Coral Productivity Is Co-Limited by Bicarbonate and Ammonium Availability
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Experimental Settings
2.2. Water Chemistry
2.3. Symbiodiniaceae Density and Chlorophylls Content
2.4. Pigment Content Analysis
2.5. Chlorophyll Fluorescence Measurements
2.6. Oxygen Exchange Measurements
2.7. Calcification and Ammonium Uptake Rates
2.8. Statistical Analysis
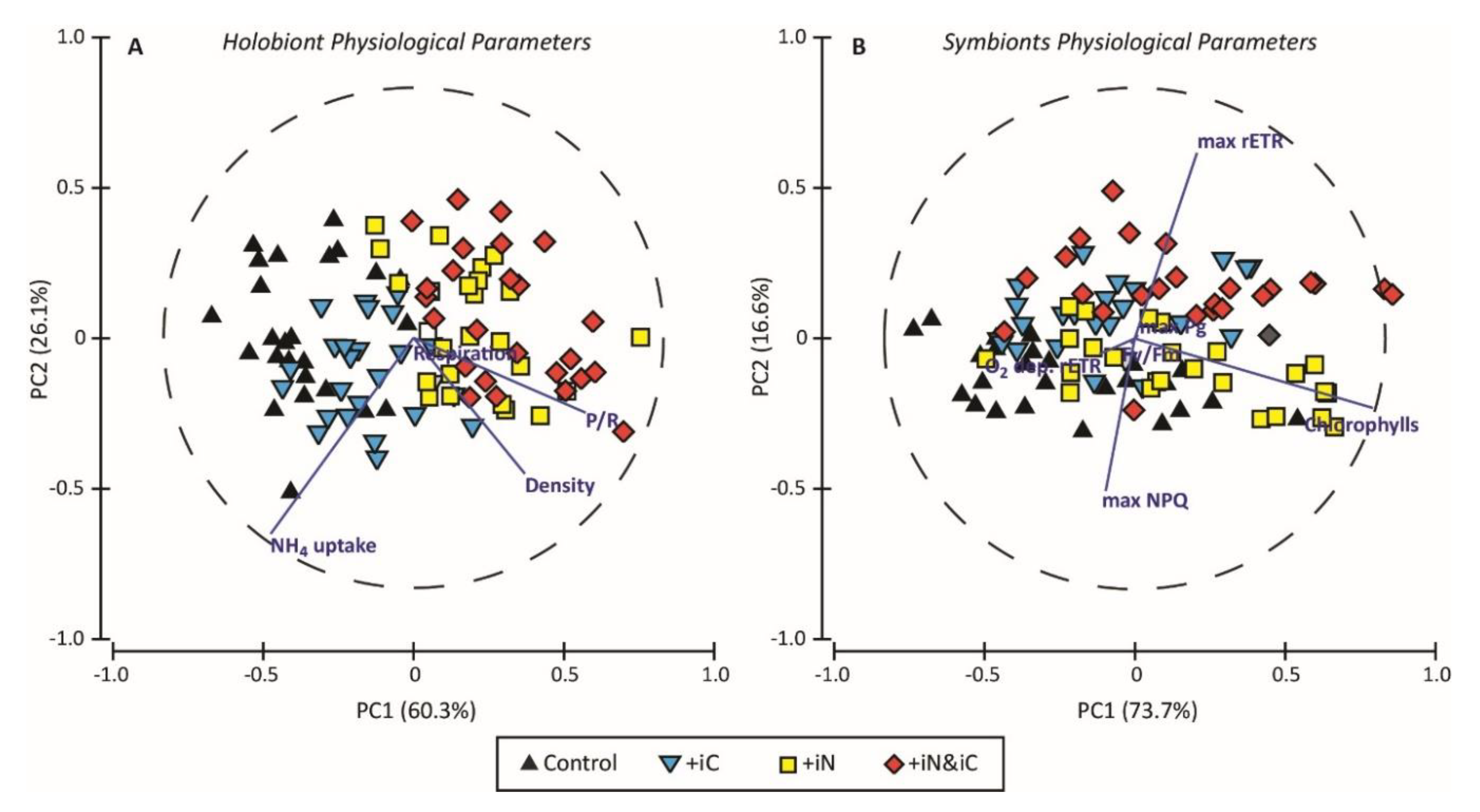
3. Results
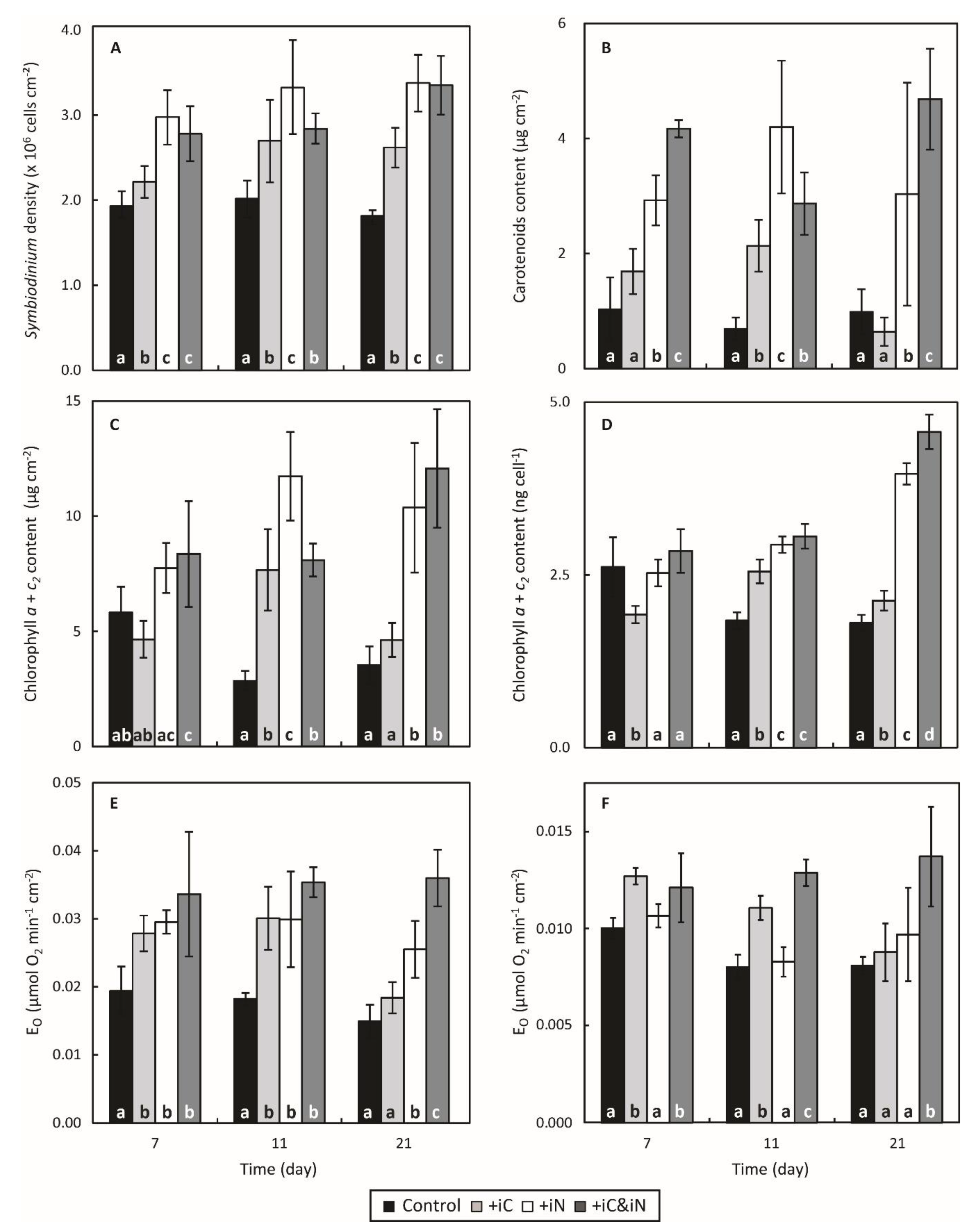
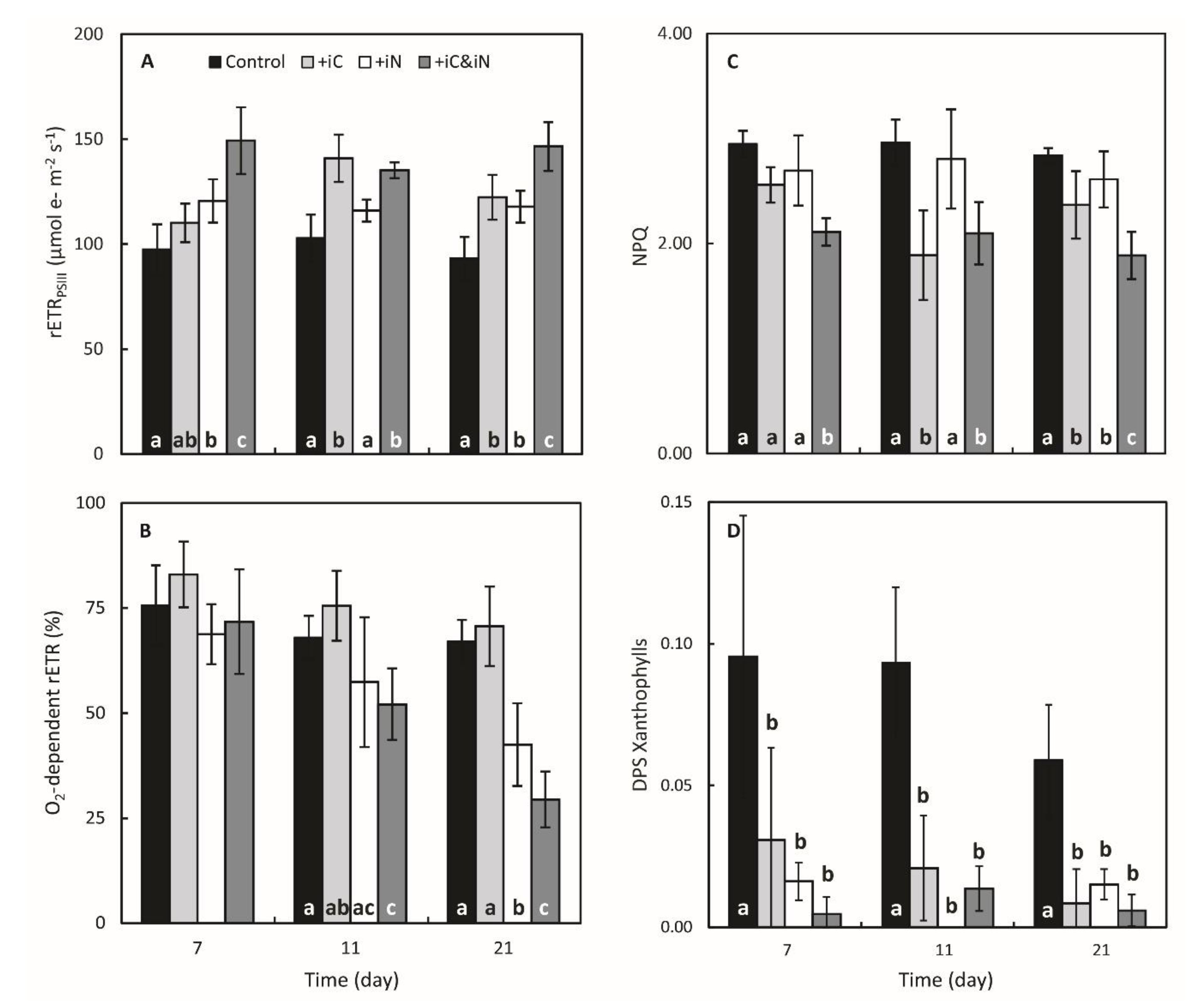
3.1. Effects of Increased Ammonium Supply Compared to Control Condition.
3.2. Effects of the Increased Bicarbonate Supply Compared to Control Condition
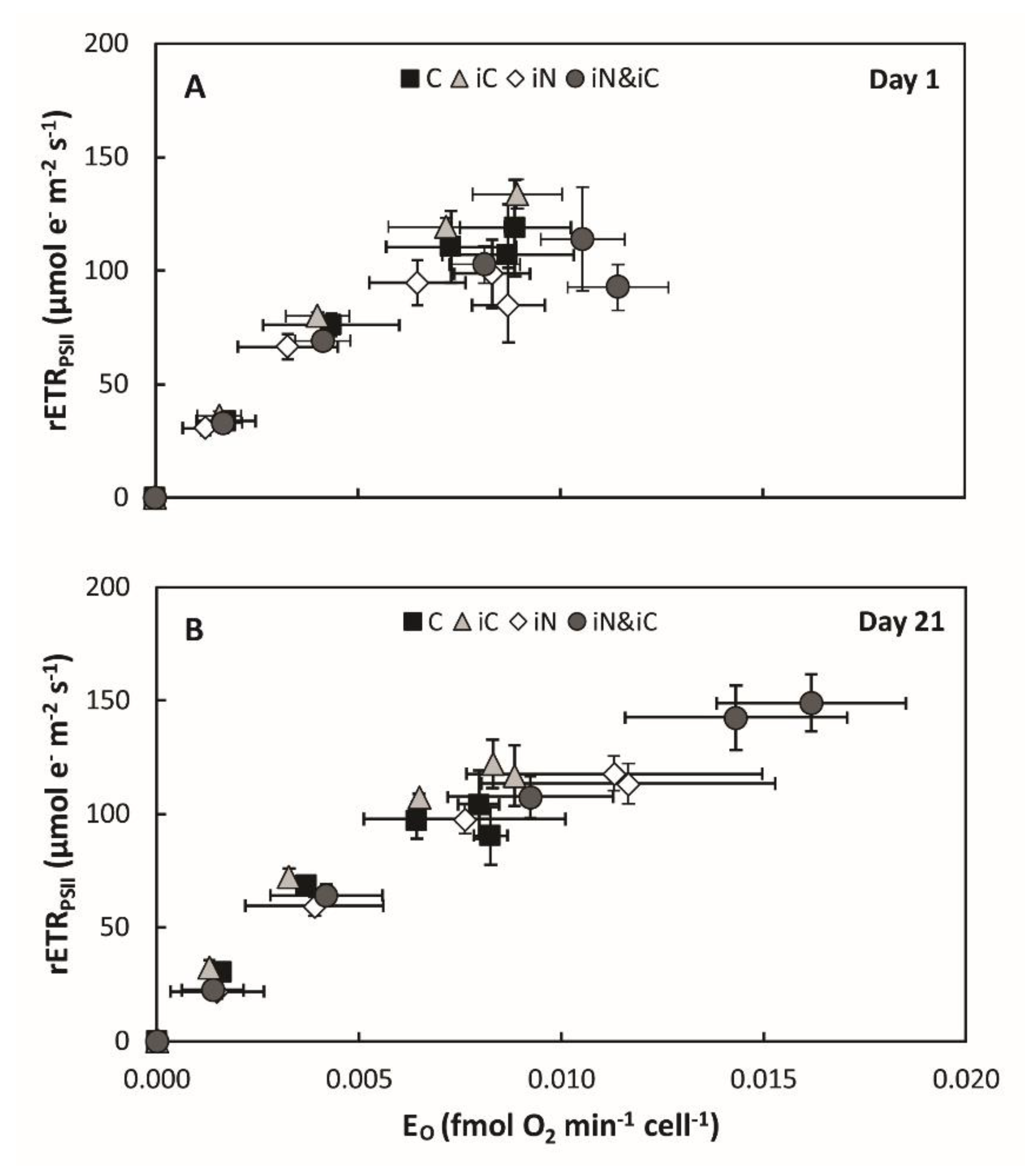
3.3. Effects of the Combined Supply of Ammonium and Bicarbonate Compared to All Conditions
4. Discussion
4.1. The Increased Availability of HCO3− and NH4+ Improves Photosynthetic Processes in Endosymbionts
4.2. The Increased Availability of HCO3− and NH4+ Sustains the Growth of the Holobiont
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melo Clavijo, J.; Donath, A.; Serôdio, J.; Christa, G. Polymorphic adaptations in metazoans to establish and maintain photosymbioses. Biol. Rev. 2018, 93, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Parkinson, J.E.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018, 28, 2570–2580.e6. [Google Scholar] [CrossRef] [PubMed]
- Muscatine, L.; Porter, J.W. Reef corals: Mutualistic symbioses adapted to nutrient-poor environments. Bioscience 1977, 27, 454–460. [Google Scholar] [CrossRef]
- Knowlton, N.; Brainard, R.E.; Fisher, R.; Moews, M.; Plaisance, L.; Caley, M.J. Coral reef biodiversity. In Life in the World’s Oceans: Diversity Distribution and Abundance; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 65–74. [Google Scholar]
- Venn, A.A.; Loram, J.E.; Douglas, A.E. Photosynthetic symbioses in animals. J. Exp. Bot. 2008, 59, 1069–1080. [Google Scholar] [CrossRef]
- Kopp, C.; Pernice, M.; Domart-Coulon, I.; Djediat, C.; Spangenberg, J.; Alexander, D.; Hignette, M.; Meziane, T.; Meibom, A. Highly dynamic cellular-level response of symbiotic coral to a sudden increase in environmental nitrogen. MBio 2013, 4. [Google Scholar] [CrossRef]
- Falkowski, P.; Dubinsky, Z.; Muscatine, L. Light and the bioenergetics of a symbiotic coral. Bio Sci. 1984, 34, 705–709. [Google Scholar] [CrossRef]
- Tremblay, P.; Grover, R.; Maguer, J.F.; Legendre, L.; Ferrier-Pagès, C. Autotrophic carbon budget in coral tissue: A new 13C-based model of photosynthate translocation. J. Exp. Biol. 2012, 215, 1384–1393. [Google Scholar] [CrossRef]
- Gibbin, E.; Banc-Prandi, G.; Fine, M.; Comment, A.; Meibom, A. A method to disentangle and quantify host anabolic turnover in photosymbiotic holobionts with subcellular resolution. Commun. Biol. 2020, 3, 14. [Google Scholar] [CrossRef]
- Wang, J.T.; Douglas, A.E. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J. Exp. Biol. 1998, 201, 2445–2453. [Google Scholar]
- Yellowlees, D.; Rees, T.A.V.; Leggat, W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef]
- Krueger, T.; Bodin, J.; Horwitz, N.; Loussert-Fonta, C.; Sakr, A.; Escrig, S.; Fine, M.; Meibom, A. Temperature and feeding induce tissue level changes in autotrophic and heterotrophic nutrient allocation in the coral symbiosis – A NanoSIMS study. Sci. Rep. 2018, 8, 12710. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Moya, A.; Tambutté, S.; Allemand, D.; Supuran, C.T.; Zoccola, D. Carbonic anhydrases in anthozoan corals—A review. Bioorganic Med. Chem. 2013, 21, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Tansik, A.; Fitt, W.; Hopkinson, B. External carbonic anhydrase in three Caribbean corals: Quantification of activity and role in CO2 uptake. Coral Reefs 2015, 34, 703–713. [Google Scholar] [CrossRef]
- Tansik, A.L.; Fitt, W.K.; Hopkinson, B.M. Inorganic carbon is scarce for symbionts in scleractinian corals. Limnol. Oceanogr. 2017, 62, 2045–2055. [Google Scholar] [CrossRef]
- Zoccola, D.; Ganot, P.; Bertucci, A.; Caminiti-Segonds, N.; Techer, N.; Voolstra, C.R.; Aranda, M.; Tambutté, E.; Allemand, D.; Casey, J.R. Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 2015, 5, 9983. [Google Scholar] [CrossRef]
- Barott, K.L.; Venn, A.A.; Perez, S.O.; Tambutté, S.; Tresguerres, M. Coral host cells acidify symbiotic algal microenvironment to promote photosynthesis. Proc. Natl. Acad. Sci. 2015, 112, 607–612. [Google Scholar] [CrossRef]
- Leggat, W.; Badger, M.R.; Yellowlees, D. Evidence for an inorganic carbon-concentrating mechanism in the symbiotic dinoflagellate Symbiodinium sp. Plant Physiol. 1999, 121, 1247–1255. [Google Scholar] [CrossRef]
- Brading, P.; Warner, M.E.; Smith, D.J.; Suggett, D.J. Contrasting modes of inorganic carbon acquisition amongst Symbiodinium (Dinophyceae) phylotypes. New Phytol. 2013, 200, 432–442. [Google Scholar] [CrossRef]
- Herfort, L.; Thake, B.; Taubner, I. Bicarbonate stimulation of calcification and photosynthesis in two hermatypic corals. J. Phycol. 2008, 44, 91–98. [Google Scholar] [CrossRef]
- Goiran, C.; Al-Moghrabi, S.; Allemand, D.; Jaubert, J. Inorganic carbon uptake for photosynthesis by the symbiotic coral/dinoflagellate association I. Photosynthetic performances of symbionts and dependence on sea water bicarbonate. J. Exp. Mar. Biol. Ecol. 1996, 199, 207–225. [Google Scholar] [CrossRef]
- Buxton, L.; Badger, M.; Ralph, P. Effects of moderate heat stress and dissolved inorganic carbon concentration on photosynthesis and respiration of Symbiodinium sp. (Dinophyceae) in culture and in symbiosis. J. Phycol. 2009, 45, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.S.; Peckol, P. Interactive effects of nitrogen and dissolved inorganic carbon on photosynthesis, growth, and ammonium uptake of the macroalgae Cladophora vagabunda and Gracilaria tikvahiae. Mar. Biol. 1995, 121, 747–753. [Google Scholar] [CrossRef]
- Di Martino Rigano, V.; Vona, V.; Di Martino, C.; Rigano, C. Effect of darkness and CO2 starvation on NH4+ and NO3− assimilation in the unicellular alga Cyanidium caldarium. Physiol. Plant. 1986, 68, 34–38. [Google Scholar] [CrossRef]
- Gordillo, F.J.; Jimenez, C.; Figueroa, F.L.; Niell, F.X. Influence of elevated CO2 and nitrogen supply on the carbon assimilation performance and cell composition of the unicellular alga Dunaliella viridis. Physiol. Plant. 2003, 119, 513–518. [Google Scholar] [CrossRef]
- Stitt, M.; Krapp, A. The interaction between elevated carbon dioxide and nitrogen nutrition: The physiological and molecular background. Plant Cell Environ. 1999, 22, 583–621. [Google Scholar] [CrossRef]
- Zheng, Z.-L. Carbon and nitrogen nutrient balance signaling in plants. Plant Signaling & Behavior 2009, 4, 584–591. [Google Scholar]
- Henley, W.J.; Levavasseur, G.; Franklin, L.A.; Osmond, C.B.; Ramus, J. Photoacclimation and photoinhibition in Ulva rotundata as influenced by nitrogen availability. Planta 1991, 184, 235–243. [Google Scholar] [CrossRef]
- Shantz, A.A.; Burkepile, D.E. Context-dependent effects of nutrient loading on the coral–algal mutualism. Ecology 2014, 95, 1995–2005. [Google Scholar] [CrossRef]
- D’Angelo, C.; Wiedenmann, J. Impacts of nutrient enrichment on coral reefs: New perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustain. 2014, 7, 82–93. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Rottier, C.; Beraud, E.; Levy, O. Experimental assessment of the feeding effort of three scleractinian coral species during a thermal stress: Effect on the rates of photosynthesis. J. Exp. Mar. Biol. Ecol. 2010, 390, 118–124. [Google Scholar] [CrossRef]
- Béraud, E.; Gevaert, F.; Rottier, C.; Ferrier-Pagès, C. The response of the scleractinian coral Turbinaria reniformis to thermal stress depends on the nitrogen status of the coral holobiont. J. Exp. Biol. 2013, 216, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Ferrier-Pages, C.; Gattuso, J.P.; Dallot, S.; Jaubert, J. Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 2000, 19, 103–113. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Smith, G.J. Influence of the population-density of zooxanthellae and supply of ammonium on the biomass and metabolic characteristics of the reef corals Seriatopora hystrix and Stylophora pistillata. Mar. Ecol. Prog. Ser. 1989, 57, 173–186. [Google Scholar] [CrossRef]
- Muscatine, L.; Falkowski, P.G.; Dubinsky, Z.; Cook, P.A.; McCloskey, L.R. The effect of external nutrient resources on the population dynamics of dooxanthellae in a reef coral. Proc. R. Soc. London Ser. B-Biol. Sci. 1989, 236, 311–324. [Google Scholar]
- Dubinsky, Z.; Stambler, N.; Ben-Zion, M.; McCloskey, L.; Muscatine, L.; Falkowski, P. The effect of external nutrient resources on the optical properties and photosynthetic efficiency of Stylophora pistillata. Proc. R. Soc. Lond. B 1990, 239, 231–246. [Google Scholar]
- Stambler, N.; Popper, N.; Dubinsky, Z.; Stimson, J. Effects of nutrient enrichment and water motion on the coral Pocillopora damicornis. Pac. Sci. 1991, 45, 299–307. [Google Scholar]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Szmant, A.M. Nutrient enrichment on coral reefs: Is it a major cause of coral reef decline? Estuaries 2002, 25, 743–766. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef]
- Weis, V.M. Effect of dissolved inorganic carbon concentration on the photosynthesis of the symbiotic sea-anemone Aiptasiapulchella C arlgren - role of carbonic-anhydrase. J. Exp. Mar. Biol. Ecol. 1993, 174, 209–225. [Google Scholar] [CrossRef]
- Holmes, R.M.; Aminot, A.; Kérouel, R.; Hooker, B.A.; Peterson, B.J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Lewis, E.; Wallace, D.; Allison, L.J. Program Developed for CO2 System Calculations; Carbon Dioxide Information Analysis Center, Managed by Lockheed Martin Energy Research Corporation for the US Department of Energy: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Dickson, A.; Millero, F. Short-term exposure to hypercapnia does not compromise feeding, acid-base balance or respiration of Patella vulgata but surprisingly is accompanied by radula damage. Deep-Sea Research 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Dickson, A.G. Standard potential of the reaction: AgCl(s) + 12H2(g) = Ag(s) + HCl(aq), and and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. The Journal of Chemical Thermodynamics 1990, 22, 113–127. [Google Scholar] [CrossRef]
- Lee, K.; Kim, T.-W.; Byrne, R.H.; Millero, F.J.; Feely, R.A.; Liu, Y.-M. The universal ratio of boron to chlorinity for the North Pacific and North Atlantic oceans. Geochim. Cosmochim. Acta 2010, 74, 1801–1811. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Stimson, J.; Kinzie, R.A. The temporal pattern and rate of release of zooxanthellae from the reef coral Pocillopora damicornis (Linnaeus) under nitrogen-enrichment and control conditions. J. Exp. Mar. Biol. Ecol. 1991, 153, 63–74. [Google Scholar] [CrossRef]
- Courtial, L.; Roberty, S.; Shick, J.M.; Houlbrèque, F.; Ferrier-Pagès, C. Interactive effects of ultraviolet radiation and thermal stress on two reef-building corals. Limnol. Oceanogr. 2017, 62, 1000–1013. [Google Scholar] [CrossRef]
- Roberty, S.; Fransolet, D.; Cardol, P.; Plumier, J.C.; Franck, F. Imbalance between oxygen photoreduction and antioxidant capacities in Symbiodinium cells exposed to combined heat and high light stress. Coral Reefs 2015, 34, 1063–1073. [Google Scholar] [CrossRef]
- Spencer Davies, P. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989, 101, 389–395. [Google Scholar] [CrossRef]
- Dang, K.V.; Pierangelini, M.; Roberty, S.; Cardol, P. Alternative Photosynthetic Electron Transfers and Bleaching Phenotypes Upon Acute Heat Stress in Symbiodinium and Breviolum spp. (Symbiodiniaceae) in Culture. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Roberty, S.; Bailleul, B.; Berne, N.; Franck, F.; Cardol, P. PSI Mehler reaction is the main alternative photosynthetic electron pathway in Symbiodinium sp., symbiotic dinoflagellates of cnidarians. New Phytol. 2014, 204, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Lehnert, E.; Jinkerson, R.E.; Clowez, S.; Kim, R.G.; DeNofrio, J.C.; Pringle, J.R.; Grossman, A.R. Symbiont population control by host-symbiont metabolic interaction in Symbiodiniaceae-cnidarian associations. Nat. Commun. 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Turpin, D.H.; Elrifi, I.R.; Birch, D.G.; Weger, H.G.; Holmes, J.J. Interactions between photosynthesis, respiration, and nitrogen assimilation in microalgae. Can. J. Bot. 1988, 66, 2083–2097. [Google Scholar] [CrossRef]
- Turpin, D.H. Effects of inorganic N availability on algal photosynthesis and carbon metabolism. J. Phycol. 1991, 27, 14–20. [Google Scholar] [CrossRef]
- Fam, R.R.; Hiong, K.C.; Choo, C.Y.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Molecular characterization of a novel algal glutamine synthetase (GS) and an algal glutamate synthase (GOGAT) from the colorful outer mantle of the giant clam, Tridacna squamosa, and the putative GS-GOGAT cycle in its symbiotic zooxanthellae. Gene 2018, 656, 40–52. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef]
- Marubini, F.; Ferrier-Pagès, C.; Furla, P.; Allemand, D. Coral calcification responds to seawater acidification: A working hypothesis towards a physiological mechanism. Coral Reefs 2008, 27, 491–499. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Population dynamics of symbiotic zooxanthellae in the coral Pocillopora damicornis exposed to elevated ammonium (NH4Cl) concentrations. Pac. Sci. 1994, 48, 263–272. [Google Scholar]
- Muller-Parker, G.; McCloskey, L.R.; Hoegh-Guldberg, O.; McAuley, P. Effect of ammonium enrichment on animal and algal biomass of the coral Pocillopora damicornis. Pac. Sci. 1994, 48, 273–283. [Google Scholar]
- Gordillo, F.J.; Niell, F.X.; Figueroa, F.L. Non-photosynthetic enhancement of growth by high CO2 level in the nitrophilic seaweed Ulva rigida C. Agardh (Chlorophyta). Planta 2001, 213, 64–70. [Google Scholar] [CrossRef]
- Lachmann, S.C.; Mettler-Altmann, T.; Wacker, A.; Spijkerman, E. Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol. Evol. 2019, 9, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Zou, D. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 2005, 250, 726–735. [Google Scholar] [CrossRef]
- Luo, Y.; Field, C.B.; Mooney, H.A. Predicting responses of photosynthesis and root fraction to elevated [CO2]a: Interactions among carbon, nitrogen, and growth*. Plant Cell Environ. 1994, 17, 1195–1204. [Google Scholar] [CrossRef]
- Krueger, T.; Horwitz, N.; Bodin, J.; Giovani, M.-E.; Escrig, S.; Fine, M.; Meibom, A. Intracellular competition for nitrogen controls dinoflagellate population density in corals. Proceedings of the Royal Society B 2020, 287, 20200049. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.A.; Voolstra, C.R.; Quigley, K.M.; Bourne, D.G.; Bay, L.K. Nutrient Availability and Metabolism Affect the Stability of Coral–Symbiodiniaceae Symbioses. Trends Microbiol. 2019, 27, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.M.; Freeman, C.J.; Wong, J.C.; Fogel, M.L.; Knowlton, N. Climate change promotes parasitism in a coral symbiosis. ISME J. 2018, 12, 921–930. [Google Scholar] [CrossRef]
- Wooldridge, S.A. Breakdown of the coral-algae symbiosis: Towards formalising a linkage between warm-water bleaching thresholds and the growth rate of the intracellular zooxanthellae. Biogeosciences 2013, 10. [Google Scholar] [CrossRef]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.-E.; Postle, A.D.; Achterberg, E.P. Nutrient enrichment can increase the susceptibility of reef corals to bleaching. Nat. Clim. Chang. 2013, 3, 160. [Google Scholar] [CrossRef]
- Ezzat, L.; Maguer, J.-F.; Grover, R.; Ferrier-Pagès, C. New insights into carbon acquisition and exchanges within the coral-dinoflagellate symbiosis under NH4+ and NO3− supply. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150610. [Google Scholar] [CrossRef]
- Tremblay, P.; Gori, A.; Maguer, J.F.; Hoogenboom, M.; Ferrier-Pagès, C. Heterotrophy promotes the re-establishment of photosynthate translocation in a symbiotic coral after heat stress. Sci. Rep. 2016, 6, 38112. [Google Scholar] [CrossRef]
- Galli, G.; Solidoro, C. ATP supply may contribute to light-enhanced calcification in corals more than abiotic mechanisms. Front. Mar. Sci. 2018, 5, 68. [Google Scholar] [CrossRef]
- Marubini, F.; Thake, B. Bicarbonate addition promotes coral growth. Limnol. Oceanogr. 1999, 44, 716–720. [Google Scholar] [CrossRef]
- Allemand, D.; Furla, P.; Benazet-Tambutte, S. Mechanisms of carbon acquisition for endosymbiont photosynthesis in Anthozoa. Can. J. Bot. Revue Can. De Bot. 1998, 76, 925–941. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyajima, T.; Koike, I.; Hayashibara, T.; Ogawa, H. Imbalanced coral growth between organic tissue and carbonate skeleton caused by nutrient enrichment. Limnol. Oceanogr. 2007, 52, 1139–1146. [Google Scholar] [CrossRef]
- Le Hénaff, M.; Muller-Karger, F.E.; Kourafalou, V.H.; Otis, D.; Johnson, K.A.; McEachron, L.; Kang, H. Coral mortality event in the Flower Garden Banks of the Gulf of Mexico in July 2016: Local hypoxia due to cross-shelf transport of coastal flood waters? Cont. Shelf Res. 2019, 190, 103988. [Google Scholar] [CrossRef]
- Bell, P.R.F.; Elmetri, I.; Lapointe, B.E. Evidence of large-scale chronic eutrophication in the Great Barrier Reef: Quantification of chlorophyll a thresholds for sustaining coral reef communities. AMBIO 2014, 43, 361–376. [Google Scholar] [CrossRef]
| Temp (°C) | pH (NDS scale) | TA (µmol kg−1) | pCO2 (µatm) | [CO2] (µmol kg−1) | [HCO3−] (µmol kg−1) | [CO32−] (µmol kg−1) | Ω aragonite | xCO2 (ppm) | [NH4+] (µM) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 25.03 ± 0.04 | 8.04 ± 0.02 | 2447.82 ± 56.69 | 609.52 ± 29.84 | 16.97 ± 0.84 | 1988.18 ± 34.11 | 186.24 ± 12.09 | 2.89 ± 0.19 | 628.80 ± 30.76 | 0.37 ± 0.24 |
| +iC | 25.21 ± 0.03 | 8.07 ± 0.05 | 6959.37 ± 745.45 | 1646.94 ± 210.85 | 45.64 ± 5.81 | 5710.35 ± 557.25 | 578.23 ± 109.01 | 8.98 ± 1.69 | 1699.63 ± 217.67 | 0.40 ± 0.34 |
| +iN | 25.04 ± 0.04 | 8.16 ± 0.03 | 2310.56 ± 37.54 | 407.70 ± 30.98 | 11.35 ± 0.87 | 1762.06 ± 34.54 | 219.27 ± 12.39 | 3.40 ± 0.19 | 420.61 ± 31.94 | 4.36 ± 1.23 |
| +iC&iN | 25.06 ± 0.09 | 8.07 ± 0.05 | 6931.74 ± 712.58 | 1625.05 ± 129.18 | 45.21 ± 3.54 | 5682.74 ± 475.26 | 578.16 ± 122.35 | 8.97 ± 1.90 | 1676.55 ± 133.43 | 4.46 ± 1.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberty, S.; Béraud, E.; Grover, R.; Ferrier-Pagès, C. Coral Productivity Is Co-Limited by Bicarbonate and Ammonium Availability. Microorganisms 2020, 8, 640. https://doi.org/10.3390/microorganisms8050640
Roberty S, Béraud E, Grover R, Ferrier-Pagès C. Coral Productivity Is Co-Limited by Bicarbonate and Ammonium Availability. Microorganisms. 2020; 8(5):640. https://doi.org/10.3390/microorganisms8050640
Chicago/Turabian StyleRoberty, Stephane, Eric Béraud, Renaud Grover, and Christine Ferrier-Pagès. 2020. "Coral Productivity Is Co-Limited by Bicarbonate and Ammonium Availability" Microorganisms 8, no. 5: 640. https://doi.org/10.3390/microorganisms8050640
APA StyleRoberty, S., Béraud, E., Grover, R., & Ferrier-Pagès, C. (2020). Coral Productivity Is Co-Limited by Bicarbonate and Ammonium Availability. Microorganisms, 8(5), 640. https://doi.org/10.3390/microorganisms8050640






