Inactivation of Shiga Toxin-Producing Escherichia coli in Refrigerated and Frozen Meatballs Using High Pressure Processing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bacterial Strains
2.2. Preparation and Inoculation of Meatballs
2.3. High Pressure Processing Treatments
2.4. Microbiological Analyses
2.5. Statistical Analyses
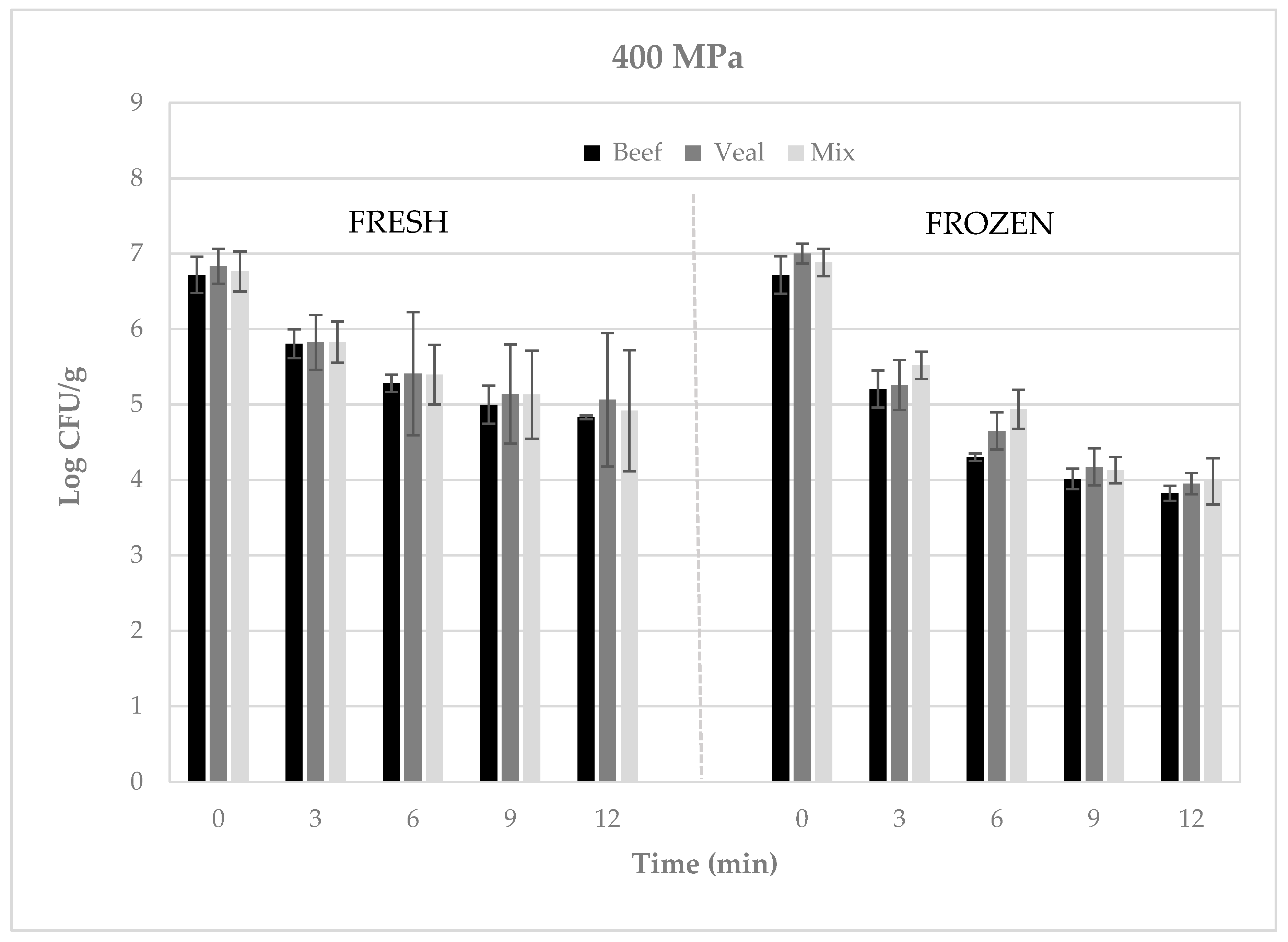
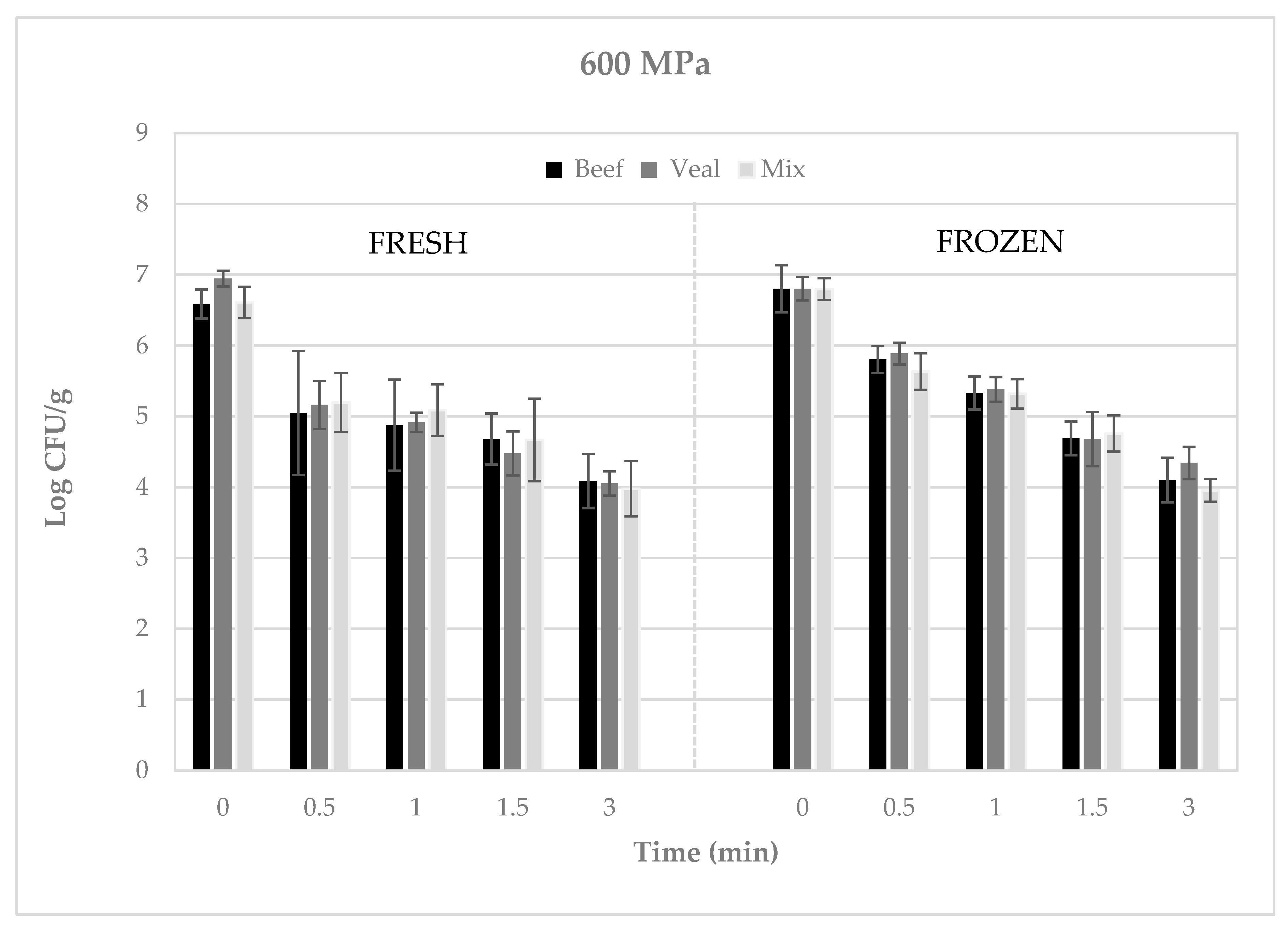
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mathusa, E.C.; Chen, Y.; Enache, E.; Hontz, L. Non-O157 Shiga toxin-producing Escherichia coli in foods. J. Food Prot. 2010, 73, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture, Food Safety and Inspection Service. USDA Targeting Six Additional Strains of E. coli in Raw Beef Trim Starting Monday. 2012. Available online: https://www.usda.gov/media/press-releases/2012/05/31/usda-targeting-six-additional-strains-ecoli-raw-beef-trim-starting (accessed on 17 January 2020).
- Gould, H.; Walsh, K.; Vieira, A.; Herman, K.; Williams, I.; Hall, A.; Cole, D. Surveillance for foodborne disease outbreaks. United States, 1998–2008. MMWR Morb. Mortal Wkly Rep. 2013, 62, 1–34. [Google Scholar]
- Simonin, H.; Duranton, F.; de Lamballerie, M. New insights into the high-pressure processing of meat and meat products. Compr. Rev. Food Sci. Food Saf. 2012, 11, 285–306. [Google Scholar] [CrossRef]
- Cutter, C.; Depasquale, D.; Hayes, J.; Seniviranthne, R.; Raines, C. Meat Science Review: HPP, Ground Beef and the ‘Big 6′ STEC. 2012. Available online: https://www.provisioneronline.com/articles/98113-meat-science-review-hpp-ground-beef-and-the-big-6-stec (accessed on 17 January 2020).
- Morales, P.; Calzada, J.; Avila, M.; Nunez, M. Inactivation of Escherichia coli O157:H7 in ground beef by single-cycle and multiple-cycle high-pressure treatments. J. Food Prot. 2008, 71, 811–815. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture, Food Safety and Inspection Service. Brown Packing Recalls Veal Products due to Possible E. coli Contamination. 2015. Available online: http://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2015/recall-104-2015-release (accessed on 19 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. Marcho Farms, Inc. Recalls Veal, Beef and Pork Products due to Possible non-O157 Shiga Toxin-Producing E. coli (STEC) Adulteration. 2017. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2017/recall-044-2017-release (accessed on 12 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. Cargill Meat Solutions Recalls Ground Beef Products due to Possible E. coli O26 Contamination. 2018. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2018/recall-081-2018-release (accessed on 17 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. Recalls and Public Health Alerts. 2019. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/current-recalls-and-alerts (accessed on 19 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. Majestic Meat Company Recalls Ground Beef Products due to Possible E. coli O157:H7 Contamination. 2018. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2018/recall-113-2018-release (accessed on 17 December 2019).
- Centers for Disease Control and Prevention. Outbreak of E. coli Infections Linked to Ground Beef. 2018. Available online: https://www.cdc.gov/ecoli/2018/o26-09-18/index.html (accessed on 19 December 2019).
- Hanbury, M. Publix Is Recalling Ground Beef Products Because of Possible E. coli Contamination. 2018. Available online: https://www.businessinsider.com/publix-ground-beef-recall-possible-e-coli-2018-8 (accessed on 19 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. King’s Command Foods, LLC. Recalls Beef Products due to Possible Foreign Matter Contamination. 2017. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2017/recall-023-2017-release (accessed on 18 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. Rich Products Corporation Recalls Beef Products due to Possible Listeria Contamination. 2018. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2018/recall-006-2018-release (accessed on 17 December 2019).
- United States Department of Agriculture, Food Safety and Inspection Service. Stino Da Napoli Recalls Various Meat Products Produced without Benefit of Inspection. 2019. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2019/recall-006-2019-release (accessed on 18 December 2019).
- Hsu, H.; Sheen, S.; Sites, J.; Cassidy, J.; Scullen, B.; Sommers, C. Effect of high-pressure processing on the survival of Shiga toxin-producing Escherichia coli (Big Six vs. O157:H7) in ground beef. Food Microbiol. 2015, 48, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Scheinberg, J.A.; Senevirathne, R.; Cutter, C.N. The efficacy of short and repeated high-pressure processing treatments on the reduction of non-O157:H7 Shiga-toxin producing Escherichia coli in ground beef patties. Meat Sci. 2015, 102, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Porto-Fett, A.C.S.; Oliver, M.; Daniel, M.; Shoyer, B.; Stahler, L.; Shane, L.; Kassama, L.S.; Jackson-Davis, A.; Luchansky, J.B. The effect of deep frying or conventional oven cooking on inactivation of Shiga toxin-producing cells of Escherichia coli (STEC) in meatballs. J. Food Prot. 2016, 79, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Porto-Fett, A.C.S.; Call, J.E.; Shoyer, B.A.; Hill, D.E.; Pshebniski, C.; Cocoma, G.J.; Luchansky, J.B. Evaluation of fermentation, drying, and/or high pressure processing on viability of Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp., and Trichinella spiralis in raw pork and Genoa salami. Int. J. Food Microbiol. 2010, 140, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Porto-Fett, A.C.S.; Shoyer, B.A.; Phillips, J.; Eblen, D.; Evans, P.; Bauer, N. Thermal inactivation of a single strain each of serotype O26:H11, O45:H2, O104:H4, O111:H-, O121:H19, O145:NM, and O157:H7 cells of Shiga toxin-producing Escherichia coli in wafers of ground beef. J. Food Prot. 2013, 76, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture, Food Safety and Inspection Service. Testing of Raw Ground Beef Component (RGBC) Samples, Including Veal, for E. coli O157:H7 and Non-O157 Shiga Toxin-Producing E. coli (STEC): Year-to-Date Totals Source and Serotype. 2013. Available online: http://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/microbiology/ec/rgbc-0157h7-and-stec-results-ytd/rgbc-stec-results-beef-veal/rgbc-stec-results-bv (accessed on 17 December 2019).
- Warren, W. Characterization of E. coli O157:H7 Subprimal Beef Cuts Prior to Mechanical Tenderization. National Cattlemens’ Beef Association Research Project Summary. 2002. Available online: https://www.beefresearch.org/CMDocs/BeefResearch/Safety_Project_Summaries/FY02_Characterization_of_E_coli_O157H7_on_Subprimal_Beef.pdf (accessed on 17 January 2020).
- Adler, J.M.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Thermal inactivation of Escherichia coli O157:H7 inoculated at different depths of non-intact blade tenderized beef steaks. J. Food Sci. 2012, 77, M108–M114. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Porto-Fett, A.C.S.; Shoyer, B.A.; Phillips, J.; Chen, V.; Kause, J.; Eblen, D.R.; Cook, V.L.; Mohr, T.B.; Esteban, E.; et al. Fate of Shiga toxin-producing O157:H7 and non-O157:H7 Escherichia coli cells within refrigerated, frozen, or frozen then thawed ground beef patties cooked on a commercial open-flame gas and a clam-shell electric grill. J. Food Prot. 2013, 76, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Luchansky, J.B.; Porto-Fett, A.C.S.; Shoyer, B.A.; Thippareddi, H.; Amaya, J.R.; Lemler, M. Thermal inactivation of Escherichia coli O157:H7 and non-O157 Shiga toxin-producing E. coli cells in mechanically tenderized veal. J. Food Prot. 2014, 77, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Ansay, S.E.; Darling, K.A.; Kasper, C.W. Survival of Escherichia coli O157:H7 in ground-beef patties during storage at 2, -2, 15 and then 12 °C and −20 °C. J. Food Prot. 1999, 62, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Phang, H.S.; Bruhn, C.M. Burger preparation: What consumers say and do in the home. J. Food Prot. 2011, 74, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Bogard, A.K.; Fuller, C.C.; Radke, V.; Selman, C.A.; Smith, K.E. Ground beef handling and cooking practices in restaurants in eight states. J. Food Prot. 2013, 76, 2132–2140. [Google Scholar] [CrossRef]
- Black, E.; Hirneisen, K.; Hoover, D.; Kniel, K. Fate of Escherichia coli O157:H7 in ground beef following high-pressure processing and freezing. J. Appl. Microbiol. 2010, 108, 1352–1360. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Food Safety and Inspection Service. Testing of Raw Ground Beef Component (RGBC) Samples for E. coli O157:H7 and Non-O157 Shiga Toxin-Producing E. coli (STEC): Year-to-Date Totals. 2018. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/microbiology/ec/rgbc-o157h7-and-stec-results-ytd/rgbc-stec-results-bv (accessed on 24 January 2020).
- Garriga, M.; Grebol, N.; Aymerich, M.T.; Monfort, J.M.; Hugas, M. Microbial inactivation after high-pressure processing at 600 MPa in commercial meat products over its shelf life. Innov. Food Sci. Emerg. Technol. 2005, 5, 451–457. [Google Scholar] [CrossRef]
- Gola, S.; Mutti, P.; Manganelli, E.; Sauarcina, N.; Rovere, P. Behaviour of E. coli O157:H7 strains in model system and in raw meat by HPP: Microbial and technological aspects. High Press. Res. 2000, 19, 481–488. [Google Scholar] [CrossRef]
- Ferrante, M. High-Pressure Processing—Keeping Foods Fresh, Healthy, and Safer. 2019. Available online: https://www.foodsafetymagazine.com/enewsletter/high-pressure-processing-keeping-foods-fresh-healthy-and-safer/ (accessed on 17 December 2019).
- Duffy, M. High-Pressure Processing: Outsourcing vs. in House. 2019. Available online: https://www.provisioneronline.com/articles/107538-high-pressure-processing-outsourcing-vs-in-house (accessed on 17 December 2019).
| State of Meat | Pressure (MPa) | Type of Meat | ||
|---|---|---|---|---|
| Veal | Beef | Mixture 1 | ||
| Refrigerated | 400 | 7.10 | 6.54 | 6.84 |
| 600 | 1.14 | 1.45 | 1.32 | |
| Frozen | 400 | 5.69 | 5.89 | 5.90 |
| 600 | 3.86 | 3.60 | 3.56 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto-Fett, A.C.S.; Jackson-Davis, A.; Kassama, L.S.; Daniel, M.; Oliver, M.; Jung, Y.; Luchansky, J.B. Inactivation of Shiga Toxin-Producing Escherichia coli in Refrigerated and Frozen Meatballs Using High Pressure Processing. Microorganisms 2020, 8, 360. https://doi.org/10.3390/microorganisms8030360
Porto-Fett ACS, Jackson-Davis A, Kassama LS, Daniel M, Oliver M, Jung Y, Luchansky JB. Inactivation of Shiga Toxin-Producing Escherichia coli in Refrigerated and Frozen Meatballs Using High Pressure Processing. Microorganisms. 2020; 8(3):360. https://doi.org/10.3390/microorganisms8030360
Chicago/Turabian StylePorto-Fett, Anna C. S., Armitra Jackson-Davis, Lamin S. Kassama, Marciauna Daniel, Michelle Oliver, YangJin Jung, and John B. Luchansky. 2020. "Inactivation of Shiga Toxin-Producing Escherichia coli in Refrigerated and Frozen Meatballs Using High Pressure Processing" Microorganisms 8, no. 3: 360. https://doi.org/10.3390/microorganisms8030360
APA StylePorto-Fett, A. C. S., Jackson-Davis, A., Kassama, L. S., Daniel, M., Oliver, M., Jung, Y., & Luchansky, J. B. (2020). Inactivation of Shiga Toxin-Producing Escherichia coli in Refrigerated and Frozen Meatballs Using High Pressure Processing. Microorganisms, 8(3), 360. https://doi.org/10.3390/microorganisms8030360





