Increasing Cytomegalovirus Detection Rate from Respiratory Tract Specimens by a New Laboratory-Developed Automated Molecular Diagnostic Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Samples and Ethics Statement
2.2. QMT Assay: QIAGEN Artus CMV RG PCR (Q-CMV PCR) Reagents Applied on BD MAX
2.3. Validation of QMT Assay
2.4. RGQ Assay and in-House PCR Assay
2.5. Roche Cobas CAP/CTM CMV Real-Time PCR Assay (Roche Assay)
2.6. Comparison of QMT Assay to the Others Assays
2.7. Statistical Analysis
3. Results
3.1. The Performance of QMT Assay in Various Clinical Specimens
3.2. CMV Nucleic Acid Detected in Simultaneous Specimens of Respiratory Tract and Plasma in Patient with Respiratory Symptoms by QMT Assay
3.3. Accuracy and Precision of QMT Assay
3.4. Analytical Sensitivity and Specificity of QMT Assay
3.5. Comparison of the Performance Between In-House, RGQ, and QMT Assays Using Frozen Residual Plasma Specimens Positive in Roche Assay
3.6. Diagnostic Performance of the In-House, RGQ, and QMT Assays with Various Clinical Specimens
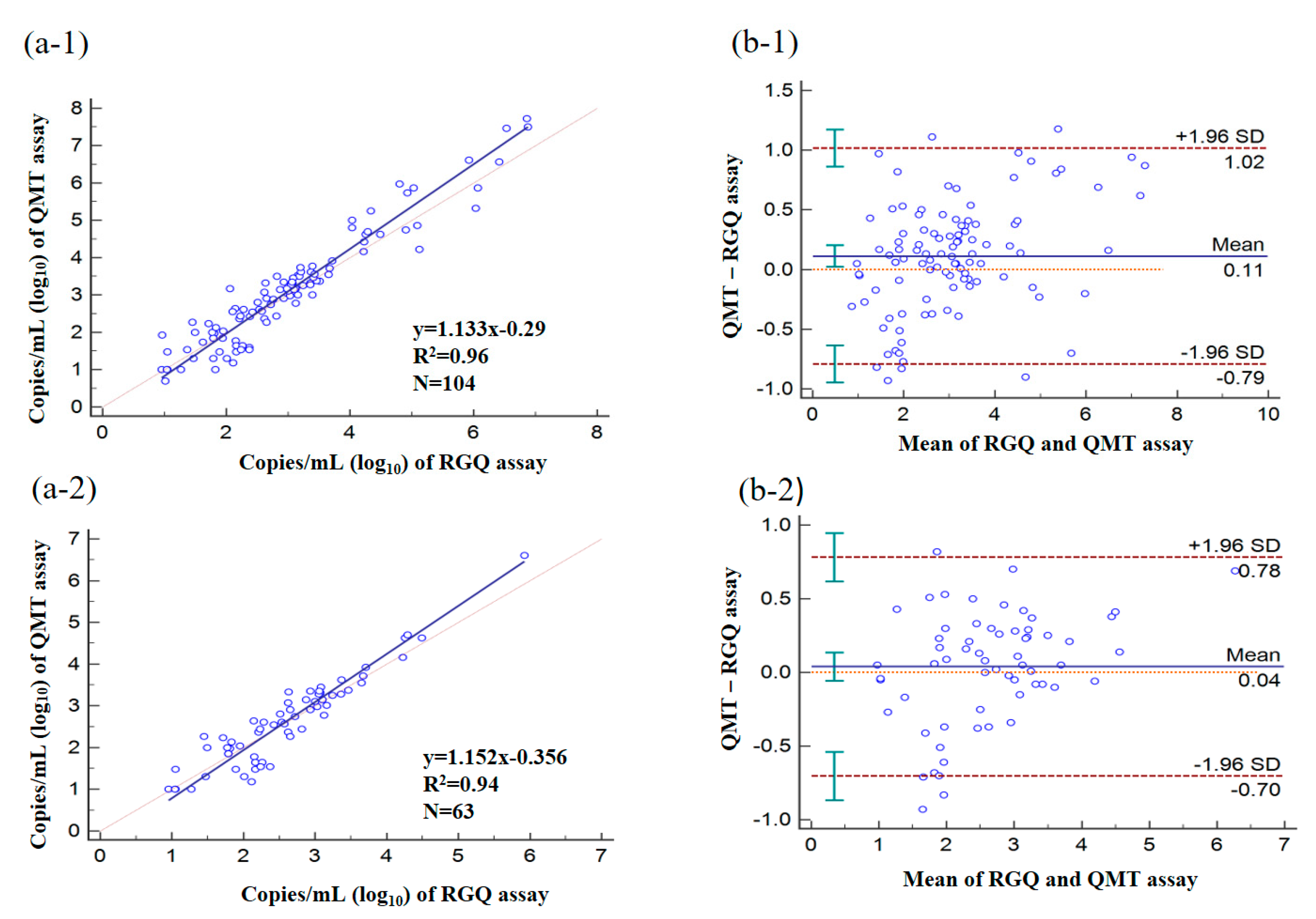
3.7. Quantitative Analysis of Clinical Specimens by Using RGQ and QMT Assays
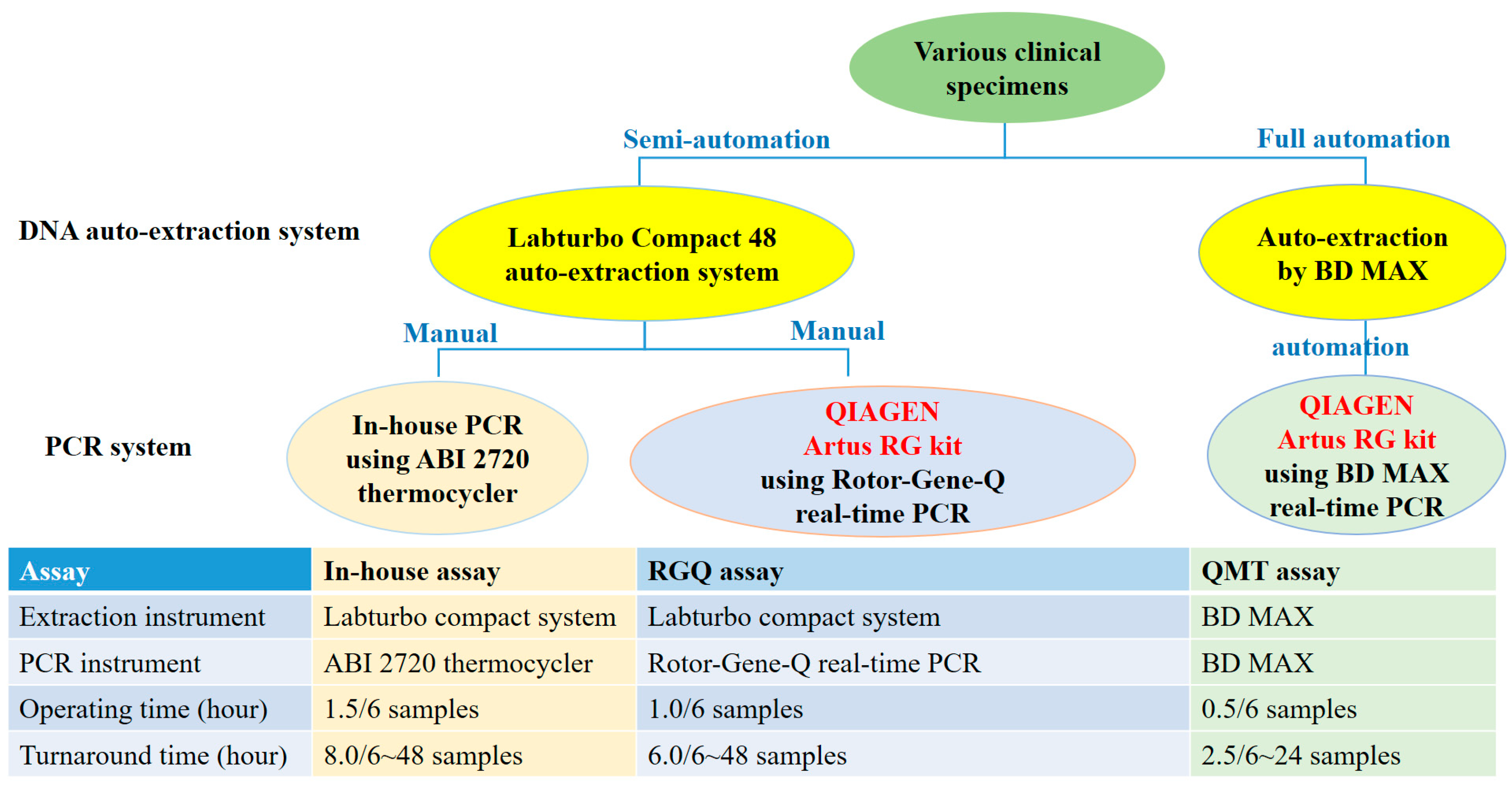
3.8. Comparison of Workflows for the Detection of CMV Nucleic Acid in Various Clinical Specimens
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boeckh, M.; Ljungman, P. How We Treat Cytomegalovirus in Hematopoietic Cell Transplant Recipients. Blood 2009, 23, 5711–5719. [Google Scholar] [CrossRef]
- Mayaphi, S.H.; Brauer, M.; Morobadi, D.M.; Mazanderani, A.H.; Mafuyeka, R.T.; Olorunju, S.A.; Tintinger, G.R.; Stoltz, A. Cytomegalovirus Viral Load Kinetics in Patients with HIV/AIDS Admitted to a Medical Intensive Care Unit: A Case for Pre-Emptive Therapy. PLoS ONE 2014, 4, e93702. [Google Scholar] [CrossRef]
- Ghaffari, S.H.; Obeidi, N.; Dehghan, M.; Alimoghaddam, K.; Gharehbaghian, A.; Ghavamzadeh, A. Monitoring of Cytomegalovirus Reactivation in Bone Marrow Transplant Recipients by Real-Time PCR. Pathol. Oncol. Res. 2008, 4, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Ross, H.; Hunt, S.; Gamberg, P.; Valantine, H.; Merigan, T.C.; Stinson, E.B. Prophylactic Ganciclovir Treatment Reduces Fungal as Well as Cytomegalovirus Infections after Heart Transplantation. Transplantation 1995, 12, 1473–1477. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Waggoner, J.J.; Pinsky, B.A. Cytomegalovirus Load at Treatment Initiation Is Predictive of Time to Resolution of Viremia and Duration of Therapy in Hematopoietic Cell Transplant Recipients. J. Clin. Virol. 2015, 69, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Gruden, J.F.; Huang, L.; Turner, J.; Webb, W.R.; Merrifield, C.; Stansell, J.D.; Gamsu, G.; Hopewell, P.C.; The Transplantation Society. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-Organ Transplantation. Transplantation 2018, 102, 900–931. [Google Scholar]
- Piñana, J.L.; Giménez, E.; Gómez, M.D.; Pérez, A.; González, E.M.; Vinuesa, V.; Hernández-Boluda, J.C.; Montoro, J.; Salavert, M.; Tormo, M.; et al. Pulmonary Cytomegalovirus (CMV) DNA Shedding in Allogeneic Hematopoietic Stem Cell Transplant Recipients: Implications for the Diagnosis of CMV Pneumonia. JInfect 2019, 78, 393–401. [Google Scholar] [CrossRef]
- Port, A.D.; Orlin, A.; Kiss, S.; Patel, S.; D’Amico, D.J.; Gupta, M.P. Cytomegalovirus Retinitis: A Review. JOcul Pharmacol. Ther. 2017, 33, 224–234. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, W.; Lv, H.; Wu, D.; Feng, Y.; Shu, H.; Jin, M.; Hu, L.; Wang, Q.; Wu, D.; et al. The Association between CMV Viremia or Endoscopic Features and Histopathological Characteristics of CMV Colitis in Patients with Underlying Ulcerative Colitis. Inflamm. Bowel. Dis. 2017, 23, 814–821. [Google Scholar] [CrossRef]
- Rabe, T.; Lazar, K.; Cambronero, C.; Goelz, R.; Hamprecht, K. Human Cytomegalovirus (HCMV) Reactivation in the Mammary Gland Induces a Proinflammatory Cytokine Shift in Breast Milk. Microorganisms 2020, 8, 289. [Google Scholar] [CrossRef]
- Caliendo, A.M.; Ingersoll, J.; Fox-Canale, A.M.; Pargman, S.; Bythwood, T.; Hayden, M.K.; Bremer, J.W.; Lurain, N.S. Evaluation of Real-Time PCR Laboratory-Developed Tests Using Analyte-Specific Reagents for Cytomegalovirus Quantification. J. Clin. Microbiol. 2007, 45, 1723–1727. [Google Scholar] [PubMed]
- Khansarinejad, B.; Soleimanjahi, H.; Samiee, S.M.; Hamidieh, A.A.; Paryan, M.; Sanahmadi, Y. Quantitation of Human Cytomegalovirus DNA in Plasma Using an Affordable in-House qPCR Assay. J. Virol. Methods 2012, 183, 170–175. [Google Scholar] [PubMed]
- Talkhabifard, M.; Javid, N.; Moradi, A.; Ghaemi, A.; Tabarraei, A. Evaluation of a Probe-Based PCR-ELISA System for Simultaneous Semi Quantitative Detection and Genotyping of Human Cytomegalovirus (HCMV) Infection in Clinical Specimens. Open Microbiol. J. 2017, 11, 83–91. [Google Scholar] [CrossRef]
- Ganzenmueller, T.; Kluba, J.; Becker, J.U.; Bachmann, O.; Heim, A. Detection of Cytomegalovirus (CMV) by Real-Time PCR in Fecal Samples for the Non-Invasive Diagnosis of CMV Intestinal Disease. J. Clin. Virol. 2014, 61, 517–522. [Google Scholar] [PubMed]
- Bravo, D.; Clari, M.A.; Costa, E.; Munoz-Cobo, B.; Solano, C.; Remigia, M.J.; Navarro, D. Comparative Evaluation of Three Automated Systems for DNA Extraction in Conjunction with Three Commercially Available Real-Time PCR Assays for Quantitation of Plasma Cytomegalovirus DNAemia in Allogeneic Stem Cell Transplant Recipients. J. Clin. Microbiol. 2011, 49, 2899–2904. [Google Scholar] [CrossRef] [PubMed]
- Forman, M.; Wilson, A.; Valsamakis, A. Cytomegalovirus DNA Quantification Using an Automated Platform for Nucleic Acid Extraction and Real-Time PCR Assay Setup. J. Clin. Microbiol. 2011, 49, 2703–2705. [Google Scholar] [PubMed]
- Tsai, H.P.; Tsai, Y.Y.; Lin, I.T.; Kuo, P.H.; Chen, T.Y.; Chang, K.C.; Wang, J.R. Comparison of Two Commercial Automated Nucleic Acid Extraction and Integrated Quantitation Real-Time PCR Platforms for the Detection of Cytomegalovirus in Plasma. PLoS ONE 2016, 11, e0160493. [Google Scholar]
- Hildenbrand, C.; Wedekind, L.; Li, G.; vonRentzell, J.E.; Shah, K.; Rooney, P.; Harrington, A.T.; Zhao, R.Y. Clinical Evaluation of Roche Cobas® Ampliprep/Cobas® Taqman® CMV Test Using Nonplasma Samples. J. Med. Virol. 2018, 90, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, J.J.; Pinsky, B.A. Comparison of Automated Nucleic Acid Extraction Methods for the Detection of Cytomegalovirus DNA in Fluids and Tissues. PeerJ 2014, 2, e334. [Google Scholar] [CrossRef]
- Lee, M.K.; Park, K.Y.; Jin, T.; Kim, J.H.; Seo, S.J. Rapid Detection of Staphylococcus Aureus and Methicillin-Resistant S. Aureus in Atopic Dermatitis by Using the BD Max StaphSR Assay. Ann. Lab Med. 2017, 37, 320–322. [Google Scholar]
- Simner, P.J.; Oethinger, M.; Stellrecht, K.A.; Pillai, D.R.; Yogev, R.; Leblond, H.; Mortensen, J. Multisite Evaluation of the BD Max Extended Enteric Bacterial Panel for Detection of Yersinia Enterocolitica, Enterotoxigenic Escherichia Coli, Vibrio, and Plesiomonas Shigelloides from Stool Specimens. J. Clin. Microbiol. 2017, 55, 3258–3266. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, B.; Williams, J.A.; Fuller, D.; Taylor, S.N.; Hook, E.W. Combined Testing for Chlamydia, Gonorrhea, and Trichomonas by Use of the BD Max CT/GC/TV Assay with Genitourinary Specimen Types. J. Clin. Microbiol. 2017, 55, 155–164. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.P.; Guerendiain, D.; Hardie, A.; Kenicer, J.; MacKenzie, L.; Templeton, K.E. Detection of Norovirus by BD MaxTM, Xpert ® Norovirus, and xTAG® Gastrointestinal Pathogen Panel in Stool and Vomit Samples. J. Clin. Virol. 2018, 105, 72–76. [Google Scholar] [PubMed]
- DeBurger, B.A.; Hanna, S.; Mortensen, J.E. Evaluation of Alternate Parasite Transport Systems for the BD Max Enteric Parasite Panel. Diagn. Microbiol. Infect Dis. 2018, 92, 204–205. [Google Scholar]
- Ellem, J.A.; Kovacevic, D.; Olma, T.; Chen, S.C. Rapid Detection of Group B Streptococcus Directly from Vaginal-Rectal Specimens Using Liquid Swabs and the BD Max GBS Assay. Clin. Microbiol. Infect. 2017, 23, 948–951. [Google Scholar] [CrossRef]
- Silbert, S.; Gostnell, A.; Kubasek, C.; Widen, R. Evaluation of the BD Max StaphSR Assay for Detecting Methicillin-Resistant Staphylococcus Aureus (MRSA) and Methicillin-Susceptible S. Aureus (MSSA) in ESwab-Collected Wound Samples. J. Clin. Microbiol. 2017, 55, 2865–2867. [Google Scholar] [CrossRef][Green Version]
- Cardenas, A.M.; Edelstein, P.H.; Alby, K. Development and Optimization of a Real-Time PCR Assay for Detection of Herpes Simplex and Varicella-Zoster Viruses in Skin and Mucosal Lesions by Use of the BD Max Open System. J. Clin. Microbiol. 2014, 52, 4375–4376. [Google Scholar] [CrossRef]
- Koller, T.; Kurze, D.; Lange, M.; Scherdin, M.; Podbielski, A.; Warnke, P. Implementation and Evaluation of a Fully Automated Multiplex Real-Time PCR Assay on the BD Max Platform to Detect and Differentiate Herpesviridae from Cerebrospinal Fluids. PLoS ONE 2016, 11, e0153991. [Google Scholar]
- Pillet, S.; Verhoeven, P.O.; Epercieux, A.; Bourlet, T.; Pozzetto, B. Development and Validation of a Laboratory-Developed Multiplex Real-Time PCR Assay on the BD Max System for Detection of Herpes Simplex Virus and Varicella-Zoster Virus DNA in Various Clinical Specimens. J. Clin. Microbiol. 2015, 53, 1921–1926. [Google Scholar] [PubMed]
- Souverein, D.; Euser, S.M.; van der Reijden, W.A.; Herpers, B.L.; Kluytmans, J.; Rossen, J.W.A.; den Boer, J.W. Clinical Sensitivity and Specificity of the Check-Points Check-Direct ESBL Screen for BD Max, a Real-Time PCR for Direct ESBL Detection from Rectal Swabs. J. Antimicrob. Chemother. 2017, 72, 2512–2518. [Google Scholar]
- Rocchetti, T.T.; Silbert, S.; Gostnell, A.; Kubasek, C.; Jerris, R.; Vong, J.; Widen, R. Rapid Detection of Four Non-Fermenting Gram-Negative Bacteria Directly from Cystic Fibrosis Patient’s Respiratory Samples on the BD Max System. Pract. Lab Med. 2018, 12, e00102. [Google Scholar] [CrossRef] [PubMed]
- Dalpke, A.H.; Hofko, M.; Zimmermann, S. Development of a Real-Time PCR Protocol Requiring Minimal Handling for Detection of Vancomycin-Resistant Enterococci with the Fully Automated BD Max System. J. Clin. Microbiol. 2016, 54, 2321–2329. [Google Scholar] [CrossRef]
- Shibata, D.; Martin, W.J.; Appleman, M.D.; Causey, D.M.; Leedom, J.M.; Arnheim, N. Detection of Cytomegalovirus DNA in Peripheral Blood of Patients Infected with Human Immunodeficiency Virus. J. Infect. Dis. 1988, 158, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Fahle, G.A.; Fischer, S.H. Comparison of Six Commercial DNA Extraction Kits for Recovery of Cytomegalovirus DNA from Spiked Human Specimens. J. Clin. Microbiol. 2000, 38, 3860–3863. [Google Scholar] [CrossRef] [PubMed]
- Binnicker, M.J.; Espy, M.E. Comparison of Six Real-Time PCR Assays for Qualitative Detection of Cytomegalovirus in Clinical Specimens. J. Clin. Microbiol. 2013, 51, 3749–3752. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR Inhibitors-Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Al-Soud, W.A.; Radstrom, P. Purification and Characterization of PCR-Inhibitory Components in Blood Cells. J. Clin. Microbiol. 2001, 39, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Seet, H.; Khan, Y.; Wright, C.; Nadarajah, R. Comparison of QIAGEN Automated Nucleic Acid Extraction Methods for CMV Quantitative PCR Testing. Am. J. Clin. Pathol. 2010, 133, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Mantovani, S.; Balloco, C.; Sidoti, F.; Fop, F.; Cavallo, R. Comparison of Two Nucleic Acid Extraction and Testing Systems for HCMV-DNA Detection and Quantitation on Whole Blood Specimens from Transplant Patients. J. Virol. Methods 2013, 193, 579–582. [Google Scholar] [CrossRef] [PubMed]
| Test | Lysis Time (min) | Lysis Temperature (°C) | Sample Volume for Extraction (µL) | Wash Volume (µL) | Specimen Volume Added to SPT a (µL) |
|---|---|---|---|---|---|
| TM_CMV_STOOL | 20 | 50 | 300 | 500 | 10 |
| TM_CMV_CSF/VTM b/URINE | 9 | 62 | 700 | 500 | 200 |
| TM_CMV_SERUM/PLASMA | 10 | 37 | 700 | 500 | 200 |
| TM_CMV_SPUTUM | 20 | 50 | 700 | 500 | 200 |
| TM_CMV_BAL c | 20 | 62 | 700 | 500 | 200 |
| Assayed | QMT Assay (n = 1067) | Detection Rate of QMT Assay [%(A/B) a] | Invalid Rate of Internal Positive Control PCR [%(A/B) b] | |
|---|---|---|---|---|
| Specimen (Total Number Tested) | CMV Positive | CMV-Negative | ||
| Plasma (426) | 110 | 316 (6) # | 25.8 (110/426) * | 1.4 (6/426) |
| Respiratory tract specimens (293) | 127 | 166 | 43.3 (127/293) | |
| BAL c (167) | 80 | 87 (14) # | 47.9 (80/167) * | 8.4 (14/167) |
| Sputum (57) | 29 | 28 (6) # | 50.9 (29/57) | 10.5 (6/57) |
| Throat swab (36) | 11 | 25 (1) # | 30.6 (11/36) | 2.8 (1/36) |
| NP swab d (33) | 7 | 26 (2) # | 21.2 (7/33) | 6.1 (2/33) |
| Stool (127) | 27 | 100 (32) # | 21.3 (27/127) | 25.2 ** (32/127) |
| Urine (18) | 5 | 13 (3) # | 27.8 (5/18) | 16.7 (3/18) |
| Breast milk (5) | 4 | 1 | 80 (4/5) | 0 (0/5) |
| Vitreous humour (90) | 17 | 73 (6) # | 18.9 (17/90) | 6.7 (6/90) |
| Bone marrow (5) | 1 | 4 (4) # | 20 (1/5) | 80 ** (4/5) |
| CSF (101) | 1 | 100 (4) # | 1.0 (1/101) | 4.0 (4/101) |
| Lung tissue (2) | 2 | 0 | 100 (2/2) | 0 (0/2) |
| Total number | 294 | 773 (78) # | 27.6 (294/1067) | 7.3 (78/1067) |
| Co-morbidity Disease | Collected Specimens from Same Patients for QMT Assay | Detection Rate of QMT Assay | ||||
|---|---|---|---|---|---|---|
| RTS+ and P+ | RTS+ and P− | RTS− and P+ | RTS | p | p Value ** | |
| Transplant—10 # (10 @) | 6 (6) | 4 (4) | 0 | 100% (10/10) a | 60% (6/10) | <0.0001 |
| Malignancy—32 (31) | 20 (19) | 12 (12) | 0 | 100% (32/32) | 62.5 (20/32) | <0.0001 |
| Immunodeficiency—13 (11) | 8 (8) | 5 (3) | 0 | 100% (13/13) | 61.5 (8/13) | <0.0001 |
| Diabetes mellitus, hypertension, cardiovascular disease—33 (32) | 23 (22) | 8 (8) | 2 (2) | 93.9 % (31/33) | 78.1% (25/32) | 0.0043 |
| Other—6 (6) | 2 (2) | 3 (3) | 1 (1) | 83.3% (5/6) | 50% (3/6) | <0.0001 |
| Total episodes & (All patients)—94 (91) | 59 (58) | 32 (30) | 3 (3) | 93.6% (88/94) | 65.9% (62/94) | <0.0001 |
| Qualitative Analysis | |||
|---|---|---|---|
| CAP specimen | QMT assay (log10 IU/mL) | In-house assay | CAP-PT report |
| 18-ID1-03 | Positive (6.15) | Positive | Positive |
| 17-ID1-03 | Negative | Negative | Negative |
| 17-ID1-11 | Positive (4.81) | Positive | Positive |
| 16-ID1-03 | Positive (6.36) | Positive | Positive |
| 16-ID1-11 | Positive (6.79) | Positive | Positive |
| Quantitative Analysis (log10 IU/mL) | |||
| CAP specimen a | QMT assay | Roche assay | CAP-PT mean per group |
| 18-VLS-03 | 3.66 | 3.60 | 3.76 |
| 18-VLS-04 | 4.73 | 4.92 | 4.71 |
| 18-VLS-13 | 3.46 | 3.78 | 3.64 |
| 18-VLS-14 | 4.77 | 4.67 | 4.82 |
| 17-VLS-03 | Negative | Negative | Negative |
| 17-VLS-04 | 3.73 | 3.34 | 3.83 |
| 17-VLS-13 b | 3.49 | 3.95 | 3.78 |
| 17-VLS-14 b | 4.83 | 5.02 | 4.90 |
| 16-VLS-03 | 2.01 | <2.14 | 2.25 |
| 16-VLS-04 | 3.63 | 3.17 | 3.35 |
| 16-VLS-13 | Negative | Negative | Negative |
| 16-VLS-14 | 3.39 | 3.27 | 3.43 |
| Viral Load (IU/mL) | In-House Assay | RGQ Assay | QMT Assay | |||
|---|---|---|---|---|---|---|
| n = 60 | Positive | Negative | Positive | Negative | Positive | Negative |
| <137 (n = 40) | 2 | 38 | 18 | 22 | 26 | 14 |
| 137–500 (n = 10) | 7 | 3 | 10 | 0 | 10 | 0 |
| 500–1000 (n = 10) | 10 | 0 | 10 | 0 | 10 | 0 |
| n = 205 | In-House Assay% (A/B) a | RGQ Assay % (A/B) a | QMT Assay% (A/B) a |
|---|---|---|---|
| Diagnostic sensitivity b | 65.5 (72/110) | 100 (110/110) | 99.1 (109/110) |
| Diagnostic specificity b | 100 (95/95) | 87.3 (83/95) | 91.5 (87/95) |
| Positive predictive value b | 100 (72/72) | 90.2 (110/122) | 93.1 (109/117) |
| Negative predictive value b | 71.4 (95/133) | 100 (83/83) | 98.9 (87/88) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, H.-P.; Yeh, C.-S.; Lin, I.-T.; Ko, W.-C.; Wang, J.-R. Increasing Cytomegalovirus Detection Rate from Respiratory Tract Specimens by a New Laboratory-Developed Automated Molecular Diagnostic Test. Microorganisms 2020, 8, 1063. https://doi.org/10.3390/microorganisms8071063
Tsai H-P, Yeh C-S, Lin I-T, Ko W-C, Wang J-R. Increasing Cytomegalovirus Detection Rate from Respiratory Tract Specimens by a New Laboratory-Developed Automated Molecular Diagnostic Test. Microorganisms. 2020; 8(7):1063. https://doi.org/10.3390/microorganisms8071063
Chicago/Turabian StyleTsai, Huey-Pin, Chun-Sheng Yeh, I-Ting Lin, Wen-Chien Ko, and Jen-Ren Wang. 2020. "Increasing Cytomegalovirus Detection Rate from Respiratory Tract Specimens by a New Laboratory-Developed Automated Molecular Diagnostic Test" Microorganisms 8, no. 7: 1063. https://doi.org/10.3390/microorganisms8071063
APA StyleTsai, H.-P., Yeh, C.-S., Lin, I.-T., Ko, W.-C., & Wang, J.-R. (2020). Increasing Cytomegalovirus Detection Rate from Respiratory Tract Specimens by a New Laboratory-Developed Automated Molecular Diagnostic Test. Microorganisms, 8(7), 1063. https://doi.org/10.3390/microorganisms8071063





