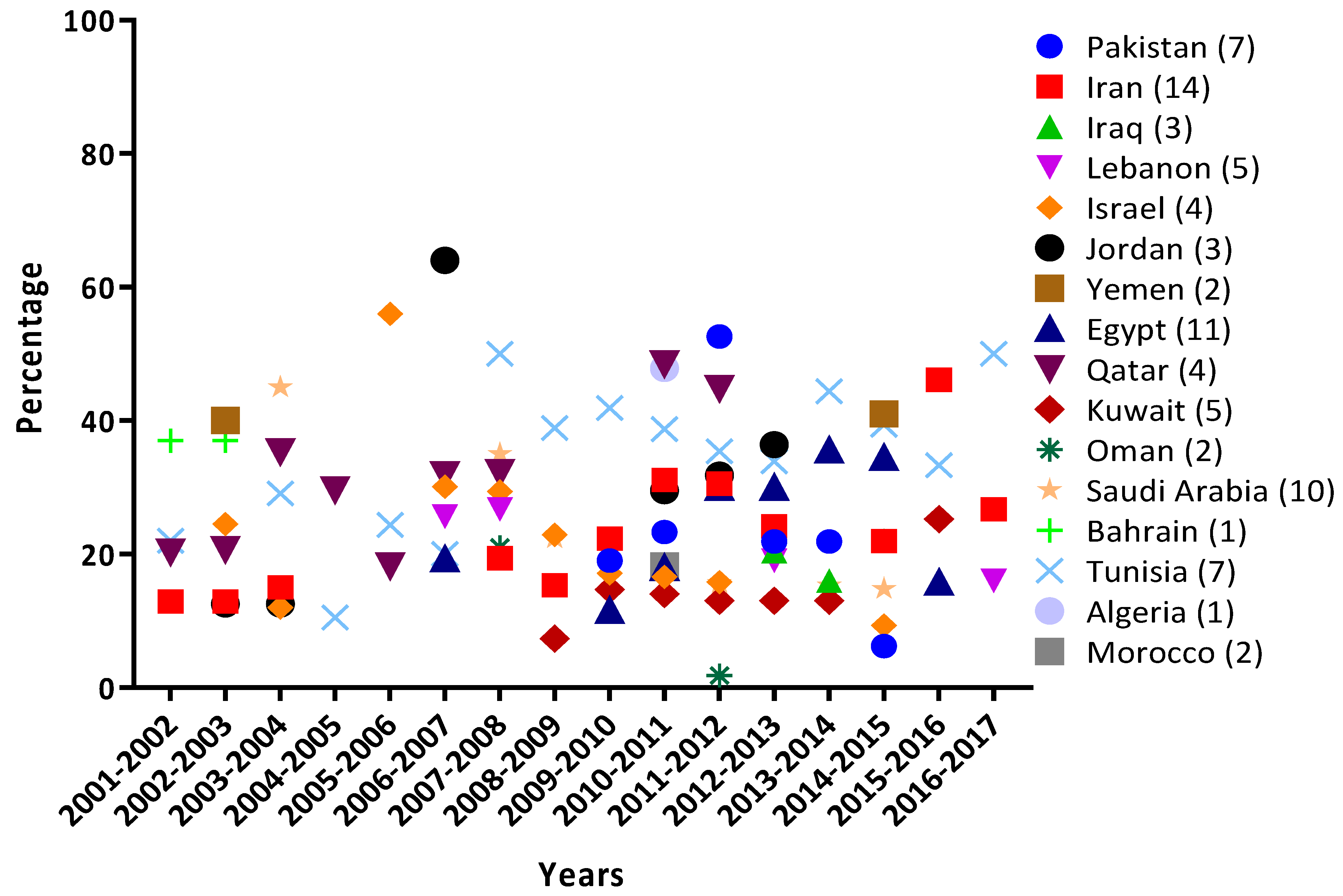
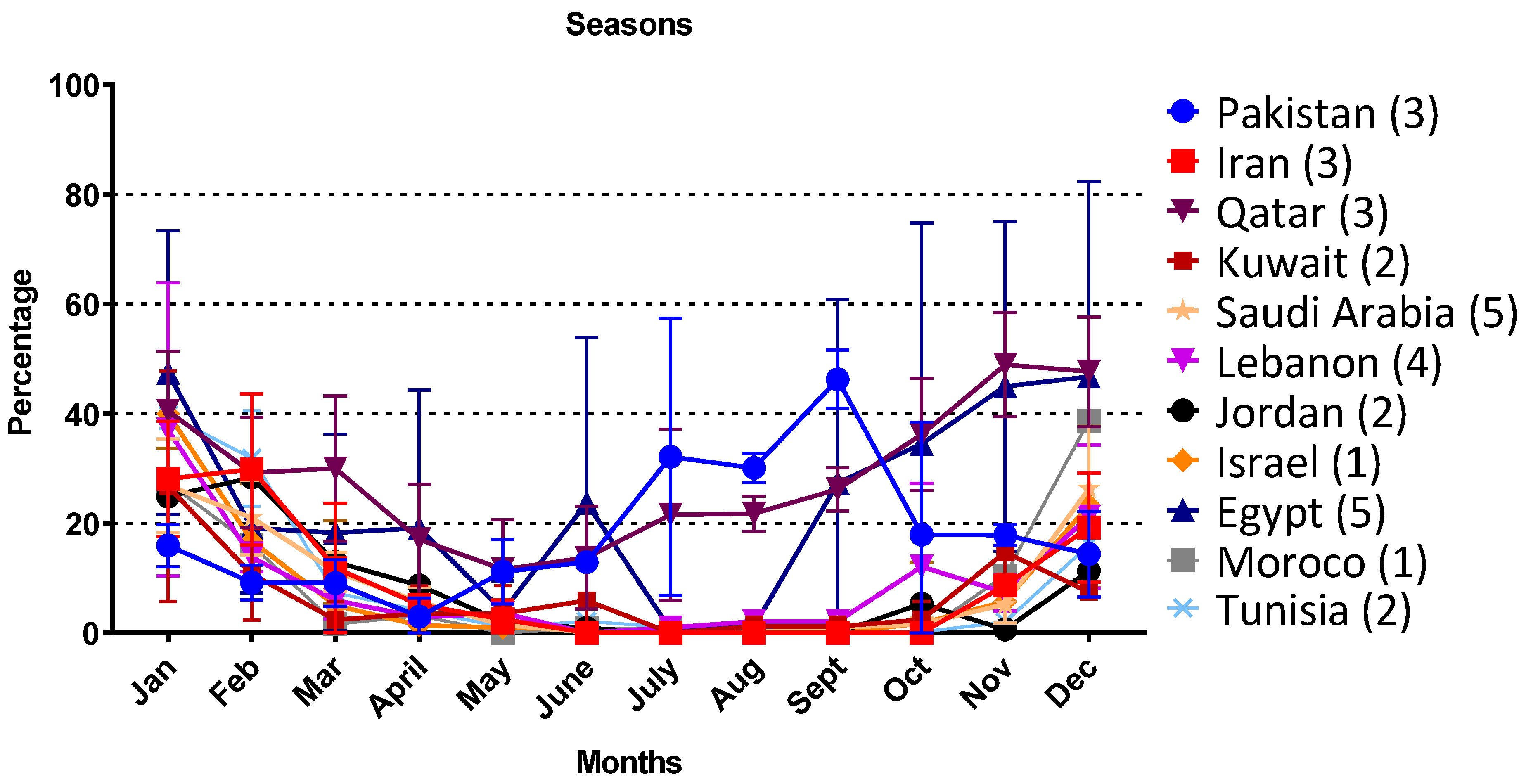
3.3. RSV Monthly Prevalence in the MENA Region
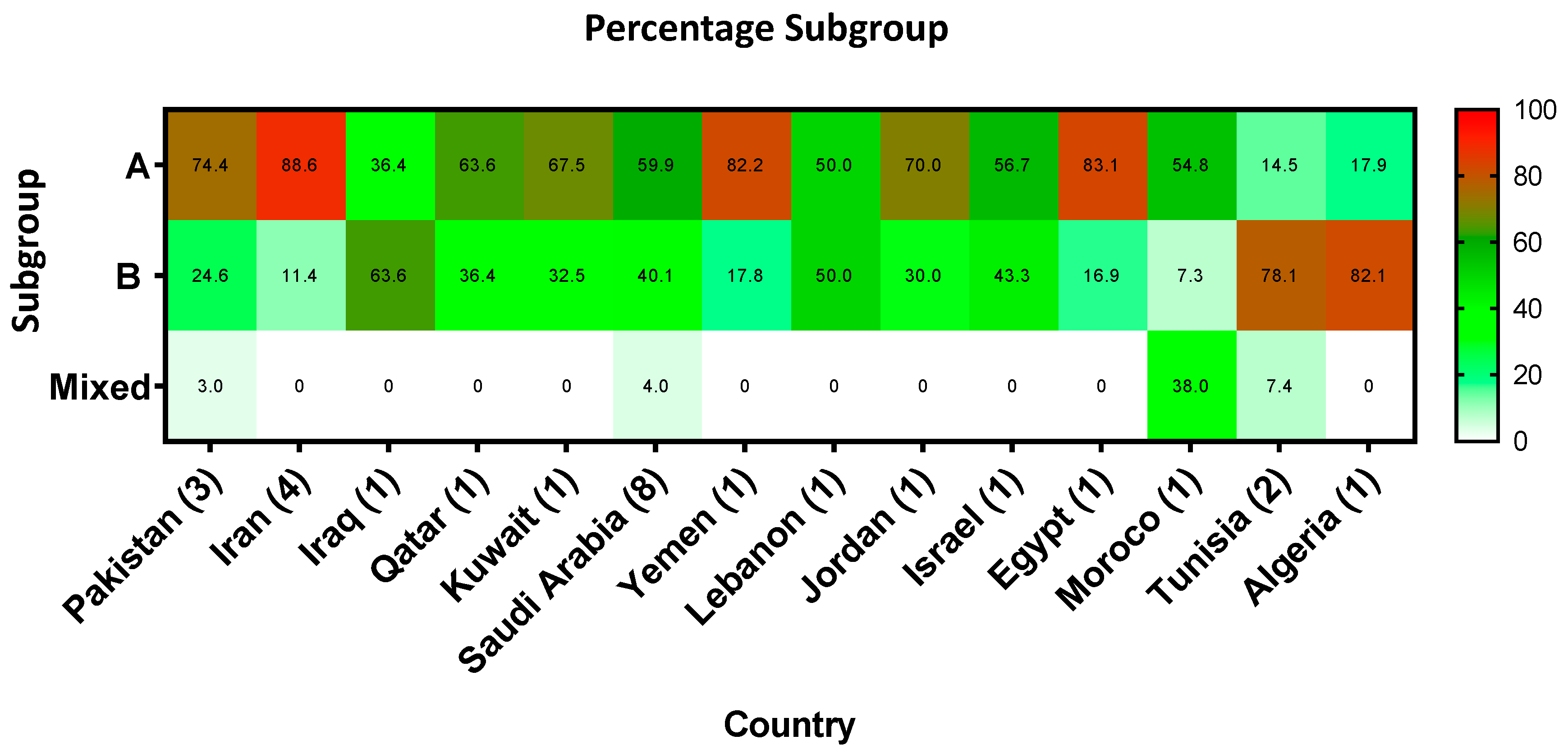
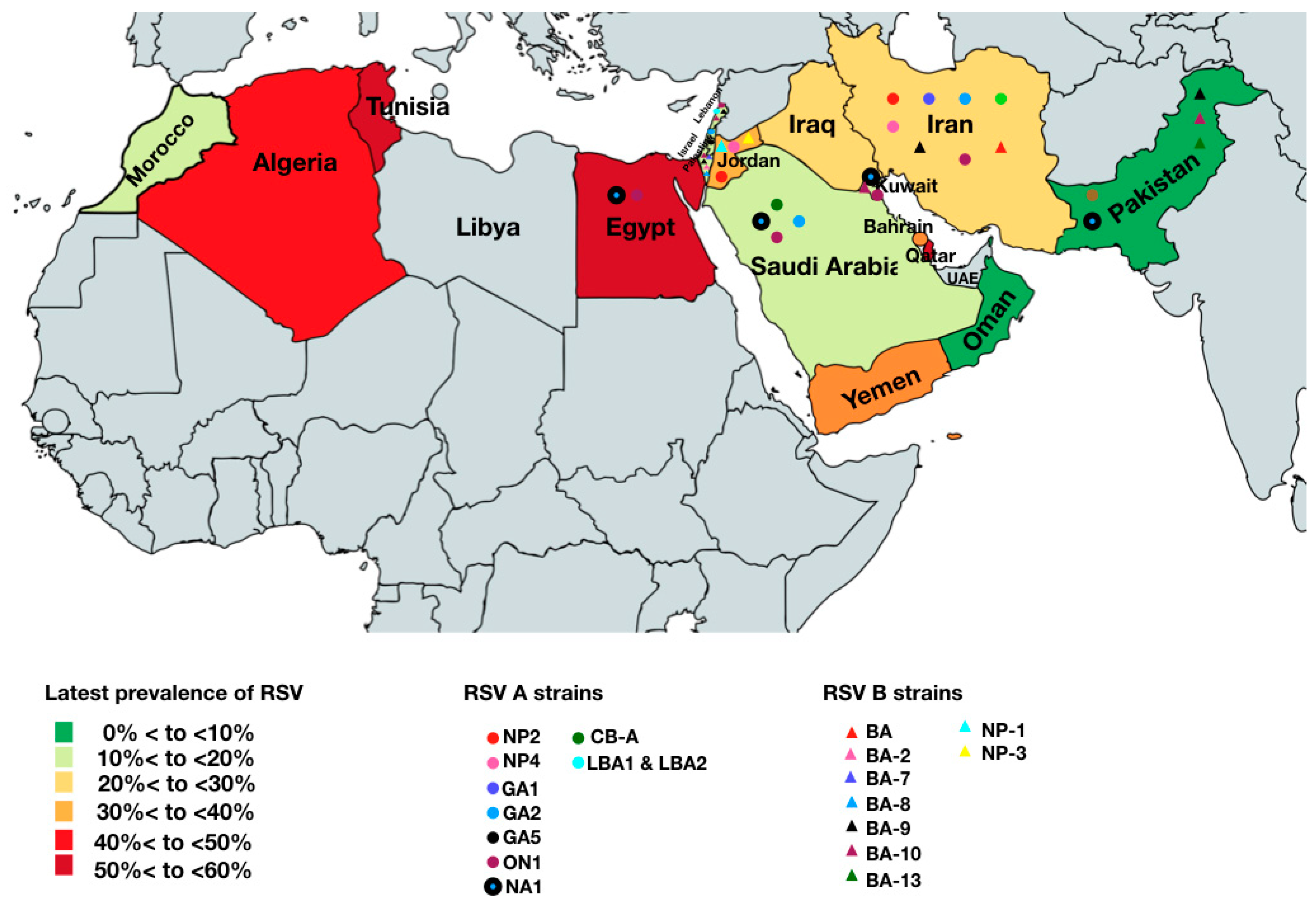
3.4. RSV Subgroups and Strains Circulating in the MENA Region
4. Discussion
4.1. The prevalence of RSV in the MENA Region
4.2. Seasonal Distribution of RSV in the MENA Region
4.3. RSV Subgroup and Strains Circulating in the MENA Region
4.4. Coinfection RSV in the MENA Region
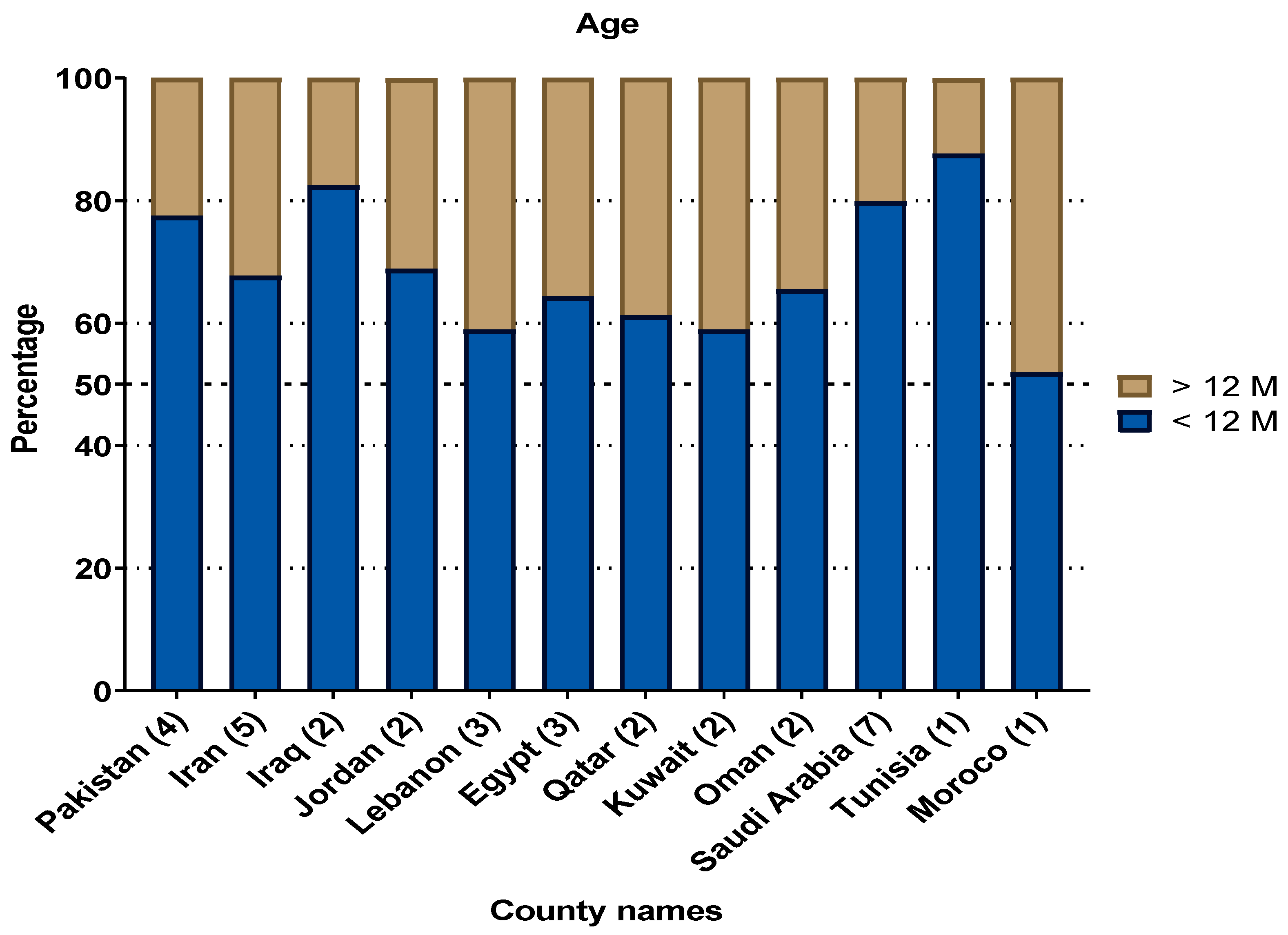
4.5. Age Distribution of RSV Infections in the MENA Region
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Country | Study Period | Age Yrs | Sample Size | RSV Positive | Male | Female | Symptoms | Detection Method | Subgroup A | Genotype | Subgroup B | Genotype | Coinfection | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iran | 2001–2003 | <5 | 202 | 26 | NR | NR | Wheezing, cough, fever | RT-PCR | NR | NR | NR | NR | NR | [122] |
| 2003–2004 | <2 | 261 | 39 | 59% | 41% | Cough, dyspnea, sneezing, the runny nose and fever | RT-PCR | 38 (97.4%) | NP4, NP2 | 1 (2.6%) | NR | 3 (7.7%) | [77] | |
| 2009 | <2 | 107 | 24 | NR | NR | Wheezing, cough, fever | RT-PCR | 16 (66.6%) | GA1, GA2 | 8 (33.4%) | BA | NR | [57] | |
| 2008–2009 | <6 | 202 | 34 | NR | NR | Wheezing, cough, fever | multiplex RT-PCR | NR | NR | NR | NR | 11 (32%) | [116] | |
| 2008–2009 | <5 | 100 | 9 | 57% | 43% | Bronchiolitis, cough,coryza, fever, chest wall retraction, wheezing, cyanosis | RT-PCR | NR | NR | NR | NR | NR | [123] | |
| 2008–2010 | <4 | 180 | 40 | NR | NR | Cough, difficulty in breathing, tachypnea, retraction, crackles and wheezing on lung auscultation. | NR | NR | NR | NR | NR | NR | [124] | |
| 2007–2013 | <2 | 485 | 94 | 59% | 41% | ALRTI symptoms | RT-PCR | 85 (90.43%) | GA1, GA2, GA5 | 9 (9.57%) | BA | [22] | ||
| 2011–2013 | <5 | 280 | 84 | 55.9% | 44.1% | Bronchiolitis, wheezing, and cough | RT-PCR | NR | NR | NR | NR | 10 (11.9%) | [125] | |
| 2010–2013 | <5 | 158 | 49 | 44.8% | 55.2% | Fever and respiratory distress | RT-PCR | NR | NR | NR | NR | 0 | [126] | |
| 2012 | <5 | 232 | 40 | 67.5% | 32.5% | Tachypnea, chest retraction and wheezing | RT-PCR | NR | NR | NR | NR | NR | [127] | |
| 2014–2015 | <17 | 60 | 5 | NR | NR | Wheezing episodes | RT-PCR | NR | NR | NR | NR | [128] | ||
| 2014–2015 | <15 | 156 | 56 | 43.5% | 56.5% | ARI symptoms | RT-PCR | NR | NR | NR | NR | 4 (7.1%) | [129] | |
| 2015–2016 | <2 | 180 | 55 | 57.8% | 42.2% | Cough, dyspnea, sneezing, the runny nose and fever | RT-PCR | 55 (100%) | ON-1 | 0 | none | NR | [58] | |
| 2016–2017 | <3 | 75 | 20 | 40% | 60% | Fever, wheezing, coughing, hypoxia, dyspnea, and rhinorrhea | RT-PCR | NR | NR | NR | NR | 4 (20%) | [130] | |
| Lebanon | 2004–2014 | <5 | 319 | 194 | 56.2% | 43.8% | NR | NR | NR | NR | NR | NR | NR | [131] |
| 2008 | <6 | 120 | 32 | NR | NR | Rhinorrhoea and dyspnea | RT-PCR | NR | NR | NR | NR | NR | [132] | |
| 2008 | 39 | 10 | NR | NR | Fever ≥38 °C, cough, rhinorrhea, or sore throat | SYBR Green RT-PCR | NR | NR | NR | NR | NR | [133] | ||
| 2013–2014 | <16 | 236 | 45 | NR | NR | Fever, cough, runny nose, shortness of breath | RT-PCR | NR | NR | NR | NR | 10 (22.2%) | [134] | |
| 2016/17 | <3 | 519 | 83 | 54.2% | 45.8% | NR | NR | 27 (50%) | ON1, LBA1,LBA2 (novel) | 27 (50%) | BA9, BA10 | NR | [23] | |
| Israel | 2003–2004 | <5 | 613 | 76 | NR | NR | Bronchiolitis | RT-PCR analysis and sequencing | NR | NR | NR | NR | NR | [135] |
| 2005–2006 | <2 | 465 | 346 | NR | NR | NR | NR | NR | NR | NR | NR | NR | [136] | |
| 2005–2006 | 557 | 230 | NR | NR | Shortage of breath, fever, cough, rhinorrhea, dyspnea, lack of appetite, vomiting, diarrhea and sore throat | RT-PCR | 77 (78.6%) | GA5, GA2 | 21 (21.6%) | BA 2,7,8,9,10 | NR | [48] | ||
| 2006–2007 | 617 | 186 | 14 (17.1%) | 68 (82.9%) | NR | |||||||||
| 2007–2008 | 558 | 164 | 80 (83.6%) | 16 (16.7%) | NR | |||||||||
| 2008–2009 | 903 | 207 | 79 (38.0%) | 128 (62%) | NR | |||||||||
| 2009–2010 | 1403 | 240 | 139 (59.9%) | 95 (40.1) | NR | |||||||||
| 2010–2011 | 4151 | 691 | 324 (67.1%) | 159 (32.9%) | NR | |||||||||
| 2011–2012 | 2829 | 448 | 143 (46.1%) | 167 (53.9%) | NR | |||||||||
| 2015–2016 | 0-70 | 1910 | 178 | NR | NR | fever, cough and dyspnea | RT-PCR | NR | NR | NR | NR | NR | [137] | |
| Yemen | October 2002 to May 2003 | <2 | 604 | 266 | NR | NR | NR | RT-PCR | 171 (82%) | NR | 37 (18%) | NR | NR | [138] |
| 2014–2015 | NR | 1346 | 552 | NR | NR | ALRTI | NR | NR | NR | NR | NR | NR | [139] | |
| Bahrain | 2000–2003 | 235 | 88 | 60% | 40% | NR | NR | NR | NR | NR | NR | NR | [140] | |
| Saudi Arabia | 2003–2004 | <1 | 282 | 128 | 57.1% | 42.9% | Cough and tachypnea | Direct fluorescein-labeled monoclonal antibody assay. | NR | NR | NR | NR | NR | [141] |
| 2005–2010 | <17 | 643 | 295 | 49.5% | 50.5% | NR | Direct immunofluorescence assays | NR | NR | NR | NR | NR | [142] | |
| 2007–2008 | <3 | 200 | 70 | 54.3% | 45.7% | NR | Monospecific and duplex RT-PCR | 40 (57.1%) | NR | 30 (42.9%) | NR | NR | [143] | |
| 2008–2009 | <3 | 174 | 39 | 66.7% | 33.3% | Bronchitis and pneumonia | RT-PCR | 23 (58.6%) | NR | 16 (41.4%) | NR | 8 (20.5%) | [144] | |
| 2007–2009 | <3 | 175 | 39 | NR | NR | NR | multiplex RT-PC | 23 (59%) | GA2, NA-1, CB-A | 16 (41%) | NR | NR | [73] | |
| 2011 | <1 | 2154 | 338 | 69.2 | 30.8 | NR | IMAGEN immunofluorescence test | NR | NR | NR | NR | 7 (21.2%) | [145] | |
| 2012–2013 | <5 | 135 | 33 | 69.7 | 30.3 | Rhinitis, pharyngitis, cough, earache, hoarseness of voice, rhonchi, crepitations, or wheezy chest | multiplex RT-PCR | 30 (90.9%) | NR | 3 (9.1%) | NR | NR | [146] | |
| 2012–2013 | <13 | 2235 | 514 | NR | NR | NR | Seeplex RV15 kit | 381 (74.4%) | NR | 131 (25.6%) | NR | NR | [147] | |
| November 2013 and January 2014 | All ages | 182 | 12 | 72% | 28% | NR | RT-PCR and multiplex microarray | 3 (3.4%) | NR | 9 (10.2%) | NR | 4 (33.3%) | [120] | |
| 2013–2014 | <14 | 4611 | 1086 | 54.8 | 45.2 | ARTI symptoms | Immunofluorescence assays | NR | NR | NR | NR | NR | [121] | |
| 2014 | <5 | 130 | 34 | NR | NR | NR | RT-PCR | 27 (77%) | NA1, ON1, | 8 (23%) | BA9 | NR | [69] | |
| 2014–2015 | 0 to 14 years | 2266 | 336 | NR | NR | NR | Anyplex II RV16 detection kit | 124 (37%) | NR | 212 (63%) | NR | RSV A: 32 (3.7) RSV B: 75(8.7%) | [83] | |
| Iraq | 2012–2013 | <15 | 269 | 55 | NR | NR | Fever of ≥38 °C on admission and with clinical signs and symptoms of an upper and/or lower respiratory tract infection | xTAG Respiratory Virus Panel Fast assay | NR | NR | NR | NR | 18 (32.7) | [148] |
| 2013 | 1-15 | 80 | 30 | NR | NR | NR | RT-PCR and fluorescent assay | NR | NR | NR | NR | NR | [149] | |
| 2014–2015 | <10 | 250 | 22 | NR | NR | NR | RT-PCR | 8 (36.3%) | NR | 14 (63.7%) | NR | NR | [82] | |
| Egypt | 2006–2007 | <5 | 427 | 70 | NR | NR | Cough and difficult breathing or tachypnea | Immunofluorescent assay (IFA) | NR | NR | NR | NR | none | [74] |
| 2006–2007 | <5 | 450 | 107 | 57.4 | 41.2 | Cough, difficulty breathing, fever, chest indrawing, and rapid breathing | rt-RT-PCR | NR | NR | NR | NR | 22 (25.9%) | [150] | |
| 2009–2013 | >65 | 5768 | 669 | NR | NR | Cough, sore throght, tachypnea, sputum production, chest pain, dyspnea | rRT-PCR | NR | NR | NR | NR | NR | [151] | |
| 2010–2011 | 2-12 | 130 | 28 | 55.6 | 44.4 | cough, tachypnea, sputum, hemoptysis, chest pain, sore throat, and shortness of breath | PCR | NR | NR | NR | NR | none | [152] | |
| 2011–2014 | <1 | 153 | 69 | NR | NR | NR | RT-PCR | NR | NR | NR | NR | NR | [153] | |
| 2013–2014 | <2 | 127 | 59 | 47.5 | 452.5 | ALRTI symptoms, severe bronchiolitis or pneumonia, tachypnea, chest indrawing | PCR | 11 (18.6%) | NR | 46 (78%) | NR | Coinfection of type A and B in 2 patients (3.4%) | [84] | |
| 2010–2014 | All ages | 3207 | 485 | 47 | 53 | Fever, Cough | RT-PCR | NR | NR | NR | NR | 3 cases were positive for Mycoplasma and were coinfected with RSV, one case of Chlamydia was coinfected with RSV | [150] | |
| 2014–2015 | <5 | 223 | 77 | NR | NR | NR | RT-PCR | 64 (83.1%) | NA1, ON1, | 13 (16.8%) | NR | NR | [59] | |
| 2015–2016 | <5 | 120 | 12 | NR | NR | NR | multiplex PCR | NR | NR | NR | NR | 6(50%) | [154] | |
| 2016–2017 | <2 | 55 | 30 | 61.9 | 38.1 | Cough, tachypnea, wheezes and crackles on auscultation, and hyperinflation | PCR | NR | NR | NR | NR | 9 (30%) | [155] | |
| Qatar | 2010–2011 | 2 weeks- 2 years | 369 | 189 | 59.8 | 40.2 | Bronchiolitis,ever, rhinitis, tachypnoea, cough, wheezing, crackles | PCR | NR | NR | NR | NR | 58 (30.7%) | [156] |
| 2010–2012 | <3 | 770 | 304 | 59.5 | 40.5 | Bronchiolitis | RT-PCR | NR | NR | NR | NR | NR | [157] | |
| 2010–2012 | <2 | 769 | 352 | NR | NR | NR | Real-time PCR | NR | NR | NR | NR | NR | [158] | |
| 2002 | <2 | 241 | 50 | 20 | 21.3 | Respiratory distress | RT-PCR | NR | NR | NR | NR | NR | [109] | |
| 2003 | <2 | 680 | 240 | 32.8 | 38.9 | NR | NR | NR | NR | NR | ||||
| 2004 | <2 | 716 | 212 | 30.1 | 29 | NR | NR | NR | NR | NR | ||||
| 2005 | <2 | 674 | 123 | 17.5 | 19.3 | NR | NR | NR | NR | NR | ||||
| 2006 | <2 | 470 | 150 | 32.4 | 31.3 | NR | NR | NR | NR | NR | ||||
| 2007 | <2 | 340 | 45 | 13.5 | 12.9 | NR | NR | NR | NR | NR | ||||
| Oman | 2007–2008 | <5 | 259 | 56 | 66 | 34 | Runny nose, cough, sore throat, Earache, Fever, Wheezing, Tachypnoea, Chest indrawing | Multiplex PCR | NR | NR | NR | NR | NR | [159] |
| 2011–2012 | 2 months to 13 yrs | 373 | 7 | NR | NR | NR | PCR | NR | NR | NR | NR | 2 (5.9%) | [32] | |
| Kuwait | 2008–2010 | <76 | 1014 | 106 | NR | NR | Bronchiolitis, Croup, pneumonia | RT-PCR, confirmed with hybridization | NR | NR | NR | NR | NR | [160] |
| 2009 | <2 | 460 | 13 | NR | NR | NR | Real-time RT-PCR | NR | NR | NR | NR | 0 | [161] | |
| 2010–2013 | <76 | 735 | 42 | 59.5 | 40 | Throat (pharyngitis),nasopharynx (nasopharyngitis), sinuses (sinusitis), larynx (laryngitis) and trachea (tracheitis) | Multiplex PCR | NR | NR | NR | NR | NR | [162] | |
| 2010–2014 | <80 | 351 | 46 | NR | NR | NR | RT-PCR | NR | NR | NR | NR | 11 (23.9%) | [115] | |
| 2016 | 0-60 | 305 | 77 | 42.9 | 57.1 | NR | RT-PCR | 52 (67.5%) | NA1, ON1 | 25 (32.5%) | Twelve (55%) strains clustered with the BA10 and the rest (45%) were clustered into three groups of untyped strains that do not belong to any of the known group B genotypes | 17 (22%) | [67] | |
| Jordan | 2002–2004 | <2 | 200 | 25 | 72 | 28 | Bronchiolitis, hypoxemia, retractions, tachypnea | Immunofluorescence analysis | NR | NR | NR | NR | NR | [3] |
| 2003–2004 | <5 | 326 | 140 | NR | NR | NR | RT-PCR | 94 (70%) | NP2, NP4 | 41 (30%) | NP1, NP3 | 67 (48%) | [76] | |
| 2007 | <5 | 728 | 467 | 55 | 45 | Cough, poor appetite, trouble breathing, post-tussive emesis, and wheezing | RT-PCR | NR | NR | NR | NR | 126 (27%) | [163] | |
| 2010–2013 | <2 | 3168 | 1394 | 60 | 40 | ALRTI symptoms and fever | RT-PCR | NR | NR | NR | NR | 669 (48%) | [10] | |
| Tunisia | 2000–2002 | <5 | 815 | 176 | NR | NR | NR | RT-PCR | 7 (17.5%) | NR | 33 (82.5%) | NR | NR | [78] |
| 2000–2002 | <35 days | 268 | 62 | 58.7 | 41.3 | Cough, wheezing and dyspnoea, and cyanosis and apnoea | Direct immunofluorescence assay and RT PCR | 2 (13%) | NR | 13 (87%) | NR | NR | [79] | |
| 2005 | <1 | 81 | 81 | 56.8 | 43.2 | NR | RT-PCR | 9 (11.1%) | NR | 60 (74.1%) | NR | NR | [80] | |
| 2009–2010 | 368 | 157 | NR | NR | NR | Indirect immunofluorescence assay and PCR | NR | NR | NR | NR | NR | [163] | ||
| 2013–2014 | <5 | 372 | 123 | NR | NR | ALRTI symptoms by wheezing, tachypnea, and signs of respiratory distress such as nasal flaring, intercostal/subcostal retractions, and central cyanosis. | multiplex RT-PCR | NR | NR | NR | NR | NR | [164] | |
| 2013–2014 | <1 | 515 | 171 | NR | NR | NR | multiplex qRT-PCR | NR | NR | NR | NR | 73 (42.7%) | [165] | |
| 2003–2015 | <=5 | 5131 | 1769 | NR | NR | ALRTI symptoms including wheezing, tachypnea, and signs of respiratory distress such as nasal flaring, intercostal/subcostal retractions, and central cyanosis | Direct immunofluorescence assay | NR | NR | NR | NR | NR | [166] | |
| Algeria | 2010–2011 | <2 | 117 | 56 | NR | NR | Fever or hypothermia associated with at least one of the following symptoms: polypnoea, wheezing, abnormalities in pulmonary listening or friction | RT-PCR | 10 (17.9%) | NR | 46 (82.1%) | NR | 19 (33.9%) | [81] |
| Morocco | 2010–2011 | 2-59 months | 683 | 124 | 62.9 | 37.1 | Breathing difficulty, chest indrawing, | Real-time PCR | NR | NR | NR | NR | 54 (43.5%) | [167] |
| 2010–2011 | <5 | 700 | 126 | NR | NR | Cough, breathing difficulty and increased respiratory rate (RR) according to age and chest indrawing. | RT-PCR | NR | NR | NR | NR | NR | [168] | |
| Pakistan | 2009–2012 | <5 | 223 | 223 | NR | NR | Fever, cough, wheezing, poor appetite, shortness of breath, sore throat | Monoplex RT-PCR assay | NR | NR | NR | NR | NR | [169] |
| 2010–2011 | <5 | 797 | 236 | 54.2 | 45.8 | cough, fever and sore throat | RT-PCR | 206 (87.3%) | NA1, GA2 | 30 (12.7%) | BA-9, BA-10 and the new BA-13 genotype | NR | [85] | |
| 2011–2012 | <5 | 610 | 119 | 91 (76.5%) | 28 (23.5%) | NR | ||||||||
| 2012–2013 | <5 | 534 | 117 | 70 (59.8%) | 47 (40.2%) | NR | ||||||||
| 2010–2011 | 6 weeks to 2 years | 169 | 30 | 66.7 | 33.3 | Severe pneumonia | PCR | NR | NR | NR | NR | NR | [170] | |
| 2011–2012 | <2 | 155 | 104 | NR | NR | coughing, runny nose, plus one of the following: wheezing, tachypnea, dyspnea, cyanosis, intercostal retractions, congestion, and/or crepitations on lung auscultation | NR | 59 (58.9%) | NR | 42 (41.1%) | NR | 7 (6.7%) | [171] | |
| 2011–2012 | <2 | 105 | 75 | 53.3 | 46.7 | Bronchiolitis or pneumonia | Nested RT-PCR | 71 (94.7%) | GA2, NA1 | 4 (5.3%) | BA | 4 (5.3%) | [172] | |
| 2012–2013 | NR | 130 | 23 | NR | NR | NR | NR | NR | NR | NR | NR | NR | [173] | |
| 2014–2015 | NR | 712 | 48 | NR | NR | NR | Magpix platform | NR | NR | NR | NR | NR | [174] | |
| Total Number | 69,981 | 17,106 | 2643 | 1561 | 1336 |
References
- World Health Organization. WHO Strategy to Pilot Global Respiratory Syncytial Virus Surveillance Based on the Global Influenza Surveillance and Response System (GISRS); WHO: Geneva, Switzerland, 2017.
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Al-Toum, R.; Bdour, S.; Ayyash, H. Epidemiology and clinical characteristics of respiratory syncytial virus infections in Jordan. J. Trop. Pediatr. 2006, 52, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Douglas, R.G., Jr. Modes of transmission of respiratory syncytial virus. J. Pediatr. 1981, 99, 100–103. [Google Scholar] [CrossRef]
- El Kholy, A.A.; Mostafa, N.A.; El-Sherbini, S.A.; Ali, A.A.; Ismail, R.I.; Magdy, R.I.; Hamdy, M.S.; Soliman, M.S. Morbidity and outcome of severe respiratory syncytial virus infection. Pediatr. Int. 2013, 55, 283–288. [Google Scholar] [CrossRef]
- Thorburn, K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch. Dis. Child. 2009, 94, 99–103. [Google Scholar] [CrossRef]
- Sow, F.B.; Gallup, J.M.; Krishnan, S.; Patera, A.C.; Suzich, J.; Ackermann, M.R. Respiratory syncytial virus infection is associated with an altered innate immunity and a heightened pro-inflammatory response in the lungs of preterm lambs. Respir. Res. 2011, 12, 106. [Google Scholar] [CrossRef]
- Buonocore, G.; Bracci, R.; Weindling, M. Neonatology: A Practical Approach to Neonatal Diseases; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Halasa, N.; Williams, J.; Faouri, S.; Shehabi, A.; Vermund, S.H.; Wang, L.; Fonnesbeck, C.; Khuri-Bulos, N. Natural history and epidemiology of respiratory syncytial virus infection in the Middle East: Hospital surveillance for children under age two in Jordan. Vaccine 2015, 33, 6479–6487. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chung, C.-H.; Chi, H.; Lin, C.-H. Six-monthly palivizumab prophylaxis effectively reduced RSV-associated hospitalization rates of preterm infants in a subtropical area: A population-based cohort study. Pediatr. Res. 2019, 86, 628–634. [Google Scholar] [CrossRef]
- Schickli, J.H.; Dubovsky, F.; Tang, R.S. Challenges in developing a pediatric RSV vaccine. Hum. Vaccines 2009, 5, 582–591. [Google Scholar] [CrossRef]
- Fulginiti, V.; Eller, J.; Sieber, O.; Joyner, J.; Minamitani, M.; Meiklejohn, G. Respiratory virus immunization: A field trial of two inactivated respiratory virus vaccines.; an aqueous trivalent paratnfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 1969, 89, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R.; Moyes, J.; Hirve, S.; Campbell, H.; Jackson, S.; Moen, A.; Nair, H.; Simões, E.A.; Smith, P.G.; Wairagkar, N. Approaches to use the WHO respiratory syncytial virus surveillance platform to estimate disease burden. Influenza Other Respir. Viruses 2019. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Middle East and North Africa (MENA). Investopedia 2020. Available online: https://www.investopedia.com/terms/m/middle-east-and-north-africa-mena.asp (accessed on 20 March 2020).
- Al-Thani, A. Strain Variation of Respiratory Syncytial Virus in Qatar and Its Relationship to B-Cell Epitopes from the Attachment (G) Protein of RSV (B) Strain; London School of Hygiene & Tropical Medicine: London, UK, 2005. [Google Scholar]
- Simoes, E. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J. Pediatrics 2003, 143, S118–S126. [Google Scholar] [CrossRef]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Dean, A.; Soe, M.M. OpenEpi: A web-based epidemiologic and statistical calculator for public health. Public Health Rep. 2009, 124, 471–474. [Google Scholar] [CrossRef]
- Lowry, R. VassarStats: Website for Statistical Computation 2012. 2014. Available online: http://vassarstats.net/ (accessed on 20 March 2020).
- Faghihloo, E.; Yavarian, J.; Jandaghi, N.Z.; Shadab, A.; Azad, T.M. Genotype circulation pattern of human respiratory syncytial virus in Iran. Infect. Genet. Evol. 2014, 22, 130–133. [Google Scholar] [CrossRef]
- Abou-El-Hassan, H.; Massaad, E.; Soudani, N.; Assaf-Casals, A.; Shaker, R.; Lteif Khoury, M.; Ghanem, S.; Karam, M.; Andary, R.; Saito, R.; et al. Detection of ON1 and novel genotypes of human respiratory syncytial virus and emergence of palivizumab resistance in Lebanon. PLoS ONE 2019, 14, e0212687. [Google Scholar] [CrossRef]
- Abushahin, A.; Janahi, I.; Tuffaha, A. Effectiveness of palivizumab immunoprophylaxis in preterm infants against respiratory syncytial virus disease in Qatar. Int. J. Gen. Med. 2018, 11, 41–46. [Google Scholar] [CrossRef]
- Anderson, E.J.; Carosone-Link, P.; Yogev, R.; Yi, J.; Simões, E.A. Effectiveness of palivizumab in high-risk infants and children: A propensity score weighted regression analysis. Pediatr. Infect. Dis. J. 2017, 36, 699–704. [Google Scholar] [CrossRef]
- Higgins, D.; Trujillo, C.; Keech, C. Advances in RSV vaccine research and development–A global agenda. Vaccine 2016, 34, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Meqdam, M.M.; Nasrallah, G.K. Enhanced detection of respiratory syncytial virus by shell vial in children hospitalised with respiratory illnesses in northern Jordan. J. Med. Virol. 2000, 62, 518–523. [Google Scholar] [CrossRef]
- Ncube, M.; Anyanwu, J.C.; Hausken, K. Inequality, economic growth and poverty in the Middle East and North Africa (MENA). Afr. Dev. Rev. 2014, 26, 435–453. [Google Scholar] [CrossRef]
- Roudi, F. Population Trends and Challenges in the Middle East and North Africa. 2001. Available online: https://www.prb.org/populationtrendsandchallengesinthemiddleeastandnorthafrica/ (accessed on 20 March 2020).
- Shahraz, S.; Forouzanfar, M.H.; Sepanlou, S.G.; BESc, P.N.; Pourmalek, F.; Lozano, R.; Asadi-Lari, M.; Sayyari, A.-A.; Naghavi, M. Population health and burden of disease profile of Iran among 20 countries in the region: From Afghanistan to Qatar and Lebanon. Arch. Iran. Med. 2014, 17, 336–342. [Google Scholar]
- Khuri-Bulos, N.; Williams, J.V.; Shehabi, A.A.; Faouri, S.; Jundi, E.A.; Abushariah, O.; Chen, Q.; Ali, S.A.; Vermund, S.; Halasa, N.B. Burden of respiratory syncytial virus in hospitalized infants and young children in Amman, Jordan. Scand. J. Infect. Dis. 2010, 42, 368–374. [Google Scholar] [CrossRef]
- Abdelmogheth, A.-A.A.; Al-Nair, A.M.A.; Balkhair, A.A.S.; Mahmoud, A.M.; El-Naggari, M. Pattern of Viral Infections among Infants and Children Admitted to the Paediatric Intensive Care Unit at Sultan Qaboos University Hospital, Oman. Sultan Qaboos Univ. Med. J. 2014, 14, e546–e550. [Google Scholar]
- Abubakar, A.; Malik, M.; Pebody, R.; Elkholy, A.; Khan, W.; Bellos, A.; Mala, P. Burden of acute respiratory disease of epidemic and pandemic potential in the WHO Eastern Mediterranean Region: A literature review. Emhj-East. Mediterr. Health J. 2016, 22, 509–522. [Google Scholar] [CrossRef]
- Mullins, J.A.; Lamonte, A.C.; Bresee, J.S.; Anderson, L.J. Substantial variability in community respiratory syncytial virus season timing. Pediatr. Infect. Dis. J. 2003, 22, 857–863. [Google Scholar] [CrossRef]
- Yusuf, S.; Piedimonte, G.; Auais, A.; Demmler, G.; Krishnan, S.; Van Caeseele, P.; Singleton, R.; Broor, S.; Parveen, S.; Avendano, L. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol. Infect. 2007, 135, 1077–1090. [Google Scholar] [CrossRef]
- Panda, S.; Mohakud, N.K.; Suar, M.; Kumar, S. Etiology, seasonality, and clinical characteristics of respiratory viruses in children with respiratory tract infections in Eastern India (Bhubaneswar, Odisha). J. Med. Virol. 2017, 89, 553–558. [Google Scholar] [CrossRef]
- Broberg, E.K.; Waris, M.; Johansen, K.; Snacken, R.; Penttinen, P.; Network, E.I.S. Seasonality and geographical spread of respiratory syncytial virus epidemics in 15 European countries, 2010 to 2016. Eurosurveillance 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Klaiber-Franco, R.; Paradiso, P.R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 1987, 68, 2521–2524. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Hruska, J. Monoclonal antibodies to respiratory syncytial virus proteins: Identification of the fusion protein. J. Virol. 1983, 47, 171–177. [Google Scholar] [CrossRef]
- Liang, B.; Kabatova, B.; Kabat, J.; Dorward, D.W.; Liu, X.; Surman, S.; Liu, X.; Moseman, A.P.; Buchholz, U.J.; Collins, P.L.; et al. Effects of Alterations to the CX3C Motif and Secreted Form of Human Respiratory Syncytial Virus (RSV) G Protein on Immune Responses to a Parainfluenza Virus Vector Expressing the RSV G Protein. J. Virol. 2019, 93, e02043-18. [Google Scholar] [CrossRef]
- Korsun, N.; Angelova, S.; Tzotcheva, I.; Georgieva, I.; Lazova, S.; Parina, S.; Alexiev, I.; Perenovska, P. Prevalence and genetic characterisation of respiratory syncytial viruses circulating in Bulgaria during the 2014/15 and 2015/16 winter seasons. Pathog. Glob. Health 2017, 111, 351–361. [Google Scholar] [CrossRef]
- Cui, G.; Zhu, R.; Qian, Y.; Deng, J.; Zhao, L.; Sun, Y.; Wang, F. Genetic Variation in Attachment Glycoprotein Genes of Human Respiratory Syncytial Virus Subgroups A and B in Children in Recent Five Consecutive Years. PLoS ONE 2013, 8, e75020. [Google Scholar] [CrossRef]
- Ren, L.; Xiao, Q.; Zhou, L.; Xia, Q.; Liu, E. Molecular characterization of human respiratory syncytial virus subtype B: A novel genotype of subtype B circulating in China. J. Med. Virol. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Sullender, W.M. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef]
- Arbiza, J.; Delfraro, A.; Frabasile, S. Molecular epidemiology of human respiratory syncytial virus in Uruguay: 1985-2001-a review. Mem. Inst. Oswaldo Cruz 2005, 100, 221–230. [Google Scholar] [CrossRef]
- Baek, Y.H.; Choi, E.H.; Song, M.S.; Pascua, P.N.; Kwon, H.I.; Park, S.J.; Lee, J.H.; Woo, S.I.; Ahn, B.H.; Han, H.S.; et al. Prevalence and genetic characterization of respiratory syncytial virus (RSV) in hospitalized children in Korea. Arch. Virol. 2012, 157, 1039–1050. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Du, L.-N.; Chen, X.; Zhao, Y.; Liu, E.-M.; Yang, X.-Q.; Zhao, X.-D. Genetic variability of respiratory syncytial viruses (RSV) prevalent in Southwestern China from 2006 to 2009: Emergence of subgroup B and A RSV as dominant strains. J. Clin. Microbiol. 2010, 48, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, S.; Hindiyeh, M.; Kolet, L.; Regev, L.; Sherbany, H.; Yaary, K.; Mendelson, E.; Mandelboim, M. Epidemiological Changes of Respiratory Syncytial Virus (RSV) Infections in Israel. PLoS ONE 2014, 9, e90515. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Moore, M.L. Influence of respiratory syncytial virus strain differences on pathogenesis and immunity. In Challenges and Opportunities for Respiratory Syncytial Virus Vaccines; Springer: Berlin, Germany, 2013; pp. 59–82. [Google Scholar]
- Cane, P.A.; Pringle, C.R. Evolution of subgroup A respiratory syncytial virus: Evidence for progressive accumulation of amino acid changes in the attachment protein. J. Virol. 1995, 69, 2918–2925. [Google Scholar] [CrossRef]
- Cane, P.A.; Matthews, D.A.; Pringle, C.R. Analysis of relatedness of subgroup A respiratory syncytial viruses isolated worldwide. Virus Res. 1992, 25, 15–22. [Google Scholar] [CrossRef]
- Roca, A.; Loscertales, M.P.; Quinto, L.; Perez-Brena, P.; Vaz, N.; Alonso, P.L.; Saiz, J.C. Genetic variability among group A and B respiratory syncytial viruses in Mozambique: Identification of a new cluster of group B isolates. J. Gen. Virol. 2001, 82, 103–111. [Google Scholar] [CrossRef]
- Cane, P.A. Molecular epidemiology of respiratory syncytial virus. Rev. Med. Virol. 2001, 11, 103–116. [Google Scholar] [CrossRef]
- Eshaghi, A.; Duvvuri, V.R.; Lai, R.; Nadarajah, J.T.; Li, A.; Patel, S.N.; Low, D.E.; Gubbay, J.B. Genetic variability of human respiratory syncytial virus A strains circulating in Ontario: A novel genotype with a 72 nucleotide G gene duplication. PLoS ONE 2012, 7, e32807. [Google Scholar] [CrossRef]
- Hendry, R.M.; Talis, A.L.; Godfrey, E.; Anderson, L.J.; Fernie, B.F.; McIntosh, K. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. J. Infect. Dis. 1986, 153, 291–297. [Google Scholar] [CrossRef]
- Reiche, J.; Schweiger, B. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J. Clin. Microbiol. 2009, 47, 1800–1810. [Google Scholar] [CrossRef]
- Faghihloo, E.; Salimi, V.; Rezaei, F.; Naseri, M.; Mamishi, S.; Mahmoodi, M.; Mokhtari-Azad, T. Genetic Diversity in the G Protein Gene of Human Respiratory Syncytial Virus among Iranian Children with Acute Respiratory Symptoms. Iran. J. Pediatr. 2011, 21, 58–64. [Google Scholar]
- Malekshahi, S.S.; Razaghipour, S.; Samieipoor, Y.; Hashemi, F.B.; Manesh, A.A.R.; Izadi, A.; Faghihloo, E.; Ghavami, N.; Mokhtari-Azad, T.; Salimi, V. Molecular characterization of the glycoprotein and fusion protein in human respiratory syncytial virus subgroup A: Emergence of ON-1 genotype in Iran. Infect. Genet. Evol. 2019, 71, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.S.; Soliman, M.S.; Kamel, M.M.; El-Kholy, A.A. Sequence analysis of the G gene of hRSVA ON1 genotype from Egyptian children with acute respiratory tract infections. J. Med. Microbiol. 2018, 67, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Cane, P.A. Analysis of linear epitopes recognised by the primary human antibody response to a variable region of the attachment (G) protein of respiratory syncytial virus. J. Med. Virol. 1997, 51, 297–304. [Google Scholar] [CrossRef]
- Garcia, O.; Martin, M.; Dopazo, J.; Arbiza, J.; Frabasile, S.; Russi, J.; Hortal, M.; Perez-Brena, P.; Martinez, I.; Garcia-Barreno, B.; et al. Evolutionary pattern of human respiratory syncytial virus (subgroup A): Cocirculating lineages and correlation of genetic and antigenic changes in the G glycoprotein. J. Virol. 1994, 68, 5448–5459. [Google Scholar] [CrossRef]
- Martinez, I.; Dopazo, J.; Melero, J.A. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J. Gen. Virol. 1997, 78 Pt 10, 2419–2429. [Google Scholar] [CrossRef]
- Rueda, P.; Delgado, T.; Portela, A.; Melero, J.A.; García-Barreno, B. Premature stop codons in the G glycoprotein of human respiratory syncytial viruses resistant to neutralization by monoclonal antibodies. J. Virol. 1991, 65, 3374–3378. [Google Scholar] [CrossRef]
- Grondahl, B.; Ankermann, T.; von Bismarck, P.; Rockahr, S.; Kowalzik, F.; Gehring, S.; Meyer, C.; Knuf, M.; Puppe, W. The 2009 pandemic influenza A(H1N1) coincides with changes in the epidemiology of other viral pathogens causing acute respiratory tract infections in children. Infection 2014, 42, 303–308. [Google Scholar] [CrossRef]
- Green, H.K.; Ellis, J.; Galiano, M.; Watson, J.M.; Pebody, R.G. Critical care surveillance: Insights into the impact of the 2010/11 influenza season relative to the 2009/10 pandemic season in England. Euro Surveill. 2013, 18. [Google Scholar] [CrossRef]
- Debiaggi, M.; Canducci, F.; Ceresola, E.R.; Clementi, M. The role of infections and coinfections with newly identified and emerging respiratory viruses in children. Virol. J. 2012, 9, 247. [Google Scholar] [CrossRef]
- Madi, N.; Chehadeh, W.; Asadzadeh, M.; Al-Turab, M.; Al-Adwani, A. Analysis of genetic variability of respiratory syncytial virus groups A and B in Kuwait. Arch. Virol. 2018, 163, 2405–2413. [Google Scholar] [CrossRef]
- Trento, A.; Galiano, M.; Videla, C.; Carballal, G.; Garcia-Barreno, B.; Melero, J.A.; Palomo, C. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 2003, 84, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Haider, S.H.; Parveen, S.; Arshad, M.; Alsenaidy, H.A.; Baaboud, A.O.; Mobaireek, K.F.; AlSaadi, M.M.; Alsenaidy, A.M.; Sullender, W. Co-Circulation of 72bp Duplication Group A and 60bp Duplication Group B Respiratory Syncytial Virus (RSV) Strains in Riyadh, Saudi Arabia during 2014. PLoS ONE 2016, 11, e0166145. [Google Scholar] [CrossRef] [PubMed]
- al-Hajjar, S.; Akhter, J.; al Jumaah, S.; Hussain Qadri, S.M. Respiratory viruses in children attending a major referral centre in Saudi Arabia. Ann. Trop. Paediatr. 1998, 18, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Bakir, T.M.F.; Halawani, M.; Ramia, S. Viral Aetiology and Epidemiology of Acute Respiratory Infections in Hospitalized Saudi Children. J. Trop. Pediatr. 1998, 44, 100–103. [Google Scholar] [CrossRef][Green Version]
- Jamjoom, G.A.; al-Semrani, A.M.; Board, A.; al-Frayh, A.R.; Artz, F.; al-Mobaireek, K.F. Respiratory syncytial virus infection in young children hospitalized with respiratory illness in Riyadh. J. Trop. Pediatr. 1993, 39, 346–349. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Farrag, M.A.; Amer, H.M. Genetic diversity in the G protein gene of group A human respiratory syncytial viruses circulating in Riyadh, Saudi Arabia. Arch. Virol. 2014, 159, 73–81. [Google Scholar] [CrossRef]
- Fattouh, A.M.; Mansi, Y.A.; El-Anany, M.G.; El-Kholy, A.A.; El-Karaksy, H.M. Acute lower respiratory tract infection due to respiratory syncytial virus in a group of Egyptian children under 5 years of age. Ital. J. Pediatr. 2011, 37, 14. [Google Scholar] [CrossRef]
- Rajala, M.S.; Sullender, W.M.; Prasad, A.K.; Dar, L.; Broor, S. Genetic variability among Group A and B respiratory syncytial virus isolates from a large referral hospital in New Delhi, India. J. Clin. Microbiol. 2003, 41, 2311–2316. [Google Scholar] [CrossRef][Green Version]
- Kaplan, N.M.; Dove, W.; Abd-Eldayem, S.A.; Abu-Zeid, A.F.; Shamoon, H.E.; Hart, C.A. Molecular epidemiology and disease severity of respiratory syncytial virus in relation to other potential pathogens in children hospitalized with acute respiratory infection in Jordan. J. Med. Virol. 2008, 80, 168–174. [Google Scholar] [CrossRef]
- Naghipour, M.; Cuevas, L.E.; Bakhshinejad, T.; Mansour-Ghanaei, F.; Noursalehi, S.; Alavy, A.; Dove, W.; Hart, C.A. Contribution of viruses, Chlamydia spp. and Mycoplasma pneumoniae to acute respiratory infections in Iranian children. J. Trop. Pediatr. 2007, 53, 179–184. [Google Scholar] [CrossRef][Green Version]
- Fodha, I.; Vabret, A.; Trabelsi, A.; Freymuth, F. Epidemiological and antigenic analysis of respiratory syncytial virus in hospitalised Tunisian children, from 2000 to 2002. J. Med. Virol. 2004, 72, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Fodha, I.; Landolsi, N.; Vabret, A.; Sboui, H.; Trabelsi, A.; Freymuth, F. Epidemiology and clinical presentation of respiratory syncytial virus infection in a Tunisian neonatal unit from 2000 to 2002. Ann. Trop. Paediatr. 2004, 24, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Fodha, I.; Vabret, A.; Ghedira, L.; Seboui, H.; Chouchane, S.; Dewar, J.; Gueddiche, N.; Trabelsi, A.; Boujaafar, N.; Freymuth, F. Respiratory syncytial virus infections in hospitalized infants: Association between viral load, virus subgroup, and disease severity. J. Med. Virol. 2007, 79, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Derrar, F.; Izri, K.; Kaddache, C.; Boukari, R.; Hannoun, D. Virologic study of acute lower respiratory tract infections in children admitted to the paediatric department of Blida University Hospital, Algeria. New Microbes New Infect. 2019, 30, 100536. [Google Scholar] [CrossRef] [PubMed]
- Al-shebani, A.; Aubaid, A. Identifying of human metapneumovirus and its phenotype as a causative agents of pneumonia in children. Asian J. Pharm. Clin. Res. 2018, 11, 450. [Google Scholar] [CrossRef][Green Version]
- Eifan, S.A.; Hanif, A.; AlJohani, S.M.; Atif, M. Respiratory Tract Viral Infections and Coinfections Identified by Anyplex II RV16 Detection Kit in Pediatric Patients at a Riyadh Tertiary Care Hospital. Biomed. Res. Int. 2017, 2017, 1928795. [Google Scholar] [CrossRef]
- Othman, H.; Alsharany, W.; Hassan, D.; Soliman, M.; Wagih, R. Respiratory syncytial virus and human metapneumovirus in severe lower respiratory tract infections in children under two. J. Infect. Dev. Ctries. 2016, 10, 283–289. [Google Scholar] [CrossRef][Green Version]
- Aamir, U.B.; Salman, M.; Nisar, N.; Badar, N.; Alam, M.M.; Ansari, J.; Zaidi, S.S.Z. Molecular characterization of circulating respiratory syncytial virus genotypes in Pakistani children, 2010–2013. J. Infect. Public Health 2019. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Shen, K.; Xie, Z.; Sun, L.; Lu, Q.; Liu, C.; Liang, G.; Beeler, J.A.; Anderson, L.J. Genetic variability of group A and B human respiratory syncytial viruses isolated from 3 provinces in China. Arch. Virol. 2007, 152, 1425–1434. [Google Scholar] [CrossRef]
- Forcic, D.; Ivancic-Jelecki, J.; Mlinaric-Galinovic, G.; Vojnovic, G.; Babic-Erceg, A.; Tabain, I. A study of the genetic variability of human respiratory syncytial virus in Croatia, 2006–2008. J. Med. Virol. 2012, 84, 1985–1992. [Google Scholar] [CrossRef]
- Nakamura, M.; Itokazu, K.; Taira, K.; Kawaki, T.; Kudaka, J.; Nidaira, M.; Okano, S.; Koja, Y.; Tamanaha, K.; Kimura, H.; et al. Genotypic and phylogenetic analysis of the G gene of respiratory syncytial virus isolates in Okinawa, Japan, 2008. Jpn. J. Infect. Dis. 2009, 62, 326–327. [Google Scholar] [PubMed]
- Ostlund, M.R.; Lindell, A.T.; Stenler, S.; Riedel, H.M.; Wirgart, B.Z.; Grillner, L. Molecular epidemiology and genetic variability of respiratory syncytial virus (RSV) in Stockholm, 2002–2003. J. Med. Virol. 2008, 80, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rebuffo-Scheer, C.; Bose, M.; He, J.; Khaja, S.; Ulatowski, M.; Beck, E.T.; Fan, J.; Kumar, S.; Nelson, M.I.; Henrickson, K.J. Whole genome sequencing and evolutionary analysis of human respiratory syncytial virus A and B from Milwaukee, WI 1998–2010. PLoS ONE 2011, 6, e25468. [Google Scholar] [CrossRef] [PubMed]
- Viegas, M.; Mistchenko, A.S. Molecular epidemiology of human respiratory syncytial virus subgroup A over a six-year period (1999–2004) in Argentina. J. Med. Virol. 2005, 77, 302–310. [Google Scholar] [CrossRef]
- Khor, C.S.; Sam, I.C.; Hooi, P.S.; Chan, Y.F. Displacement of predominant respiratory syncytial virus genotypes in Malaysia between 1989 and 2011. Infect. Genet. Evol. 2013, 14, 357–360. [Google Scholar] [CrossRef]
- Pierangeli, A.; Trotta, D.; Scagnolari, C.; Ferreri, M.L.; Nicolai, A.; Midulla, F.; Marinelli, K.; Antonelli, G.; Bagnarelli, P. Rapid spread of the novel respiratory syncytial virus A ON1 genotype, central Italy, 2011 to 2013. Euro Surveill. 2014, 19. [Google Scholar] [CrossRef]
- Sahu, M.; Shukla, M.K.; Barde, P.V. Molecular characterization of human respiratory syncytial virus detected from central India. J. Med. Virol. 2017, 89, 1871–1874. [Google Scholar] [CrossRef]
- Dapat, I.C.; Shobugawa, Y.; Sano, Y.; Saito, R.; Sasaki, A.; Suzuki, Y.; Kumaki, A.; Zaraket, H.; Dapat, C.; Oguma, T.; et al. New genotypes within respiratory syncytial virus group B genotype BA in Niigata, Japan. J. Clin. Microbiol. 2010, 48, 3423–3427. [Google Scholar] [CrossRef]
- Bayrakdar, F.; Kocabas, C.N.; Altas, A.B.; Kavuncuoglu, H.G.; Cosgun, Y.; Misirlioglu, E.D.; Durmaz, I.; Korukluoglu, G.; Ozkul, A. Genetic variability human respiratory syncytial virus subgroups A and B in Turkey during six successive epidemic seasons, 2009–2015. J. Med. Virol. 2018, 90, 456–463. [Google Scholar] [CrossRef]
- Tabatabai, J.; Prifert, C.; Pfeil, J.; Grulich-Henn, J.; Schnitzler, P. Novel respiratory syncytial virus (RSV) genotype ON1 predominates in Germany during winter season 2012–2013. PLoS ONE 2014, 9, e109191. [Google Scholar] [CrossRef]
- Esposito, S.; Piralla, A.; Zampiero, A.; Bianchini, S.; Di Pietro, G.; Scala, A.; Pinzani, R.; Fossali, E.; Baldanti, F.; Principi, N. Characteristics and Their Clinical Relevance of Respiratory Syncytial Virus Types and Genotypes Circulating in Northern Italy in Five Consecutive Winter Seasons. PLoS ONE 2015, 10, e0129369. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Suzuki, A.; Lupisan, S.; Galang, H.; Sombrero, L.; Aniceto, R.; Okamoto, M.; Saito, M.; Fuji, N.; Otomaru, H.; et al. Genetic characterization of human respiratory syncytial virus detected in hospitalized children in the Philippines from 2008 to 2012. J. Clin. Virol. 2013, 57, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.L.; Anand, S.P.; Wadhwa, B.S.; Chadha, M.S. Genetic variability of human respiratory syncytial virus in Pune, Western India. Infect. Genet. Evol. 2013, 20, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Cane, P.A.; Matthews, D.A.; Pringle, C.R. Identification of variable domains of the attachment (G) protein of subgroup A respiratory syncytial viruses. J. Gen. Virol. 1991, 72 Pt 9, 2091–2096. [Google Scholar] [CrossRef]
- Parveen, S.; Sullender, W.M.; Fowler, K.; Lefkowitz, E.J.; Kapoor, S.K.; Broor, S. Genetic variability in the G protein gene of group A and B respiratory syncytial viruses from India. J. Clin. Microbiol. 2006, 44, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.T.; Kuypers, J.; Wald, A.; Englund, J.A. Multiple versus single virus respiratory infections: Viral load and clinical disease severity in hospitalized children. Influenza Other Respir. Viruses 2012, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Jeon, J.S.; Kim, J.W.; Rheem, I. Epidemiology of respiratory viral infection using multiplex rt-PCR in Cheonan, Korea (2006–2010). J. Microbiol. Biotechnol. 2013, 23, 267–273. [Google Scholar] [CrossRef]
- Renois, F.; Talmud, D.; Huguenin, A.; Moutte, L.; Strady, C.; Cousson, J.; Lévêque, N.; Andréoletti, L. Rapid Detection of Respiratory Tract Viral Infections and Coinfections in Patients with Influenza-Like Illnesses by Use of Reverse Transcription-PCR DNA Microarray Systems. J. Clin. Microbiol. 2010, 48, 3836. [Google Scholar] [CrossRef]
- Huang, G.; Yu, D.; Mao, N.; Zhu, Z.; Zhang, H.; Jiang, Z.; Li, H.; Zhang, Y.; Shi, J.; Zhang, S.; et al. Viral Etiology of Acute Respiratory Infection in Gansu Province, China, 2011. PLoS ONE 2013, 8, e64254. [Google Scholar] [CrossRef]
- Suryadevara, M.; Cummings, E.; Bonville, C.A.; Bartholoma, N.; Riddell, S.; Kiska, D.; Rosenberg, H.F.; Domachowske, J.B. Viral Etiology of Acute Febrile Respiratory Illnesses in Hospitalized Children Younger Than 24 Months. Clin. Pediatr. 2011, 50, 513–517. [Google Scholar] [CrossRef]
- Bicer, S.; Giray, T.; Çöl, D.; Erdağ, G.Ç.; Vitrinel, A.; Gürol, Y.; Çelik, G.; Kaspar, Ç.; Küçük, Ö. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital. J. Pediatr. 2013, 39, 22. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, A.; Elsheikh, M.; Janahi, M.; Al Nabet, A.; Caksen, H.; Bener, A. Seasonality and epidemiology of respiratory syncytial virus in Qatar. J. Pediatr. Infect. Dis. 2015, 03, 041–045. [Google Scholar] [CrossRef]
- Canducci, F.; Debiaggi, M.; Sampaolo, M.; Marinozzi, M.; Berrè, S.; Terulla, C.; Gargantini, G.; Cambieri, P.; Romero, E.; Clementi, M. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J. Med. Virol. 2008, 80, 716–723. [Google Scholar] [CrossRef]
- Drews, A.L.; Atmar, R.L.; Glezen, W.P.; Baxter, B.D.; Piedra, P.A.; Greenberg, S.B. Dual respiratory virus infections. Clin. Infect. Dis. 1997, 25, 1421–1429. [Google Scholar] [CrossRef]
- Costa, L.F.; Queiroz, D.A.; Lopes da Silveira, H.; Bernardino Neto, M.; de Paula, N.T.; Oliveira, T.F.; Tolardo, A.L.; Yokosawa, J. Human rhinovirus and disease severity in children. Pediatrics 2014, 133, e312–e321. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the Respiratory Tract: Viral Competition for Resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef]
- Calvo, C.; Garcia-Garcia, M.L.; Pozo, F.; Paula, G.; Molinero, M.; Calderon, A.; González-Esguevillas, M.; Casas, I. Respiratory Syncytial Virus Coinfections With Rhinovirus and Human Bocavirus in Hospitalized Children. Medicine 2015, 94, e1788. [Google Scholar] [CrossRef]
- Essa, S.; Owayed, A.; Altawalah, H.; Khadadah, M.; Behbehani, N.; Al-Nakib, W. Mixed Viral Infections Circulating in Hospitalized Patients with Respiratory Tract Infections in Kuwait. Adv. Virol. 2015, 2015, 714062. [Google Scholar] [CrossRef]
- Malekshahi, S.S.; Azad, T.M.; Yavarian, J.; Shahmahmoodi, S.; Naseri, M.; Rezaei, F. Molecular detection of respiratory viruses in clinical specimens from children with acute respiratory disease in Iran. Pediatr. Infect. Dis. J. 2010, 29, 931–933. [Google Scholar] [CrossRef]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol. Infect. 2015, 143, 37–47. [Google Scholar] [CrossRef]
- Oliveira, T.; Freitas, G.; Ribeiro, L.; Yokosawa, J.; Siqueira, M.M.; Portes, S.; Silveira, H.; Calegari, T.; Costa, L.; Mantese, O. Prevalence and clinical aspects of respiratory syncytial virus A and B groups in children seen at Hospital de Clínicas of Uberlândia, MG, Brazil. Mem. Inst. Oswaldo Cruz 2008, 103, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sangaré, L.; Curtis, M.P.; Ahmad, S. Hospitalization for respiratory syncytial virus among California infants: Disparities related to race, insurance, and geography. J. Pediatr. 2006, 149, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Abdulhaq, A.A.; Basode, V.K.; Hashem, A.M.; Alshrari, A.S.; Badroon, N.A.; Hassan, A.M.; Alsubhi, T.L.; Solan, Y.; Ejeeli, S.; Azhar, E.I. Patterns of Human Respiratory Viruses and Lack of MERS-Coronavirus in Patients with Acute Upper Respiratory Tract Infections in Southwestern Province of Saudi Arabia. Adv. Virol. 2017, 2017, 4247853. [Google Scholar] [CrossRef] [PubMed]
- Albogami, S.S.; Alotaibi, M.R.; Alsahli, S.A.; Masuadi, E.; Alshaalan, M. Seasonal variations of respiratory viruses detected from children with respiratory tract infections in Riyadh, Saudi Arabia. J. Infect. Public Health 2018, 11, 183–186. [Google Scholar] [CrossRef]
- Farshad, N.; Saffar, M.J.; Khalilian, A.R.; Saffar, H. Respiratory viruses in hospitalized children with acute lower respiratory tract infections, Mazandaran Province, Iran. Indian Pediatr. 2008, 45, 590–592. [Google Scholar]
- Nikfar, R.; Shamsizadeh, A.; Makvandi, M.; Khoshghalb, A. Detection of Respiratory Syncytial Virus in Hospitalized Children with Acute Lower Respiratory Tract Infections, Using RT PCR in Ahvaz, Iran. Arch. Pediatr. Infect. Dis. 2013, 1, 118–121. [Google Scholar] [CrossRef]
- Barari Sawadkohi, R.; Mohammadzade, I.; Mohammadpour-Mir, A.; Poor Nasrollah, M.; Valipour, M.; Hosseinzadeh, F.; Saeedi, F. Prevalence of Acute Lower Respiratory Tract Infections due to Respiratory Syncytial Virus in Amirkola Children’s hospital, Northern Iran during March 2008–March 2010. Iran. Red Crescent Med. J. 2012, 14, 680–683. [Google Scholar]
- Moattari, A.; Aleyasin, S.; Emami, A.; Fyruzi, M.; Pirbonyeh, N. The Prevalence of Human Metapneumovirus and Respiratory Syncytial Virus and Coinfection With Both in Hospitalized Children With Acute Respiratory Infection in South of Iran. Arch. Pediatr. Infect. Dis. 2015, 3, e21581. [Google Scholar] [CrossRef]
- Parsania, M.; Poopak, B.; Pouriayevali, M.H.; Haghighi, S.; Amirkhani, A.; Nateghian, A. Detection of Human Metapneumovirus and Respiratory Syncytial Virus by Real-Time Polymerase Chain Reaction Among Hospitalized Young Children in Iran. Jundishapur J. Microbiol. 2016, 9, e32974. [Google Scholar] [CrossRef]
- Pourakbari, B.; Mahmoudi, S.; Movahedi, Z.; Halimi, S.; Momeni, S.; Hosseinpour Sadeghi, R.; Mamishi, S. Viral etiology of acute lower respiratory tract infections in hospitalized young children in a children’s referral hospital in Iran. Turk. J. Pediatr. 2014, 56, 354–359. [Google Scholar]
- Hassanzad, M.; Nadji, S.A.; Darougar, S.; Tashayoie Nejad, S.; Boloursaz, M.; Mahdaviani, S.A.; Baghaie, N.; Ghaffaripour, H.; Velayati, A. Association of specific viral infections with childhood asthma exacerbations. Interv. Med. Appl. Sci. 2018, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Halaji, M.; Hashempour, T.; Moayedi, J.; Pouladfar, G.R.; Khansarinejad, B.; Khashei, R.; Moattari, A.; Musavi, Z.; Ghassabi, F.; Pirbonyeh, N. Viral etiology of acute respiratory infections in children in Southern Iran. Rev. Soc. Bras. Med. Trop. 2019, 52, e20180249. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Yavarian, J.; Karbasizade, V.; Moghim, S.; Esfahani, B.N.; Hosseini, N.S. Phylogenetic analysis of human bocavirus in children with acute respiratory infections in Iran. Acta Microbiol. Immunol. Hung. 2019, 66, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Naja, Z.; Fayad, D.; Khafaja, S.; Chamseddine, S.; Dbaibo, G.; Hanna-Wakim, R. Bronchiolitis Admissions in a Lebanese Tertiary Medical Center: A 10 Years’ Experience. Front. Pediatr. 2019, 7, 189. [Google Scholar] [CrossRef]
- Hamze, M.; Hlais, S.; Rachkidi, J.; Mallat, H.; Lichaa, E.; Zahab, N. Infections with respiratory syncytial virus in North Lebanon--prevalence during winter 2008. East. Mediterr. Health J. 2010, 16, 539–545. [Google Scholar] [CrossRef]
- Zaraket, H.; Dbaibo, G.; Salam, O.; Saito, R.; Suzuki, H. Influenza Virus Infections in Lebanese Children in the 2007–2008 Season. Jpn. J. Infect. Dis. 2009, 62, 137–138. [Google Scholar]
- Finianos, M.; Issa, R.; Curran, M.D.; Afif, C.; Rajab, M.; Irani, J.; Hakimeh, N.; Naous, A.; Hajj, M.J.; Hajj, P.; et al. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J. Med. Virol. 2016, 88, 1874–1881. [Google Scholar] [CrossRef]
- Regev, L.; Hindiyeh, M.; Shulman, L.M.; Barak, A.; Levy, V.; Azar, R.; Shalev, Y.; Grossman, Z.; Mendelson, E. Characterization of human metapneumovirus infections in Israel. J. Clin. Microbiol. 2006, 44, 1484–1489. [Google Scholar] [CrossRef]
- Kassis, I.; Srugo, I.; Srur, S.; Horowitz, Y.; Almagor, T.; Wolf, D.; Kra-oz, Z.; Kennes, Y.; Rishpon, S.; Miron, D. The burden and outcomes of acute bronchiolitis among young children hospitalized in Israel. Harefuah 2009, 148, 748–751, 794, 795. [Google Scholar]
- Friedman, N.; Alter, H.; Hindiyeh, M.; Mendelson, E.; Shemer Avni, Y.; Mandelboim, M. Human Coronavirus Infections in Israel: Epidemiology, Clinical Symptoms and Summer Seasonality of HCoV-HKU1. Viruses 2018, 10, 515. [Google Scholar] [CrossRef]
- Al-Sonboli, N.; Hart, C.A.; Al-Aghbari, N.; Al-Ansi, A.; Ashoor, O.; Cuevas, L.E. Human metapneumovirus and respiratory syncytial virus disease in children, Yemen. Emerg. Infect. Dis. 2006, 12, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Thabet, A.A.; Al-Kohani, A.; Shadoul, A.; Al-Mahaqri, A.; Bin Yahya, M.; Saleh, A.H.; Al-Adeemy, D.; Khan, W.; Malik, M. Characteristics of severe acute respiratory infectionassociated hospitalization in Yemen, 2014/15. East. Mediterr. Health J. 2016, 22, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Muslamani, A.; Farid, E. Clinical Profiles of Infants Hospitalized with Acute Respiratory Syncial Virus Bronchiolitis IN Bahrain. J. Bahrain Med. Soc. Majallat Jam’īyat Al-Atibbā’ Al-Bahraynīyah 2006, 18, 169–173. [Google Scholar]
- Meqdam, M.M.; Subaih, S.H. Rapid detection and clinical features of infants and young children with acute lower respiratory tract infection due to respiratory syncytial virus. FEMS Immunol. Med. Microbiol. 2006, 47, 129–133. [Google Scholar] [CrossRef]
- Bukhari, E.; Malak, M. Viral agents causing acute lower respiratory tract infections in hospitalized children at a tertiary care center in Saudi Arabia. Saudi Med. J. 2013, 34, 1151–1155. [Google Scholar]
- Almajhdi, F.; Al-Jaralla, A.; Elaeed, M.; Latif, A.; Gissmann, L.; Amer, H. Prevalence of Respiratory Syncytial Virus Infection in Riyadh During the Winter Season 2007–2008 and Different Risk Factors Impact. Int. J. Virol. 2009, 5, 154–163. [Google Scholar] [CrossRef]
- Amer, H.M.; Alshaman, M.S.; Farrag, M.A.; Hamad, M.E.; Alsaadi, M.M.; Almajhdi, F.N. Epidemiology of 11 respiratory RNA viruses in a cohort of hospitalized children in Riyadh, Saudi Arabia. J. Med. Virol. 2016, 88, 1086–1091. [Google Scholar] [CrossRef]
- Alanazi, A.; Alzahrani, N.; Almutairi, M.; Badri, M.; Aqel, H. Viruses associated with respiratory tract infections in children attending to the emergency room, King Abdulaziz Medical City, Riyadh, Saudi Arabia. World J. Med. Sci. 2013, 8, 103–106. [Google Scholar] [CrossRef]
- Al-Ayed, M.S.; Asaad, A.M.; Qureshi, M.A.; Ameen, M.S. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse-transcriptase polymerase chain reaction. Saudi Med. J. 2014, 35, 1348–1353. [Google Scholar]
- Fagbo, S.F.; Garbati, M.A.; Hasan, R.; AlShahrani, D.; Al-Shehri, M.; AlFawaz, T.; Hakawi, A.; Wani, T.A.; Skakni, L. Acute viral respiratory infections among children in MERS-endemic Riyadh, Saudi Arabia, 2012–2013. J. Med. Virol. 2017, 89, 195–201. [Google Scholar] [CrossRef]
- Hassan, D.A.; Rachid, S.K.; Ziebuhr, J. A Single-Center Study of Viral Respiratory Tract Infections in Hospitalized Children from the Kurdistan Region of Iraq. Glob. Pediatr. Health 2018, 5. [Google Scholar] [CrossRef]
- Al-Charrakh, A.; Al-mola, G.; Al-Azzawi, J. Detection of human Metapneumovirus and respiratory Syncytial Virus associated with asthmatic patients using direct fluorescent assay and Real time—PCR. Wasit J. Sci. Med. 2016, 8, 52–65. [Google Scholar]
- Hatem, A.; Mohamed, S.; Abu Elhassan, U.E.; Ismael, E.A.M.; Rizk, M.S.; El-Kholy, A.; El-Harras, M. Clinical characteristics and outcomes of patients with severe acute respiratory infections (SARI): Results from the Egyptian surveillance study 2010–2014. Multidiscip. Respir. Med. 2019, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Rowlinson, E.; Dueger, E.; Mansour, A.; Azzazy, N.; Mansour, H.; Peters, L.; Rosenstock, S.; Hamid, S.; Said, M.M.; Geneidy, M.; et al. Incidence and etiology of hospitalized acute respiratory infections in the Egyptian Delta. Influenza Other Respir. Viruses 2017, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.M.; El Basha, N.R.; El Rifai, N.M.; El Baz, M.S.; Draz, I.H.; El Kholy, A.A.; Sherif, M.M. Viral causes of acute respiratory infection among Egyptian children hospitalized with severe acute asthma exacerbation. J. Egypt Public Health Assoc. 2013, 88, 52–56. [Google Scholar] [CrossRef] [PubMed]
- El Basha, N.; Marzouk, H.; Sherif, M.; El Kholy, A. Prematurity is a significant predictor of worse outcomes in viral bronchiolitis: A comparative study in infancy. J. Egypt Public Health Assoc. 2017, 92, 188–194. [Google Scholar] [CrossRef]
- Gad, M.N.; Refaay, D.; Gad, N.; AZ, M. Viral Infections in Egyptian Hospitalized Children With Acute Respiratory Tract Infections. J. Clin. Cell. Immunol. 2017. [Google Scholar] [CrossRef]
- Thwaites, R.S.; Coates, M.; Ito, K.; Ghazaly, M.; Feather, C.; Abdulla, F.; Tunstall, T.; Jain, P.; Cass, L.; Rapeport, G.; et al. Reduced Nasal Viral Load and IFN Responses in Infants with Respiratory Syncytial Virus Bronchiolitis and Respiratory Failure. Am. J. Respir. Crit. Care Med. 2018, 198, 1074–1084. [Google Scholar] [CrossRef]
- Janahi, I.; Abdulkayoum, A.; Almeshwesh, F.; Alkuwari, M.; Al Hammadi, A.; Alameri, M. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Infect. Dis. 2017, 17, 139. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Alhammadi, A.H.; Khalifa, M.S.; Muneer, E. Does cesarean section pose a risk of respiratory syncytial virus bronchiolitis in infants and children? Asian Pac. J. Trop. Med. 2014, 7 (Suppl. S1), S134–S136. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Alhammadi, A.H.; Chandra, P.; Muneer, E.; Khalifa, M.S. Identifying agents triggering bronchiolitis in the State of Qatar. Int. J. Gen. Med. 2018, 11, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Khamis, F.A.; Al-Kobaisi, M.F.; Al-Areimi, W.S.; Al-Kindi, H.; Al-Zakwani, I. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J. Med. Virol. 2012, 84, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Khadadah, M.; Essa, S.; Higazi, Z.; Behbehani, N.; Al-Nakib, W. Respiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in Kuwait. J. Med. Virol. 2010, 82, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Al-Turab, M.; Chehadeh, W.; Al-Mulla, F.; Al-Nakib, W. Human metapneumovirus in patients with respiratory tract infection in Kuwait. J. Med. Virol. 2011, 83, 1811–1817. [Google Scholar] [CrossRef]
- Essa, S.; Owayed, A.; Altawalah, H.; Khadadah, M.; Behbehani, N.; Al-Nakib, W. The Prevalence of Human Bocavirus, Human Coronavirus-NL63, Human Metapneumovirus, Human Polyomavirus KI and WU in Respiratory Tract Infections in Kuwait. Med. Princ. Pract. 2015, 24, 382–387. [Google Scholar] [CrossRef]
- Berrajah, L.F.; Ben Slama, K.A.; Khbou, I.; Gargouri, S.; Chtourou, A.; Znazen, A.; Kassis, M.; Yaich, S.; Hammami, A.; Hachicha, M.; et al. Virus and Atypical Pathogens Detected in Community-Acquired Lower Respiratory Tract Infection in Infants and Children of Sfax Region, Tunisia. Bull. Soc. Pathol. Exot. 2018, 111, 90–98. [Google Scholar] [CrossRef][Green Version]
- Brini, I.; Guerrero, A.; Hannachi, N.; Bouguila, J.; Orth-Höller, D.; Bouhlel, A.; Boughamoura, L.; Hetzer, B.; Borena, W.; Schiela, B.; et al. Epidemiology and clinical profile of pathogens responsible for the hospitalization of children in Sousse area, Tunisia. PLoS ONE 2017, 12, e0188325. [Google Scholar] [CrossRef]
- Brini Khalifa, I.; Hannachi, N.; Guerrero, A.; Orth-Höller, D.; Bhiri, S.; Bougila, J.; Boughamoura, L.; Merchaoui, S.N.; Sboui, H.; Mahdhaoui, N.; et al. Demographic and seasonal characteristics of respiratory pathogens in neonates and infants aged 0 to 12 months in the Central-East region of Tunisia. J. Med. Virol. 2019, 91, 570–581. [Google Scholar] [CrossRef]
- Brini, I.; Bhiri, S.; Ijaz, M.; Bouguila, J.; Nouri-Merchaoui, S.; Boughammoura, L.; Sboui, H.; Hannachi, N.; Boukadida, J. Temporal and climate characteristics of respiratory syncytial virus bronchiolitis in neonates and children in Sousse, Tunisia, during a 13-year surveillance. Environ. Sci. Pollut. Res. 2018. [Google Scholar] [CrossRef]
- Jroundi, I.; Mahraoui, C.; Benmessaoud, R.; Moraleda, C.; Tligui, H.; Seffar, M.; Kettani, S.E.C.E.; Benjelloun, B.S.; Chaacho, S.; MuÑOz-Almagro, C.; et al. A comparison of human metapneumovirus and respiratory syncytial virus WHO-defined severe pneumonia in Moroccan children. Epidemiol. Infect. 2016, 144, 516–526. [Google Scholar] [CrossRef]
- Jroundi, I.; Mahraoui, C.; Benmessaoud, R.; Moraleda, C.; Tligui, H.; Seffar, M.; Kettani, S.C.; Benjelloun, B.S.; Chaacho, S.; Maaroufi, A.; et al. The Epidemiology and Aetiology of Infections in Children Admitted with Clinical Severe Pneumonia to a University Hospital in Rabat, Morocco. J. Trop. Pediatr. 2014, 60, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Yousafzai, M.T.; Waris, R.; Jafri, F.; Aziz, F.; Abbasi, I.N.; Zaidi, A. RSV associated hospitalizations in children in Karachi, Pakistan: Implications for vaccine prevention strategies. J. Med. Virol. 2017, 89, 1151–1157. [Google Scholar] [CrossRef]
- Ali, A.; Khowaja, A.R.; Bashir, M.Z.; Aziz, F.; Mustafa, S.; Zaidi, A. Role of human metapneumovirus, influenza A virus and respiratory syncytial virus in causing WHO-defined severe pneumonia in children in a developing country. PLoS ONE 2013, 8, e74756. [Google Scholar] [CrossRef] [PubMed]
- Bashir, U.; Nisar, N.; Arshad, Y.; Alam, M.M.; Ashraf, A.; Sadia, H.; Kazi, B.M.; Zaidi, S.S.Z. Respiratory syncytial virus and influenza are the key viral pathogens in children <2 years hospitalized with bronchiolitis and pneumonia in Islamabad Pakistan. Arch. Virol. 2017, 162, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Aamir, U.B.; Alam, M.M.; Sadia, H.; Zaidi, S.S.Z.; Kazi, B.M. Molecular Characterization of Circulating Respiratory Syncytial Virus (RSV) Genotypes in Gilgit Baltistan Province of Pakistan during 2011–2012 Winter Season. PLoS ONE 2013, 8, e74018. [Google Scholar] [CrossRef]
- Rasmussen, Z.; Thomas, E.D.; Bano, N.; Baker, J.M.; Jahan, A.; Azam, S.I.; Jafri, M.H.; Hartz, A.; Hussain, E.; Shah, W.H.; et al. Epidemiology of Childhood Diarrhea in Rural North Pakistan: 20-Year Follow-Up From 1989–1996 to 2012–2014. In Open Forum Infectious Diseases; Oxford University Press: England, UK, 2015; Volume 2. [Google Scholar] [CrossRef]
- Aziz, F.; Kerai, S.; Qureshi, S.; Nisar, I.; Brown, N.; Jehan, F. Microbead array based technology for detection and quantitation of viral respiratory pathogens associated with pneumonia among children. Int. J. Infect. Dis. 2016, 45, 431. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassine, H.M.; Sohail, M.U.; Younes, N.; Nasrallah, G.K. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms 2020, 8, 713. https://doi.org/10.3390/microorganisms8050713
Yassine HM, Sohail MU, Younes N, Nasrallah GK. Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms. 2020; 8(5):713. https://doi.org/10.3390/microorganisms8050713
Chicago/Turabian StyleYassine, Hadi M., Muhammad U. Sohail, Nadin Younes, and Gheyath K. Nasrallah. 2020. "Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region" Microorganisms 8, no. 5: 713. https://doi.org/10.3390/microorganisms8050713
APA StyleYassine, H. M., Sohail, M. U., Younes, N., & Nasrallah, G. K. (2020). Systematic Review of the Respiratory Syncytial Virus (RSV) Prevalence, Genotype Distribution, and Seasonality in Children from the Middle East and North Africa (MENA) Region. Microorganisms, 8(5), 713. https://doi.org/10.3390/microorganisms8050713






